Abstract
Sexual dimorphism results from dichotomous selection on male and female strategies of growth in relation to reproduction. In polygynous mammals, these strategies reflect sexual selection on males for access to females and competitive selection on females for access to food. Consequently, in such species, males display rapid early growth to large adult size, whereas females invest in condition and early sexual maturity at the expense of size. Hence, the magnitude of adult size dimorphism should be susceptible to divergence of the sexes in response to environmental factors differentially influencing their growth to reproduction. We show that divergent growth of male and female red deer after 32 years of winter warming and 15 years of contemporaneously earlier plant phenology support this prediction. In response to warmer climate during their early development, males grew more rapidly and increased in size, while female size declined. Conversely, females, but not males, responded to earlier plant phenology with increased investment in condition and earlier reproduction. Accordingly, adult size dimorphism increased in relation to warmer climate, whereas it declined in relation to forage quality. Thus, the evolutionary trajectories of growth related to reproduction in the sexes (i) originate from sexual and competitive selection, (ii) produce sexual size dimorphism, and (iii) are molded by environmental variation.
Whether attributable to epigamic or intrasexual selection (1), males and females in polygynous vertebrates exhibit widely divergent strategies of growth in relation to reproduction (2), the result of which is sexual size dimorphism (3). In polygynous birds, for example, where sexual selection on body size operates in addition to mate choice by females, males commonly grow to larger adult size and breed later than their smaller conspecific females (4). Similarly, in polygynous mammals, sexual selection favors large male size through rapid early growth and delayed maturation, whereas in females, competitive selection has favored investment in condition and early sexual maturity at the expense of size (5, 6). Consequently, in many mammals, male adult size and reproductive success depend on early development, whereas those of females depend more strongly on investment in condition and reproductive life span (7). It follows that, because compensatory growth in such species is more likely in females than in males (see, e.g., ref. 8), any factor, such as climate, that constrains early development may be more likely to constrain adult size and reproductive success in males than in females (9). If so, a given set of environmental conditions during the early postnatal development of males and females may elicit opposing growth responses in the sexes that exacerbate or constrain adult size dimorphism (10).
Previous studies have illustrated this potential through single-sex responses to environmental variation. Changes in adult dimorphism may occur, for instance, when size of males (but not females) varies with population density, as in white-tailed deer (Odocoileus virginianus) (11). Alternatively, adult dimorphism may vary when size of females (but not males) depends on environmental seasonality, as in Darwin’s finches (Geospiza conirostris) (12), or on intensity of depredation experienced by the population, as in beavers (Castor candensis) (13). To our knowledge, however, adult size dimorphism has not been shown to vary as a result of completely divergent responses of both sexes to environmental change.
Ungulates are among the most sexually dimorphic of mammals (5), and, among ungulates, red deer (Cervus elaphus) display considerable polygyny and adult size dimorphism (14). We investigated the potential for environmental variation caused by climatic fluctuation to shape adult size dimorphism in red deer in Norway through divergent influences on growth of the sexes. Because mammals in general display their greatest mass-specific growth in utero (15), and because abiotic conditions during early development can persist into adulthood (8, 16), our investigation of the influence of environmental variation on growth of red deer in Norway focused on climatic conditions during winter, when they were in utero. In the Northern Hemisphere, the North Atlantic Oscillation (NAO) determines most long-term and interannual variation in winter climate (17) and strongly influences the early development of several species of northern ungulates (18).
Recently, the dichotomous influence of the NAO on the population dynamics of male and female red deer has been demonstrated in several populations. In Norway, for example, abundance of female, but not male, red deer in two populations declined one year after positive NAO winters, whereas in a third population male, but not female, abundance declined after positive NAO winters (19). On the Isle of Rum, Scotland, numbers of red deer males declined, while numbers of females increased, after positive NAO winters (18). It follows from these observations that the NAO, and perhaps climatic fluctuation in general, may also influence the growth and ecology of the sexes differently, thereby contributing to the degree of sexual size dimorphism in adults. We tested this hypothesis by investigating relationships between the NAO and weights of 142 calves and their mothers, and of 32 cohorts of adults, from a natural population in Norway (19).
