Abstract
The impact of environmental change on animal populations is strongly influenced by the ability of individuals to plastically adjust key life-history events. There is therefore considerable interest in establishing the degree of plasticity in traits and how selection acts on plasticity in natural populations. Breeding time is a key life-history trait that affects fitness and recent studies have found that females vary significantly in their breeding time–environment relationships, with selection often favouring individuals exhibiting stronger plastic responses. In contrast, here, we show that although breeding time in the common guillemot, Uria aalge, is highly plastic at the population level in response to a large-scale environmental cue (the North Atlantic Oscillation, NAO), there is very little between-individual variation—most individuals respond to this climate cue very similarly. We demonstrate strong stabilizing selection against individuals who deviate from the average population-level response to NAO. This species differs significantly from those previously studied in being a colonial breeder, in which reproductive synchrony has a substantial impact on fitness; we suggest that counter selection imposed by a need for synchrony could limit individuals in their response and potential for directional selection to act. This demonstrates the importance of considering the relative costs and benefits of highly plastic responses in assessing the likely response of a population to the environmental change.
Keywords: phenotypic plasticity, phenology, stabilizing selection, climate change, guillemot (Uria aalge)
1. Introduction
The need to understand how individuals respond to environmental variation has become critical as large-scale environmental processes, such as climate change, continue to have demonstrable ecological effects in many natural systems (Walther et al. 2002). Determining how individuals base key life-history decisions on environmental cues is therefore crucial to predicting how these changes will affect fitness. Phenotypic plasticity, defined as the ability of a single genotype to modify its phenotype under heterogeneous environmental conditions (Houston & McNamara 1992), is fundamental to an animal's ability to deal with environmental change. However, little is known about the nature of plastic responses in wild populations or how natural selection acts on such responses (Nussey et al. 2005a; Pigliucci 2005).
The seasonal timing of reproduction is an important fitness-related trait that varies with changes in climate and temperature regimes across taxa—birds (Crick et al. 1997; Winkel & Hudde 1997; McCleery & Perrins 1998), amphibians (Beebee 1995) and mammals (Réale et al. 2003). Population-level changes in the timing of breeding could come about through several mechanisms: (i) changes over time in the pool of individuals constituting the breeding population arising through immigration of better-adapted individuals; (ii) micro-evolutionary processes occurring where changes in gene frequency across generations result from selection or genetic drift, bringing about changes in population characteristics; or (iii) individuals altering their timing of breeding in response to environmental cues within their reproductive lifetimes, leading to within-individual phenotypic plasticity (Przybylo et al. 2000). Distinguishing between these alternatives and determining the relative importance of plasticity are both essential to understand how individuals cope in a changing environment and has important implications for population dynamics and evolutionary processes (Przybylo et al. 2000; Réale et al. 2003; Nussey et al. 2005a).
Recent studies have shown that population-level changes in breeding time result from individuals responding to changing environmental cues, e.g. collared flycatchers, Ficedula albicollis, in relation to the North Atlantic Oscillation (NAO; Przybylo et al. 2000) and red squirrels, Tamiasciurus hudsonicus, in relation to pine cone abundance (Réale et al. 2003). However, only three studies have considered the possibility of between-individual variation in plasticity and explicitly tested whether individual females respond in similar or different ways to climate and food conditions (two short-lived passerine birds, Brommer et al. 2005; Nussey et al. 2005b; one ungulate, Nussey et al. 2005a). All found that females differed significantly in their breeding time–environment relationships, with some evidence for selection in favour of highly plastic individuals. However, evolutionary pressures on breeding time will vary greatly between different animal systems. In social or colonial species, breeding synchronization can be an important determinant of breeding success, and selection may therefore disfavour traits that generate asynchrony (Emlen & Demong 1975; Findlay & Cooke 1982; Ims 1990; Westneat 1992; Foley & Fitzgerald 1996; Sillero-Zubiri et al. 1998). This is true for many seabirds, a group of long-lived organisms commonly used as bio-indicators of change in the marine environment (Furness & Monaghan 1987). Synchronization of the timing of breeding and social factors are often assumed to play an important role in determining seabird reproductive success (Darling 1938; Birkhead & Harris 1985; Hatchwell 1991; Murphy & Schauer 1996). Potential benefits of synchronous breeding include a dilution of the predation risk (Birkhead 1977; Hatchwell 1991) and lower the risk of egg and/or chick losses due to interference from conspecifics when neighbouring birds are at the same stage of breeding (Murphy & Schauer 1996). Selection against asynchrony may limit the potential fitness advantage that could be gained from a large shift in response to the environmental change and this, in theory, should decrease variation in plastic responses among individuals, thereby creating a very different arena for the evolution of plasticity than that seen in less social breeding systems.
