Abstract
The Antarctic Dry Valleys are regarded as one of the harshest terrestrial habitats on Earth because of the extremely cold and dry conditions. Despite the extreme environment and scarcity of conspicuous primary producers, the soils contain organic carbon and heterotrophic micro-organisms and invertebrates. Potential sources of organic compounds to sustain soil organisms include in situ primary production by micro-organisms and mosses, spatial subsidies from lacustrine and marine-derived detritus, and temporal subsidies (‘legacies’) from ancient lake deposits. The contributions from these sources at different sites are likely to be influenced by local environmental conditions, especially soil moisture content, position in the landscape in relation to lake level oscillations and legacies from previous geomorphic processes. Here we review the abiotic factors that influence biological activity in Dry Valley soils and present a conceptual model that summarizes mechanisms leading to organic resources therein.
Keywords: Antarctica, carbon, decomposition, Dry Valleys, respiration, soil
1. Introduction
Antarctica is the Earth's fifth largest continent and covers 14×106 km2, making it approximately 50% larger than the USA. Most of the continent is south of the Antarctic circle (66°S), the majority capped by a permanent icesheet. However, about 0.35% is ice-free for some or all of the year (British Antarctic Survey 2004). The Dry Valleys on the east side of the Transantarctic Mountains in the Ross Dependency (Victoria Land), between longitudes 170°W and 170°E and adjacent to the Ross Sea and Ice Shelf, comprise the largest such area. The Dry Valleys occupy approximately 15 000 km2 and are substantially ice-free due to their isolation from the ice plateau by the barrier created by the Transantarctic Mountains. The remaining ice-free land in Antarctica is along parts of the coast line, in volcanically heated regions, on isolated nunataks, on the peaks of high mountains (some over 4000 m) and on sub-Antarctic islands close to sea level.
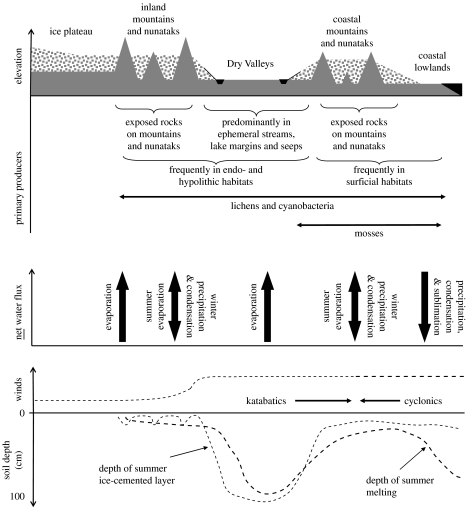
The ice plateau covering most of the continent defines the climatic conditions of Antarctica and its Dry Valleys. The prevailing katabatic winds radiate from the South Pole and, descending from the polar plateau, channel dry air at high speeds through the valleys of the Transantarctic Mountains, leading to ablation of snow and ice from the valleys. The drying influence of the wind and the low precipitation (typically only a few centimetres of water equivalent per annum) makes the Dry Valleys arguably the driest desert on Earth. Temperatures are well below 0 °C for much of the year: only during the short summer do the air and ground temperatures rise above 0 °C. Generalized relations of ice and liquid water distribution, fluxes, prevailing winds and organisms with geomorphology in continental Antarctica are summarized in figure 1.
Figure 1.
Generalized relations of ice and liquid water distribution, net water fluxes, prevailing winds and primary producers with geomorphology in continental Antarctica. The diagram has been adapted from Janetschek (1970). It should be noted that the water fluxes are indicative net fluxes, and it is not intended to imply that precipitation only occurs during the winter and that evaporation only occurs during the summer in the Dry Valleys.
The Dry Valleys include large areas that were not over-run by ice at Pleistocene glacial maximum and have been at least partially ice-free since the Mid–Late Miocene. Terrestrial habitats have, therefore, existed in this region for possibly up to 12–13 Myr (Denton et al. 1993). The major geomorphic features of Antarctica were predominantly formed by ancient water-based glaciers and have emerged comparatively recently with the partial recession of the ice-sheet (Campbell & Claridge 1987, 2000; Taylor 1922). Landforms produced by more recent fluvial and glacial processes include moraines, patterned ground, alluvial fans, outwash plains and deltas, gravel and dunes, screes and solifluction deposits (Campbell & Claridge 1987). Soils that have formed in these features are skeletal and typically characterized by absence of structure and cohesion, low moisture and organic matter contents (Beyer et al. 1999), and often high salt concentrations due to limited leaching (Bockheim 1997; Campbell & Claridge 2000). Organisms have had a relatively minor role in soil formation because biological communities in the soils are sparse, with low biomass (Bargagli et al. 1999; Treonis et al. 1999; Stevens & Hogg 2002; Moorhead et al. 2003) and their distribution is strongly influenced by water availability (Kennedy 1993).