Additionally, considering the theoretical prediction that growth and reproductive strategies of females in polygynous vertebrates should reflect competitive selection in relation to forage (20, 21), we investigated the potential influence of temporal variation in forage quality on adult size dimorphism through sex-specific growth. To this end, we analyzed weights of 15 cohorts of adults in relation to plant phenology during their first spring. The relationship between plant phenology and forage quality has been documented in many species of forbs; generally, digestibility and the accessibility and concentration of protein and nutrients decline as plant tissues age (22, 23). For this reason, the timing of plant phenology relative to early growth in ungulates is an important determinant of variation in body size among cohorts (16). We used 15 years of data on timing of flowering (24) by the most preferred forb, wood anemone (Anemone nemorosa), in diets of red deer within the range of this population (23). By concentrating our analyses on conditions during early development, we aimed to avoid the confounding influences on adult dimorphism of growth beyond sexual maturity (25), and, by controlling for age, we removed its influence on size and dimorphism in adults (26).
MATERIALS AND METHODS
NAO Index.
The NAO index is quantified for December through March as the average deviation from the long-term mean sea-level surface pressure difference between Lisbon, Portugal, and Stykkisholmer, Iceland (17). The data are at the Climate Indices website: http://www.cgd.ucar.edu:80/cas/climind. In our study site, the NAO index was positively correlated with the average winter (December–March) precipitation (r = 0.41, P = 0.02) and temperature (r = 0.65, P < 0.001), but not snowfall (r = 0.13, P = 0.50), between 1963 and 1994.
Calf-Hind and Fetal Analyses.
All red deer data derive from a single population at the northern extent of the species’ worldwide distribution, in Sør-Trøndelag, Norway (population 4 in ref. 19). We assigned weights of 71 male and 71 female red deer calves and their mothers, collected by the Norwegian Institute for Nature Research (NINA) from harvested animals, to two categories of the winter NAO preceding their birth (cold and warm). Categories were designated as negative or positive values of the NAO index. Because the NAO index is based on deviations from a long-term mean difference, by definition negative values characterize cold winters, whereas positive values characterize warm winters in the eastern Atlantic (17, 27). We estimated mean calf weights by NAO category in their own and their mother’s year of birth by using ANCOVA with the NAO category as the factor, and covariates: May–June degree-days [to account for growth during spring (16)], mother’s age (ln-transformed), mother’s weight, and ln(days since September 1) to account for temporal variation in calf weight. If both mother’s age and weight were significant, we replaced them with a cross term of mother’s weight × age (ln-transformed) to avoid violating the assumption of parallel slopes of covariates. We compared weights of calves of each sex, and of hinds, between cold and warm winters by using the F test. We similarly assigned weights of 52 male and 39 female fetuses collected by NINA between 1969 and 1997 to cold and warm NAO categories and estimated the growth rates of each sex within each category by comparing slopes of regressions of ln(weight) on ln(days since September 1) by using the t test. The distribution of the data by sex did not differ among NAO categories for calves (χ2 = 0.42, P = 0.94) or fetuses (χ2 = 6.01, P = 0.20). In analyses of calf and fetal weights, we used ANCOVA with a categorical NAO index as the factor because low sample sizes (n < 10) in most years precluded correlational or regression analysis.
Adult Sexual Size Dimorphism.
Data on eviscerated weights and mandibles were collected from harvested adult males (n = 3,203) and females (n = 2,047) by NINA between 1957–1996. Adults were assigned to cohorts based on estimates of age from dental cementum annuli; of these, cohorts 1963–1994 contained ≥30 individuals. We estimated cohort-specific mean weights of both sexes by using ANCOVA with ln(weight) as the dependent variable and cohort as the factor, with age and May–June degree-days in the year of birth as covariates. Cohort-specific adult size dimorphism was calculated as ln(♂weight)/ln(♀weight) by year of birth. To test for the influence of climate on cohort-specific adult size dimorphism, estimates were weighted by cohort size and regressed against the NAO index of the winter preceding birth of the cohort. To test for the influence of plant phenology on cohort-specific adult size dimorphism, cohort-weighted estimates were regressed against the first flowering date of A. nemorosa (24) observed near Trondheim in the cohort’s year of birth for cohorts 1963–1977. Significance of correlations was adjusted for autocorrelation (see ref. 18).