Here, we use data from a well-studied seabird, the common guillemot (Uria aalge), to investigate phenotypic plasticity in breeding time in a colonially breeding species. Where a population shows an average plastic response to an environmental gradient, there are two possible scenarios: either individuals respond in the same way or there is variation in individual plastic responses and reaction norms (Pigliucci 2005). These scenarios can be distinguished statistically by quantifying the interaction between individual responses and environmental cues, using the linear reaction norm approach (de Jong 1995); Brommer et al. 2005; Nussey et al. 2005a,b). We use records from a long-term intensive study of common guillemots to test: (i) whether the population shows, on average, a plastic adjustment of laying dates in response to a large-scale atmospheric phenomenon known to be an important predictor of likely spring conditions, the winter NAO index; (ii) whether females differ in their individual plastic responses to this environmental variation; and (iii) whether stronger plastic responses lead to higher breeding success and hence if selection acts on this plasticity. We show that, contrary to the previous findings, virtually no between-individual variation in plasticity could be detected in relation to NAO, despite an overall plastic response at the population level. This suggests that females respond in a remarkably similar fashion to this environmental cue. We then demonstrate that stabilizing selection appears to act against females deviating from the average population-level response, given that breeding synchronization is an important component of fitness in this highly social and colonial species.
2. Material and methods
(a) Study area and population
The common guillemot (hereafter guillemot) is a long-lived seabird occurring in both the North Atlantic and North Pacific and the most abundant seabird in the UK. The data used here were collected on the Isle of May, Firth of Forth, Scotland (56°11′ N, 2°33′ W), each breeding season from 1981 to 2005. The study population occupies six topographically discrete areas dispersed along ca 100 m of the cliff. All 1412 unique breeding sites in the areas were followed each year, though not all sites were occupied in every year, to give a total of 23 258 breeding records (see Harris et al. 1996 for a full description of breeding site characteristics). A subset of 245 individually colour-ringed females was followed in five of the areas from 1982 to 2005. Laying dates at all sites were recorded each year. The species has a single egg clutch, but will lay a replacement egg if the first one is lost. Here, we consider only the laying of the first egg. Approximately 75% of all first eggs are laid during a 7–10 day period. Details of the study population and data collection methods are given in Harris & Wanless (1988).
(b) Plasticity of laying date in relation to North Atlantic Oscillation
The phenology of common guillemots on the Isle of May up to 2002 correlated with the winter NAO index, with laying tending to be earlier in positive NAO years (Frederiksen et al. 2004). Winter NAO strongly predicts large-scale climatic conditions and weather patterns in the northern Atlantic and adjoining landmasses (Hurrell 1995). Positive NAO values indicate warm, wet winters dominated by westerly winds in northwestern Europe and vice versa. NAO has been used in many ecological studies of a range of species as an environmental correlate of biological traits (Stenseth et al. 2003). In species such as guillemots that spend the winter far from the breeding grounds, winter NAO may act as a useful signal that allows birds to anticipate likely spring conditions in the breeding areas in advance of returning (Frederiksen et al. 2004). No significant linear or cyclical trends in NAO were apparent over the time period considered in this study (NAO data taken from http://www.cru.uea.ac.uk/cru/data/nao.htm; see Jones et al. 1997). We also examined the effects of sea surface temperature (SST) as a more local environmental cue on laying date; there was neither any correlation at the population level between SST and laying date (Frederiksen et al. 2004), nor any evidence for individual variation in slopes (unpublished data from the present study). SST was therefore not considered further.