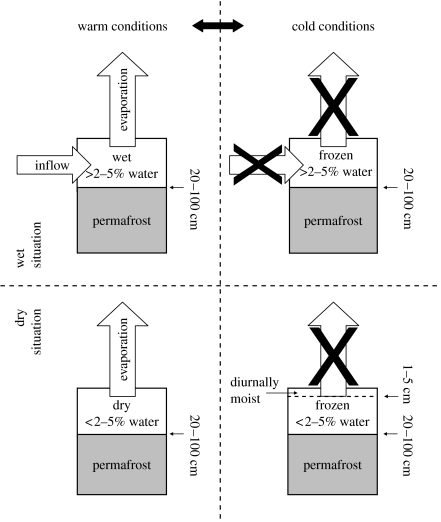
As with hot deserts (Noy-Meir 1973; Ludwig & Whitford 1981), availability of water in Antarctic Dry Valley soils varies in time and space. However, unlike hot deserts in which the liquid–vapour phase transition determines biologically available water, the liquid–ice interface is critical to soil biota in the Dry Valleys. Liquid water is available in summer through melting of near-surface permafrost and light snowfalls, in streams and rivers through glacial melting, and on lake margins through thawing of ice caps and the development of moats. When temperatures hover around 0 °C, lake-edge moats undergo diurnal freeze–thaw cycles. Furthermore, lakes with outgoing drainage experience a diurnal ‘tide’ as inputs of glacial meltwater temporarily exceed drainage when glacier surfaces are warmed by the sun and then subsequently decline when feeder glaciers are in shade as the sun moves around the horizon (or sets). Both processes provide water inputs to the soils at the lake margins. The ephemerality of available water is driven by the interaction of position in the landscape and weather conditions (figure 2). At generally wet, downslope sites, along shallow streambeds and at lake margins, warm sunny conditions maximize inflows of meltwater and wet surface soils favour biota, while cool cloudy conditions reduce inflows and freeze surface soil. By contrast, in dry, upslope habitats, soils are severely dehydrated by sunny conditions rapidly evaporating almost all permafrost meltwater, but their surfaces can be diurnally moist over the permafrost during cooler, cloudy conditions. Evaporative losses from hillslopes are ameliorated by desert pavements of pebbles that mulch the soil surface, as has been observed in hot deserts (Cooke et al. 1993). Where these pavements consist of translucent quartzitic fragments, hypolithic communities of cyanobacteria and eukaryotic algae can be active, even under very sunny conditions (Schlesinger et al. 2003). During unusually warm summers, extensive melting of snowfields and permafrost generate subsurface flows of water down hillslopes, leading to seeps and springs (Lyons et al. 2005), and thereby create sudden yet ephemeral streams to which biota respond rapidly (McKnight et al. 1999; Gooseff et al. 2003).
Figure 2.
Summary of soil moisture and water fluxes for soils under different environmental conditions in the Dry Valleys. For justification of the 2–5% cut-off in water content see Treonis et al. (1999), Elberling (2003), Barrett et al. (2004) and Elberling et al. (in press).
2. Heterotrophic organisms in the Dry Valleys
There have been few comprehensive surveys of the diversity of organisms in the Dry Valleys, but Adams et al. (in press) recently summarized the limited information with a view to establishing a baseline to facilitate future biodiversity and functional ecological investigations. Nevertheless, the Dry Valleys are highly significant sites in ongoing studies of ecosystem processes, because of their presumed relative biological simplicity, and for monitoring the effects of environmental changes, because their biota operates at environmental extremes.
Despite the limited information on organisms in Dry Valley soils, there is clear evidence that these soils support biologically mediated processes and communities of heterotrophic organisms. Respiration, nitrogen (N) mineralization and a variety of substrate-induced responses have been measured under laboratory conditions (Barrett et al. 2002, 2005, in press; Hopkins et al. in press). Small but consistent net emissions of CO2 from Dry Valley soil surfaces are reported in situ (Burkins et al. 2002; Parsons et al. 2004; Elberling et al. in press) indicating heterotrophic activity, but responses to exotic substrates (cotton strips) are not consistently observed (Treonis et al. 2002).