Because both time series for adult dimorphism and the NAO index from 1963–1994 were nonstationary, the assumption of independence of error terms was tested and confirmed by plotting the residuals of the regression of these two series against time; there was no relationship (r = 0.26, P = 0.15). Nonetheless, including the term “year” as an independent variable in the regression of adult dimorphism on the NAO index removed the significance of the NAO term, so we cannot exclude the possibility that, in addition to the influence of climate, dimorphism has also changed through the study period with some other variable. However, we emphasize that temporal trends in both series on dimorphism and the NAO were due to outlying endpoints. When these outliers were excluded, a reanalysis of the remaining stationary subset (1964–1991) revealed that the NAO term remained significant (r = 0.53, P = 0.004), while “year” did not enter the regression.
RESULTS
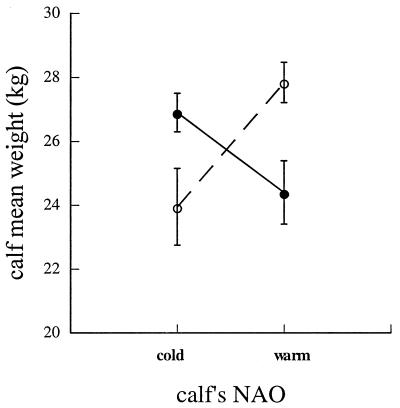
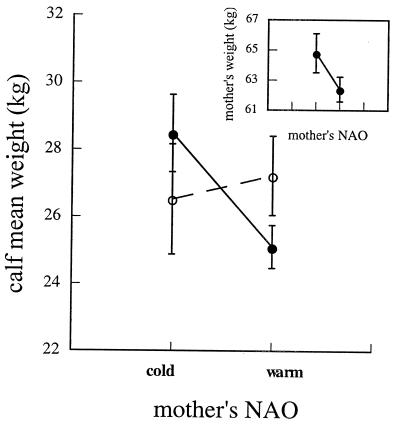
Weights of male and female calves born between 1967 and 1996 displayed completely divergent responses to the state of the NAO during their own fetal development and during the fetal development of their mothers. Whereas weights of male calves increased from those born after cold winters to those born after warm winters, weights of female calves declined along the same gradient (Fig. 1). Like their daughters, hinds born after cold winters were larger than those born after warm winters (Fig. 2 Inset). Additionally, a strong maternal effect on weights of female calves operated in this population as it did in Scottish red deer (16), because small hinds produced small daughters (Fig. 2). In contrast, weights of sons did not vary significantly with the state of the NAO while their mothers were in utero (Fig. 2).
Figure 1.
Least-squares mean weights of male (○) and female (●) red deer calves in relation to the type of NAO winter (c = cold, w = warm) preceding their birth. Males: F(c − w) = 7.86, P = 0.007. Females: F(c − w) = 4.33, P = 0.041.
Figure 2.
Least-squares mean weights of male (○) and female (●) red deer calves in relation to the type of NAO winter preceding the birth of their mother. Males: F(c − w) = 0.25, P = 0.62. Females: F(c − w) = 5.49, P = 0.02. (Inset) Least-squares mean weights of mothers in relation to the type of NAO winter preceding their birth. F(c − w) = 2.15, P = 0.14.
The greater weights of male calves born after warm winters reflected higher fetal growth rates of males carried in utero during warm winters (4.74 ± 0.24) than during cold winters (3.69 ± 0.26) (t = 5.15, P < 0.001). In contrast, fetal growth rates of females did not vary between cold (4.69 ± 0.11) and warm (4.52 ± 0.42) winters (t = 0.96, P > 0.20), so the smaller size of females born after warm winters was related to postnatal growth.
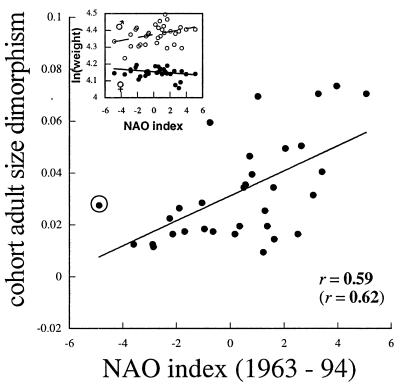
The pattern of divergent fetal growth rates and weights of male and female calves in relation to the state of the NAO while they were in utero was also apparent in cohort-specific mean weights of adults (controlled for age) from the same population. Males born after warm NAO winters were larger as adults, whereas weights of adult females in this sample declined, although nonsignificantly, with the NAO index while they were in utero (Fig. 3 Inset). Consequently, sexual size dimorphism of adults increased with the NAO index of the winter preceding their birth (Fig. 3).