The cross-sectional analysis (i.e. considering mean laying dates of all individuals each year) was first updated using all records up to 2005 by regressing annual mean Julian laying date against winter NAO. Mean laying dates each year were calculated from the complete dataset of all breeding sites followed. Birds breeding for the first and second time (ca 5–7 years of age) lay later in the season than more experienced birds (Hedgren 1980), hence to remove any possible initial age-dependent variation in phenology, first and second breeding records for all individuals, regardless of actual age, were excluded from analyses using individually known birds. Breeding experience, or number of years since a female was first recorded as a breeder, was then entered into analyses as a covariate.
To test whether the observed correlation between laying date and NAO represented a plastic adjustment of phenology by female guillemots, the following restricted maximum-likelihood (REML) linear mixed-effects model (LMM):
where NAO, area and breeding experience were fixed effects and female identity (ID) and year were multi-level random effects, was fitted to the data in a longitudinal analysis (i.e. where the laying dates each year of individuals breeding in multiple years are considered).
Only laying dates of females breeding in 4 or more years were considered. NAO and breeding experience were entered as continuous fixed effects. Laying patterns tended to vary consistently between areas (Wanless & Harris 1988); hence, area was entered as a factor in the fixed model and an interaction between NAO and area was included to determine whether birds in different areas responded differently. The random factor ID accounts for the cumulative effects of individual-specific properties, such as genes, maternal effects and developmental factors, thereby allowing the main effect of NAO on laying date to be estimated independently (Przybylo et al. 2000). It also accounts for repeated measures on individual females.
Because females have such long breeding lifespans (mean=10.7 breeding records per female, range=4–25 in this dataset), they will experience a wide range of NAO conditions across years. Therefore, one can infer that trends will be present within females as well as across females; if the longitudinal analysis revealed a significant overall main effect of NAO of similar magnitude on the cross-sectional analysis, the population-level correlation would be largely due to phenotypic plasticity, rather than to different females experiencing different NAO conditions. The first model assumed that all females responded in a similar fashion to NAO, i.e. the variation due to differences between females in their individual responses to NAO was 0. To test whether females varied in their individual responses, a second LMM was fitted:
This time, a random interaction term for ID×NAO was included. ID estimates the variance component due to differences between females in their mean trait values in the average environment (elevations), while the random interaction term estimates the variance component resulting from differences between females in their laying date–NAO relationship (slopes). Comparing the deviance of models with and without this interaction term allows one to test whether females differ significantly in their plastic responses. Again, only females, which bred in 4 or more years, were used for the analysis in order to generate meaningful slopes. Further, restricting the analysis to females with at least five or six observations yielded very similar results.
(c) Selection analysis
If selection favours increased plasticity, females that show a response greater than average should achieve higher fitness. However, if synchrony is important, a plastic response that takes individuals far from the average response could decrease the success of these individuals and be counter-selected for. Stabilizing selection would thus act to reduce any variation in plasticity that might exist in the population. To quantify individual plastic responses, coefficients for elevation and slope were obtained from a linear regression model, where a separate regression of residual laying date against NAO was calculated for each female (n=245). Residual laying dates were the residuals from an ANCOVA model of laying date against year and area, with year as a covariate and area as a factor. (Using residual laying date controls for the effects of year and area on laying date, allowing laying date to be modelled against NAO independently; however, using residuals from a model of laying date against area only (i.e. ignoring the effect of year) or simply modelling raw laying dates against NAO (i.e. ignoring the effects of year and area) produced very similar results to those presented here, both qualitatively and quantitatively.) Separate regressions for each female generate individual estimates for elevation, a female's expected laying date response in the average environment and slope, which measures the strength of its plastic response to the NAO (Nussey et al. 2005c). Again, only females that bred in 4 or more years were used to remove potential extreme values.