Dry Valley soils contain a diverse range of heterotrophs (reviewed by Adams et al. in press), including bacteria and fungi, protozoa, tardigrades, rotifers, collembola, acari and notably microbivorous and detritivorous nematodes. The last group are the most widely studied (Freckman & Virginia 1997; Treonis et al. 1999; Virginia & Wall 1999; Coutright et al. 2001; Doran et al. 2002). All these consumers form the highest trophic layer in the terrestrial community (‘McMurdo's equivalent of elephants and tigers’; Wilson 2002; Wall 2005), and their presence indicates a reliable supply of organic substrates.
Estimates of organic carbon (C) turnover in the Dry Valleys are in the range 20–130 years (Burkins et al. 2002; Elberling et al. in press), and Barrett et al. (2005) reported that large proportions of the organic C and N in Dry Valley soils are potentially mineralizable within a relatively short period (90 days) under optimal conditions. The estimates of C mineralization in the Dry Valleys are fast by comparison with soils in temperate regions, which are typically in the range of centuries to millennia (Kirschbaum 2000; Hopkins & Gregorich 2005).
Spatial differences of a hundredfold or more in soil organic C concentration (Elberling et al. in press), respiration flux (Elberling et al. in press; Hopkins et al. in press) and microbial biomass concentration (Barrett et al. in press) show the biological heterogeneity of the Dry Valley landscapes. This heterogeneity ranges from microenvironments within a soil profile (Gregorich et al. in press) to macroenvironments within a mosaic of landscape elements (Elberling et al. in press), and relates to both horizontal and vertical spatial patterns in availability of liquid water and soil temperature (figure 2). In addition, there is evidence for an assemblage of anaerobic micro-organisms (Hopkins et al. in press), especially in the wetter sites where methanogenesis and denitrification have been detected (Gooseff et al. 2004; Gregorich et al. in press). However, unlike less extreme terrestrial ecosystems, in which primary producers are usually abundant and macroscopic, and their spatial patterning across the landscape conspicuous, the provenance of organic resources to support the heterotrophic organisms in the Dry Valleys is more complex (Barrett et al. 2004).
3. In situ terrestrial primary production
The occurrence of significant net primary production depends on environmental conditions and the distribution of organisms. Figure 1 summarizes relationships between some soil and climatic factors and primary producers along a transect from the polar plateau through the Transantarctic Mountains towards the coast. Within the Dry Valleys, primary producers adopt different habits, including development of epilithic, hypolithic and endolithic communities—respectively on, under and inside rock and soil surfaces—on hillslopes and desert pavements in dry habitats and microbial mat and filamentous communities in wetter habitats, such as lake margins and in stream beds (figure 3). Cyanobacteria often dominate the primary producer biomass, with, for example, Nostoc commune forming conspicuous films and mats in ephemeral streams and at lake margins during summer (Vincent 1988), as well as growing epiphytically on mosses (Alfinito et al. 1998) and with Gloeocapsa sp. adapted to extremely dry conditions by growing endolithically (Friedmann & Ocampo 1976; Friedmann 1982; Friedmann et al. 1993). Eukaryotic algae are widely reported in Antarctica from most ice-free areas, including in ephemeral streams and at lake margins, on wetter soils, as epiphytes on mosses, in cyanobacterial mats and predominantly in lakes (Cathey et al. 1981; Hawes & Schwarz 1999; Pocock et al. 2004). Mosses in the Dry Valleys form conspicuous communities at wet seeps and springs, and less obviously beneath a thin layer of translucent soil particles and on the soil surface fringing stones sheltered from the wind.
Figure 3.
Examples of sources of organic resources for heterotrophic organisms in the Dry Valleys: (a) surficial lichen; (b) hypolithic algal/cyanobacterial community (the stone was buried to the depth indicated by the arrow); (c) endolithic community in sandstone (the arrow indicates organisms (black coloration) exposed by exfoliation of the sandstone); (d) hypolithic moss; (e) surficial moss in ephemeral stream bed; (f) remains of Adélie penguin; (g) mummified crab-eater seal; (h) area of lake shore from which cyanobacterial mat has been removed (presumably by wind); (i) wind-blown foam (presumed to be derived from decomposing lacustrine detritus) accumulation at lake shore; (j) lacustrine detritus stranded close to lake shore following a recent fall in lake level; (k) cyanobacterial mat from ancient lake shore exposed following erosion of the overlying soil/sediment.