Figure 3.
Relationship between cohort-specific adult sexual size dimorphism [ln(male wt)/ln(female wt)] and the NAO index of the winter preceding birth of the cohort. The circled point significantly influenced the regression, and the correlation excluding it is reported in parentheses. (Inset) Least-squares mean estimates of cohort-specific weights of adult males and females in relation to the NAO index of the winter preceding their birth (males: r = 0.36, P = 0.04; females: r = −0.27, P = 0.14). Bold, P < 0.05.
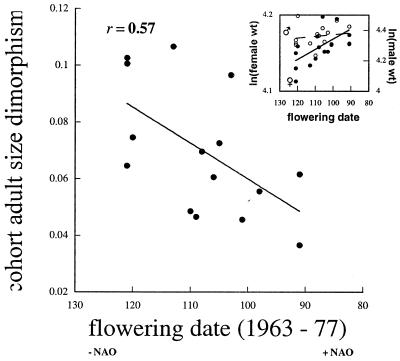
In response to earlier plant phenology after warmer winters (rNAO,phenology = −0.55, P = 0.03), however, adult sexual size dimorphism declined. Cohorts born in years of early plant phenology were less sexually dimorphic as adults than were cohorts born in years of delayed plant phenology (Fig. 4). This relationship reflected an increase in weights of adult females with earlier phenology in their year of birth, and a corresponding lack of such a relationship for males (Fig. 4 Inset). That the increase in female adult weight with early phenology in their year of birth reflects greater investment in condition is supported by two further observations: they lived up to 5 years longer (Y = 20.15 − 0.12x; R2 = 0.41, P = 0.01)‖, and they were up to 25% more likely to breed as yearlings, than females born in years of delayed phenology (18).
Figure 4.
Relationship between cohort-specific adult sexual size dimorphism [ln(male wt)/ln(female wt)] and plant phenology (the first flowering date of A. nemorosa) in the cohort’s year of birth (Julian flowering date). Note the x axis is reversed to represent the same NAO gradient as in Fig. 3. (Inset) Least-squares estimates of cohort-specific mean weights of adult males and females in relation to plant phenology in their year of birth (males: r = −0.16, P = 0.56; females: r = −0.61, P = 0.017). Bold, P < 0.05.
DISCUSSION
Theory predicts that the degree of sexual dimorphism in polygynous species should be determined by the comparative influence of a given phenotypic trait on reproductive success in the sexes (28). Where this trait is size at sexual maturity, an adjunct line of reasoning would predict that the degree of dimorphism should be determined by factors differentially influencing size at maturity in males and females. This is precisely what the results reported here demonstrate. However, interpretation of divergent growth of males and females in response to environmental variation as adaptive requires consideration of the manner in which these responses are likely to influence lifetime reproductive success of the sexes, rather than just size at age of maturity.
In both sexes, natural selection should favor rapid development to sexual maturity, because the sooner an organism matures, the more likely it is to reproduce before dying (29, 30). For males in polygynous species, this means rapid growth to large adult size resulting from sexual selection (6, 7). For females, however, this means investment in condition to achieve fecundity as early as possible, because lifetime reproductive success of females relates strongly to reproductive life span (7). The greater size of males born after warmer winters documented here must confer a selective advantage, because larger males are more likely to survive to adulthood (31) and, as adults, are likely to sire more offspring than small males (32). Increased investment in condition by females in response to early forage availability after warm winters, at the expense of size, is likely to contribute substantially to their lifetime reproductive success (12, 33). Females born after warm NAO winters were smaller, but more likely to conceive as yearlings (18) in addition to living, on average, up to 5 years longer than females born after cold winters. Considering that adult fecundity of females in this population is nearly constant among cohorts (34), such differences in reproductive lifespan could translate into a lifetime production of as many as 5 more offspring per female among those born after warm winters.
That female weight declined with increasingly warm winters in the year of birth (Fig. 3), but increased along the same climatic gradient in relation to plant phenology (Fig. 4) could reflect a stronger influence of plant phenology than of climate on female growth, independent of the timing of these influences. Alternatively, variation in weights of female offspring may depend more on environmental conditions during postnatal growth (spring plant phenology) than during growth in utero (winter climate). Although both of these hypotheses seem plausible, the fact that fetal growth rates of females did not differ between warm and cold winters, together with the strong maternal effect on female offspring weight (Fig. 2), appears to support the latter.