A generalized linear model (GLIM) with a logit link function and binomial errors was constructed to test for a statistically significant relationship between breeding success and the estimates of slope and elevation in a weighted logistic regression:
where breeding success is a binomial proportion consisting of a vector of ‘successes’ (i.e. number of breeding attempts in which a chick was successfully raised to fledging) and ‘failures’ (i.e. number of failed breeding attempts). The quadratic terms test for nonlinear selection and the interaction for correlational selection between slope and elevation. If these two traits are highly correlated, then selection on elevation could also cause a correlated response in slope, even if selection does not act directly on slope itself. For comparison with other studies, standardized selection gradients were subsequently obtained using relative breeding success, where breeding success, expressed as the proportion of breeding attempts per individual that were successful, was standardized by dividing by the mean for all individuals. Elevation and slope were standardized to have a mean of 0 and a standard deviation of 1 and then entered into a linear regression, weighted by the total number of breeding attempts per female, assuming a normal error distribution (Lande & Arnold 1983). This gives parameter estimates, which can be taken to be the standardized selection gradients; these are the selection gradient values reported in the results, whereas the significance of terms is obtained from the formal GLIM that tests for selection on elevation and slope.
All models were fitted using REML methods in GENSTAT 8th edition (VSN International) or R v. 2.1 (R development team 2005). Continuous explanatory variables were centred on their mean values prior to inclusion in the models (Pinheiro & Bates 2000).
3. Results
(a) Plasticity in relation to North Atlantic Oscillation
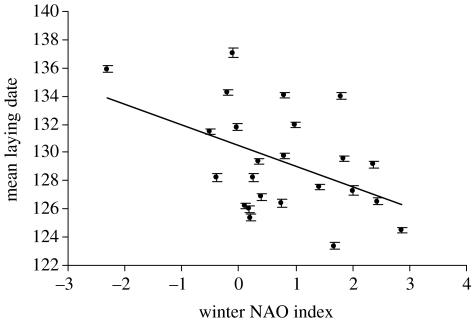
The cross-sectional analysis showed that annual mean laying date was negatively correlated with NAO (figure 1). The results (table 1) showed that this negative main effect of NAO persisted in the full LMMs after other significant terms had been accounted for (model 1, b=−1.43±0.59). Birds in different areas also responded slightly differently to NAO, as evidenced by the significant interaction between NAO and area, but in each area, NAO always had a negative effect. There was no effect of breeding experience on laying date, though there were strong effects of year and female identity (table 1).
Figure 1.
Annual mean laying date in the colony, in number of days since January 1 (±s.e.), plotted against NAO values for each year, showing a significant negative relationship (b=−1.56±0.59, R2=0.24, p=0.015). This effect of NAO on laying date is independent of year.
Table 1.
Linear mixed effects model of laying date with random effects for year and (a) female identity only, where females are all assumed to respond in the same way to NAO and (b) female identity plus a female identity×NAO random interaction term, which allows for different individual responses to NAO (n=2597 breeding records for 245 females). (The significance of adding each subsequent random effect to the models was assessed using log-likelihood test statistics, where the change in deviance (−2logLik) is compared to a chi-squared distribution with appropriate degrees of freedom. Only significant fixed effects are shown, as when added last to the model (type III tests). ***p<0.001. Year and NAO had independent effects in both models.)
| variance components for random effects in final model: | |||||
|---|---|---|---|---|---|
| component | s.e. | d.f. | deviance | log-likelihood test statistics | |
| year | 10.60 | 3.32 | 2582 | 10221.32 | |
| (a) female identity | 8.72 | 0.91 | 2581 | 9303.94 | 917.38*** |
| (b) female identity×NAO | 0.01 | 0.06 | 2580 | 9303.91 | 0.03 |
| fixed effects: | |||||
|---|---|---|---|---|---|
| coefficient | s.e. | d.f. | Wald statistic | p-value | |
| NAO | −1.434 | 0.586 | 1 | 0.013 | |
| area | 129.3 | 0.8 | 4 | 37.88 | <0.001 |
| NAO×area | — | — | 4 | 7.96 | <0.001 |
In the second LMM, a random interaction term ID×NAO was included to determine whether females varied significantly in their plastic responses. This model estimated a non-significant variance component for this random interaction term, which was very close to 0 (0.01±0.06), indicating very little variation between females in their responses to NAO. Inclusion of this random interaction term resulted in a very slight drop in deviance and neither improved the explanatory power of the model significantly nor had any effect on the fixed effects (change in deviance=0.03, d.f.=1, p=0.86).
(b) Selection analysis
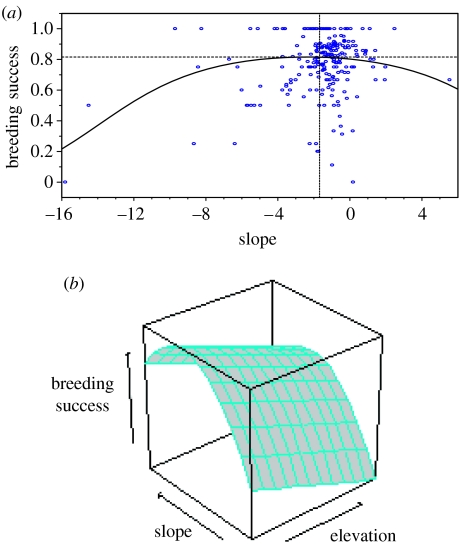
Once all non-significant terms were removed from the GLIM, the only terms that remained significant were elevation and the square of slope. This indicates directional selection on elevation (figure 2b), favouring earlier laying dates on average, as evidenced by a negative selection gradient (table 2). The fact that there was no direct selection on slope, but on the square term for slope, shows that stabilizing selection acted on plasticity, where the average slope has optimal breeding success, which declines as one moves away from this optimum in either direction (figure 2a,b), i.e. there was selection against females whose plastic responses deviated strongly from the average response. The interaction between elevation and slope was not significant, indicating that selection on the slope was not affected by whether individuals were on average late or early breeders over their lifetime.
Figure 2.
(a) Results from the selection analysis showing how breeding success, measured as the proportion of total breeding attempts per female, where a chick was produced, depends on slope (actual slopes used in GLIM and not standardized values). Each data point (n=245) represents an individual female. The curved line shows the relationship between breeding success and slope as predicted by the GLIM, where elevation is held constant at the average value. The average slope has optimum breeding success, as indicated by the dashed lines, and breeding success declines while moving away from this optimum in either direction. (b) Three-dimensional surface plot showing the relation of both elevation and slope to breeding success, as predicted by the GLIM, indicating directional selection on elevation and stabilizing selection on slope.
Table 2.
Results of selection analysis GLIM using individual regression coefficients for elevation, slope, their squared terms and interaction for n=245 females. (Significance of terms was assessed using F-deletion tests to compensate for over-dispersion (there was some over-dispersion in the data, as a result of the residual scaled deviance being larger than the residual degrees of freedom by a factor of 1.68; this was overcome by specifying quasi-binomial errors that makes use of a scaled deviance parameter (Crawley 2002)); p-values of significant terms are from final model, where non-significant terms have been removed. An intercept was also fitted but is not shown. Coefficient estimates and their standard errors are standardized selection gradients from a linear regression (see §2 for details).)
| coefficient | estimate | s.e. | p-value |
|---|---|---|---|
| elevation | −0.05 | 0.01 | 0.001 |
| (elevation)2 | −0.006 | 0.01 | 0.773 |
| slope | −0.01 | 0.02 | 0.193 |
| (slope)2 | −0.02 | 0.007 | 0.019 |
| elevation×slope | −0.01 | 0.01 | 0.607 |
4. Discussion
Here, we show that population-level changes in phenology, in response to a large-scale atmospheric phenomenon, arose from individuals plastically adjusting their laying date. However, in contrast to previous studies, we found very little between-individual variation in plasticity, indicating that individuals responded in a remarkably similar fashion to the NAO. We demonstrate that stabilizing selection acts on plasticity and suggest that selection against asynchronous breeding may prevent individuals deviating far from the population mean response, despite potential benefits of early breeding.