The divergent responses of males and females to environmental variation observed here may be best understood by considering the dichotomous selection regimes shaping life histories of the sexes in polygynous mammals, which in males reflect selection for access to females and in females reflect selection for access to food (20). As a consequence, responses of the sexes to environmental variation directed the magnitude of sexual size dimorphism in this polygynous species. These results suggest to us that global warming, particularly increases in average winter temperatures in northern latitudes (35), may pose consequences for sexual selection in large mammals such as red deer. Just as the intensity of sexual selection may vary in response to changes in density and adult sex ratios independently of environment (36), the positive influence of warmer winters on growth rates of male red deer in utero and postpartum may, independent of changes in population density, promote female choice for larger males. Conversely, the negative association between cold winter temperatures and male adult body size documented here suggests that environment can operate as a constraint on sexual selection if abiotic conditions are not favorable for increases in size of males.
That sexual dimorphism presents a challenge to understanding evolution by means of natural selection is evident in Darwin’s formulation (37) and extensive treatment (3) of sexual selection to explain the existence of traits that appeared to contradict natural selection (38). Furthermore, the complexity of relationships influencing sexual dimorphism has engendered debate that persists to this day over the contributions of environment and competitive selection to both individual and intersexual variation (39, 40). In fact, of the three monumental debates between Darwin and Wallace, the least reconcilable was that on the roles of competitive selection and environmental variation in the evolution of sexual dimorphism (41, 42). Clearly, as Darwin (3) and later Fisher (29) demonstrated, female choice can operate almost without limit on variation in male phenotypes, independently of environment, to increase male size. Our results illustrate, however, that once arisen, sex differences in strategies of growth related to reproduction can promote divergent responses of males and females to environmental variation that ultimately shape adult size dimorphism.
Acknowledgments
We thank R. T. Bowyer, T. H. Clutton-Brock, M. Festa-Bianchet, P. Harvey, G. P. Sætre, R. Shine, J. A. Stamps, K. E. Weber, and Professor John Maynard Smith and two anonymous referees for insightful suggestions and comments that greatly improved the paper. These data constitute part of a long-term monitoring study of red deer in Norway that has benefited from the assistance and support of wildlife boards throughout the country and the Norwegian Institute for Nature Research. This research was funded by National Science Foundation Grant DBI-9804178 to E.P., a grant from the Danish National Research Council (SNF) to M.C.F., and a grant from the Norwegian Science Council (NFR) to N.C.S.
ABBREVIATION
- NAO
North Atlantic Oscillation
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
We used MANOVA to estimate mean cohort-specific ages of adult females, controlled for weight by including weight as a covariate, for cohorts born in years 1963–1977. We regressed these against the first flowering date of A. nemorosa (x) in the cohort’s year of birth to get predicted values of cohort mean age (Y) as a function of plant phenology. The resulting regression equation was used to estimate the mean ages of cohorts born in the years of earliest and latest flowering; their difference was 5 years.
References
- 1.Huxley J S. Am Nat. 1938;72:416–433. [Google Scholar]
- 2.Clutton-Brock T H, Guinness F E, Albon S D. Red Deer: Behavior and Ecology of Two Sexes. Univ. of Chicago Press; 1982. [Google Scholar]
- 3.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 4.Selander R K. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago: Aldine; 1972. pp. 180–230. [Google Scholar]
- 5.Glucksmann A. Biol Rev. 1974;49:423–475. doi: 10.1111/j.1469-185x.1974.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 6.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 7.Clutton-Brock T H, editor. Reproductive Success. Univ. of Chicago Press; 1988. [Google Scholar]
- 8.Post E, Stenseth N C, Langvatn R, Fromentin J-M. Proc R Soc London B. 1997;264:1317–1324. doi: 10.1098/rspb.1997.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose K E, Clutton-Brock T H, Guinness F E. J Anim Ecol. 1998;67:979–986. doi: 10.1046/j.1365-2656.1998.6760979.x. [DOI] [PubMed] [Google Scholar]
- 10.Slatkin M. Evolution. 1984;38:622–630. doi: 10.1111/j.1558-5646.1984.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Leberg P L, Smith M H. J Mammal. 1993;74:723–731. [Google Scholar]
- 12.Downhower J F. Nature (London) 1976;263:558–563. doi: 10.1038/263558a0. [DOI] [PubMed] [Google Scholar]
- 13.Boyce M S. J Appl Ecol. 1981;18:749–753. [Google Scholar]
- 14.Weckerly F W. J Mammal. 1998;79:33–52. [Google Scholar]
- 15.Robbins C T, Robbins B L. Am Nat. 1979;114:101–116. [Google Scholar]
- 16.Albon S D, Clutton-Brock T H, Guinness F E. J Anim Ecol. 1987;56:69–81. [Google Scholar]
- 17.Hurrell J W, Van Loon H. Clim Change. 1997;36:310–326. [Google Scholar]
- 18.Post, E. & Stenseth, N. C. (1999) Ecology80, in press.
- 19.Forchhammer M C, Stenseth N C, Post E, Langvatn R. Proc R Soc London B. 1998;265:341–350. doi: 10.1098/rspb.1998.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivers R L. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- 21.Ims R A. Nature (London) 1988;335:541–543. doi: 10.1038/335541a0. [DOI] [PubMed] [Google Scholar]
- 22.Klein D R. Rangifer Spec Issue. 1990;3:123–130. [Google Scholar]
- 23.Albon S D, Langvatn R. Oikos. 1992;65:502–513. [Google Scholar]
- 24.Lauscher A, Lauscher F. Phänologie Norwegens, teil IV. Vienna: Eigen; 1990. [Google Scholar]
- 25.Stamps J A. Biol J Linn Soc. 1993;50:123–145. [Google Scholar]
- 26.Shine R. Am Nat. 1990;135:278–283. [Google Scholar]
- 27.Hurrell J W. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- 28.Clutton-Brock T H. In: Evolution From Molecules to Men. Bendall D S, editor. Cambridge, U.K.: Cambridge Univ. Press; 1983. pp. 457–482. [Google Scholar]
- 29.Fisher R A. The Genetical Theory of Natural Selection. Oxford: Oxford Univ. Press; 1930. [Google Scholar]
- 30.Williams G C. Adaptation and Natural Selection. Princeton: Princeton Univ. Press; 1966. [Google Scholar]
- 31.Loison A, Langvatn R. Oecologia. 1998;116:489–500. doi: 10.1007/s004420050614. [DOI] [PubMed] [Google Scholar]
- 32.Clutton-Brock T H, Albon S D, Guinness F E. In: Reproductive Success. Clutton-Brock T H, editor. Univ. of Chicago Press; 1988. pp. 325–343. [Google Scholar]
- 33.Clutton-Brock T H, Harvey P. In: Advances in the Study of Mammalian Behavior. Eisenberg J F, Kleiman D G, editors. Washington, D.C.: Am. Soc. Mammal.; 1983. pp. 632–663. [Google Scholar]
- 34.Albon S D, Clutton-Brock T H, Langvatn R. In: The Biology of Deer. Brown R D, editor. Berlin: Springer; 1992. pp. 15–21. [Google Scholar]
- 35.Maxwell B. In: Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective. Chapin F S III, Jeffries R L, Reynolds J F, Shaver G R, Svoboda J, editors. San Diego: Academic; 1992. pp. 11–34. [Google Scholar]
- 36.Clutton-Brock T H, Rose K E, Guinness F E. Proc R Soc London B. 1997;264:1509–1516. doi: 10.1098/rspb.1997.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darwin C. On the Origin of Species. London: Murray; 1859. [Google Scholar]
- 38.Mayr E. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago: Aldine; 1972. pp. 87–104. [Google Scholar]
- 39.Hedrick A V, Temeles E J. Trends Ecol Evol. 1989;4:136–138. doi: 10.1016/0169-5347(89)90212-7. [DOI] [PubMed] [Google Scholar]
- 40.Shine R. Q Rev Biol. 1989;64:419–461. doi: 10.1086/416458. [DOI] [PubMed] [Google Scholar]
- 41.Kottler M J. Proc Am Philos Soc. 1980;124:203–226. [PubMed] [Google Scholar]
- 42.Kottler M J. In: The Darwinian Heritage. Kohn D, editor. Princeton: Princeton Univ. Press; 1985. pp. 367–434. [Google Scholar]