Breeding was on average earlier in years when NAO was positive, indicative of warmer and wetter winter conditions. In winter, guillemots from the Isle of May disperse throughout the North Sea and thus the onset of reproduction in spring is expected to be informed by cues operating both over large distances and during a period well in advance of when birds actually return to the colony, allowing birds to adequately predict likely conditions (Frederiksen et al. 2004). Alternatively, NAO could act as a constraint on the timing of breeding, whereby climatic conditions determine food supply and hence body condition in the pre-breeding period. Although the actual mechanisms by which individual birds respond are unclear, the overall population-level response to NAO was largely explained by individual phenotypic plasticity. Other explanations that could underlie this type of population shift in breeding time, such as immigration of more adapted individuals, micro-evolutionary processes or some association between different values of NAO and the average laying date (Przybylo et al. 2000), could be discounted.
Analysis of data from females who had bred for at least 4 years revealed that females behaved in an extremely similar manner in relation to NAO, with very little variation in their plastic responses. The formal mixed model indicated that this variation was not significantly different from 0, implying that the variance due to any differences in plasticity between individuals was not large enough to be statistically significant relative to other sources of variance in the model; individuals therefore appeared to respond very similarly. This represents a novel result, since previous studies that have considered between-individual variation in plasticity in breeding time have all found significant differences between individuals: in collared flycatchers in Sweden (Brommer et al. 2005; Nussey et al. 2005b), great tits, Parus major, in The Netherlands (Nussey et al. 2005b) and red deer, Cervus elaphus, in Scotland (Nussey et al. 2005a). In contrast, we have shown that the opposite is true for guillemots with females exhibiting a strong response to NAO, but all to a similar extent.
This may arise from their colonial lifestyle. Breeding guillemots are characterized by a high degree of breeding synchrony; they typically breed at extremely high densities (in this population, often more than 40 pairs per m2) and low mortality and high levels of site and mate fidelity mean that pairs are likely to breed alongside the same neighbours from year to year (Harris et al. 1996). Reproductive synchrony appears to have a number of social benefits: actively breeding close neighbours may be less likely to flush and dislodge eggs when disturbed than non-breeders or late breeders not yet settled on eggs or brooding chicks (Murphy & Schauer 1996) and synchronization of breeding between groups of neighbouring pairs may accrue benefits via a dilution of predation risk—this may be important for the advantages of predator swamping to apply throughout the season (Birkhead 1977; Hatchwell 1991). The general importance of reproductive synchrony in guillemots may therefore limit selection on an ability to respond to environment cues; in this study, the average plastic response, which appears to be closely followed by the majority of females, has optimal fitness. Guillemots laying consistently early or late shift their laying date by the same amount when the environment changes, maintaining the ranking of individuals’ laying dates relative to each other (repeatability of individual laying dates, expressed relative to area means, equals 0.494 in this colony). This is despite evidence for significant directional selection for earlier breeding (females with earlier average laying dates, relative to others in the colony, had higher breeding success than later breeding females). Stabilizing selection thus acts to reduce between-individual variation in plasticity. We suggest that for a colonially breeding seabird, the ability to modify the phenotype in line with the rest of the population and to remain synchronous may be of primary importance, rather than the strength of plastic response per se, which is more likely to be determined by the level of environmental variation. This stabilizing selection may explain our observation that the component of variance due to differences in slopes in a mixed model was not statistically significant, in marked contrast to previous studies (Brommer et al. 2005; Nussey et al. 2005a,b).
A number of environmental factors could in general explain this type of result; indeed, recent evidence from great tits in southern England would also seem to suggest a lack of significant variation in plastic responses, for reasons as yet undetermined (A. Charmantier 2006, personal communication). In some species, environmental conditions could impose a limited time window during which successful reproduction is possible; if the timing of this window varies among years, then this could also limit selection away from an average response and individuals would follow the same reaction norm. However, the short-time window hypothesis seems unlikely for our particular result, as guillemots are not necessarily constrained by external conditions to breed in such a contracted period. For example, other seabird species breeding on the Isle of May, such as shags (Phalacrocorax aristotelis), also rely on lesser sandeels (Ammodytes marinus) as their main prey items and face similar conditions, but have a much more extended breeding season; shags do not breed in dense colonies like guillemots, and therefore, synchrony may not be as important. The social constraints argument rather seems more plausible, given the highly social and colonial lifestyle of guillemots. If the increased need for reproductive synchrony in guillemots plays a key role in determining selection pressures, this may limit the expression of highly variable responses. Evidence from recent studies of free-living vertebrate populations suggests that there is an underlying heritable component to breeding time plasticity (Brommer et al. 2005; Nussey et al. 2005a,b); therefore, from an evolutionary standpoint, stabilizing selection and the consequent erosion of variation could be the important phenomena to take into account when investigating the evolution of plastic responses. This is a crucial aspect to consider in social species and highlights the importance of evaluating the costs as well as the benefits of a highly plastic response when analysing how populations of animals might respond to climatic and other types of environmental change.
Acknowledgments
The authors wish to thank many people who collected field data over the years, and Scottish Natural Heritage for allowing us to work on the Isle of May National Nature Reserve. The fieldwork was funded by the Natural Environment Research Council and the Joint Nature Conservation Committee's integrated Seabird Monitoring Programme. We also thank Dan Nussey, Alistair Wilson and Anne Charmantier for helpful discussion and Matt Robinson for comments on the manuscript. The work was supported by a Principal's studentship to T.E.R. from the University of Edinburgh, a Leverhulme Emiritus Fellowship to M.P.H. and Royal Society fellowships to E.J.A.C. and L.E.B.K.
References
- Beebee T.J.C. Amphibian breeding and climate. Nature. 1995;374:219–220. doi:10.1038/374219a0 [Google Scholar]
- Birkhead T.R. The effect of habitat and density on breeding success in the common guillemot (Uria aalge) J. Anim. Ecol. 1977;46:751–764. [Google Scholar]
- Birkhead T.R, Harris M.P. Ecological adaptations for breeding in the Atlantic Alcidae. In: Nettleship D.N, Birhkead T.R, editors. The Atlantic Alcidae: the evolution, distribution and biology of the auks inhabiting the Atlantic Ocean and adjacent areas. Academic Press; London, UK: 1985. pp. 205–231. [Google Scholar]
- Brommer J.E, Merilä J, Sheldon B.C, Gustafsson L. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution. 2005;59:1362–1371. [PubMed] [Google Scholar]
- Crawley M.J. Wiley; New York, NY: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Crick H.Q.P, Dudley C, Glue D.E, Thomson D.L. UK birds are laying eggs earlier. Nature. 1997;388:526. doi:10.1038/41453 [Google Scholar]
- Darling F.F. Cambridge University Press; Cambridge, UK: 1938. Bird flocks and the breeding cycle. [Google Scholar]
- de Jong G. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 1995;145:493–512. doi: 10.1086/285542. doi:10.1086/285752 [DOI] [PubMed] [Google Scholar]
- Emlen S.T, Demong N.J. Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis. Science. 1975;188:1029–1031. doi: 10.1126/science.1145188. [DOI] [PubMed] [Google Scholar]
- Findlay C.S, Cooke F. Breeding synchrony in the lesser snow goose (Anser caerulescens caerulescens). 1. Genetic and environmental components of hatch date variability and their effects on hatch synchrony. Evolution. 1982;36:342–351. doi: 10.1111/j.1558-5646.1982.tb05050.x. doi:10.2307/2408053 [DOI] [PubMed] [Google Scholar]
- Foley R.A, Fitzgerald C.M. Is reproductive synchrony an evolutionarily stable strategy for hunter-gatherers? Curr. Anthropol. 1996;37:539–545. doi:10.1086/204516 [Google Scholar]
- Frederiksen M, Harris M.P, Daunt F, Rothery P, Wanless S. Scale-dependent climate signals drive breeding phenology of three seabird species. Global Change Biol. 2004;10:1214–1221. doi:10.1111/j.1529-8817.2003.00794.x [Google Scholar]
- Furness R.W, Monaghan P. Blackie; London, UK: 1987. Seabird ecology. [Google Scholar]
- Harris M.P, Wanless S. The breeding biology of guillemots Uria aalge on the Isle of May over a 6 year period. Ibis. 1988;130:172–192. [Google Scholar]
- Harris M.P, Wanless S, Barton T.R. Site use and fidelity in the common guillemot Uria aalge. Ibis. 1996;138:399–404. [Google Scholar]
- Hatchwell B.J. An experimental study of the effects of timing of breeding on the reproductive success of common guillemots (Uria aalge) J. Anim. Ecol. 1991;60:721–736. [Google Scholar]
- Hedgren S. Reproductive success of guillemots Uria aalge on the island of Stora Karlsö. Ornis Fenn. 1980;57:49–56. [Google Scholar]
- Houston A.I, McNamara J.M. Phenotypic plasticity as a state-dependent life-history decision. Evol. Ecol. 1992;6:243–253. doi:10.1007/BF02214164 [Google Scholar]
- Hurrell J.W. Decadal trends in the North-Atlantic Oscillation: regional temperatures and precipitation. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- Ims R.A. The ecology and evolution of reproductive synchrony. Trends Ecol. Evol. 1990;5:135–140. doi: 10.1016/0169-5347(90)90218-3. doi:10.1016/0169-5347(90)90218-3 [DOI] [PubMed] [Google Scholar]
- Jones P, Jonsson T, Wheeler D. Extension to the North Atlantic Oscillation using early instrumental pressure observations from Gibraltar and south-west Iceland. Int. J. Climatol. 1997;17:1433–1450. doi:10.1002/(SICI)1097-0088(19971115)17:13<1433::AID-JOC203>3.0.CO;2-P [Google Scholar]
- Lande R, Arnold S.J. The measurement of selection on correlated traits. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. doi:10.2307/2408842 [DOI] [PubMed] [Google Scholar]
- McCleery R.H, Perrins C.M. Temperature and egg-laying trends. Nature. 1998;391:30–31. doi:10.1038/34073 [Google Scholar]
- Murphy E.C, Schauer J.H. Synchrony in egg-laying and reproductive success of neighboring common murres, Uria aalge. Behav. Ecol. Sociobiol. 1996;39:245–258. doi:10.1007/s002650050287 [Google Scholar]
- Nussey D.H, Clutton-Brock T.H, Elston D.A, Albon S.D, Kruuk L.E.B. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 2005a;74:387–396. doi:10.1111/j.1365-2656.2005.00941.x [Google Scholar]
- Nussey D.H, Postma E, Gienapp P, Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005b;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Clutton-Brock T.H, Albon S.D, Pemberton J.P, Kruuk L.E.B. Constraints on plastic responses to climate variation in red deer. Biol. Lett. 2005c;1:457–460. doi: 10.1098/rsbl.2005.0352. doi:10.1098/rsbl.2005.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M. Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 2005;20:481–486. doi: 10.1016/j.tree.2005.06.001. doi:10.1016/j.tree.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York, NY: 2000. Mixed-effects models in S and S-Plus. [Google Scholar]
- Przybylo R, Sheldon B.C, Merila J. Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J. Anim. Ecol. 2000;69:395–403. doi:10.1046/j.1365-2656.2000.00401.x [Google Scholar]
- Réale D, McAdam A.G, Boutin S, Berteaux D. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. B. 2003;270:591–596. doi: 10.1098/rspb.2002.2224. doi:10.1098/rspb.2002.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillero-Zubiri C, Johnson P.J, Macdonald D.W. A hypothesis for breeding synchrony in Ethiopian wolves (Canis simensis) J. Mammal. 1998;79:853–858. [Google Scholar]
- Stenseth N.C, Ottersen G, Hurrell J.W, Mysterud A, Lima M, Chan K.S, Yoccoz N.G, Ådlandsvik B. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. doi:10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Wanless S, Harris M.P. The importance of relative laying date on breeding success of the guillemot Uria aalge. Ornis Scand. 1988;19:205–211. [Google Scholar]
- Westneat D.F. Nesting synchrony by female red-winged blackbirds: effects on predation and breeding success. Ecology. 1992;73:2284–2294. doi:10.2307/1941475 [Google Scholar]
- Winkel W, Hudde H. Long-term trends in reproductive traits of tits (Parus major, P. caeruleus) and pied flycatchers (Ficedula hypoleuca) J. Avian Biol. 1997;28:187–190. [Google Scholar]