There are estimates of net primary production for selected organisms and for the biomass of some groups of primary producers at selected sites (Green et al. 1992, 1998; Schwarz et al. 1992; Kappen et al. 1998; Pannewitz et al. 2005, 2006). However, these data are sparse on an areal basis and over a range of environmental conditions; thus, estimating net primary production for Dry Valley soils is difficult. Friedmann et al. (1993) estimated the net primary production at between 11 and 17 kg C ha−1 yr−1 from the endolithic microbial community, of which 50–85 g C ha−1 yr−1 enters microbial biomass and the remaining metabolites percolate into soils and rocks and are potentially available to heterotrophs. However, they stress that this production may be restricted to as little as 20% of the surface area in the Dry Valleys. It is generally assumed that the growth rate of primary producers in the Dry Valleys is slow (Johnston & Vestal 1991) and Bonani et al. (1988) made a preliminary report that crypotendolithic organisms from the Dry Valleys may be thousands of years old. However, more recent evidence suggests a mean age for such material of less than a century (B. Büdel et al. 2005, unpublished work). At sites of ephemeral water flow, which may represent only a few per cent of the land surface (Elberling et al. in press), are significant hot-spots of productivity capable of rapid responses to incoming water (McKnight et al. 1999; Conovitz et al. 2006). For comparison, Noy-Meir (1973) estimated above- and below-ground productivity in excess of 1 t C ha−1 yr−1 for a range of hot desert ecosystems worldwide and up to ten times as much for semi-desert ecosystems.
Nitrification has been detected in Dry Valleys soils, but the potential rates are very small and it is presumed that chemoautotrophic sources of organic C are negligible (Hopkins et al. in press), except perhaps at localized sites of large NH4+ concentrations such as at lake margins (Gregorich et al. in press).
4. Temporal subsidies (legacies)
Considerable reference has been made to the role of ancient lake sediments as sources of soil organic matter in the Dry Valleys, which has been redistributed by geomorphic processes (the so-called ‘legacy C’; Burkins et al. 2000). The principal evidence for its presence comes from the Taylor Valley, which contained palaeolake Washburn between about 10 000 and 23 000 years ago (Péwé 1960). The evidence comprises C and N stable isotope signatures, which distinguish ancient C from contemporary sources, and indirect geomorphic evidence (Burkins et al. 2000; Higgins et al. 2000). Large lakes with levels far higher than present may also have occurred in both the Wright and Victoria Valleys (Hall et al. 2001, 2002; Hall & Denton 2002, 2005), the sediments from which may also contribute to contemporary C cycling. Indeed, there are conspicuous deposits of algal detritus on lake terraces above Lake Vanda, the current lake in the Wright Valley (Hall et al. 2001), but their quantitative contribution to contemporary C cycling has not been investigated and there are apparently no radiocarbon dates for soil organic matter.
Estimates of the turnover of contemporary organic C in other Dry Valleys soils are astonishingly fast, ranging between 20 and 120 years (Burkins et al. 2002; Elberling et al. in press), supported by rapid mineralization of soil C and N in laboratory studies (Barrett et al. 2002, 2005; Hopkins et al. in press). The implications of these fast turnover times are either that legacy C deposits may be about to be exhausted, or that the legacy C is either so stable or protected that it contributes little to contemporary C cycling. If the latter is true, contemporary C cycling in Dry Valleys soils must be sustained largely, if not exclusively, by modern sources of organic matter.
5. Spatial subsidies
(a) Imports of resources
The Dry Valleys benefit potentially from imports of organic resources in the form of bird guano and feathers (Greenfield 1992; Marshall 1998; Legrand et al. 1998), wind-blown marine detritus and the arrival of inopportune seals and penguins that wander into the Dry Valleys and die, presumably from starvation or exhaustion (figure 3). Mummified seal carcasses are relatively frequent occurrences in the Dry Valleys and have persisted because of the cold and drying conditions (Péwé et al. 1959; Claridge 1961; Webb & Leckie 1977). Although apparent radiocarbon ages up to millennia have been reported for mummified seals and penguins in the Dry Valleys, the large 14C depletion of the Antarctic marine reservoir makes these substantial over-estimates, and ages in the range of a few decades to two to three centuries are more realistic (Dort 1971, 1981; Mabin 1986). At the sites of deposition these subsidies could make a major contribution to biological processes in soils, at least when and where there is liquid water, and Lewis-Smith (1997) hypothesized that nutrient enrichment adjacent to mummified soils encourages colonization of the soil surface by lichens.
(b) Redistribution of resources within valleys
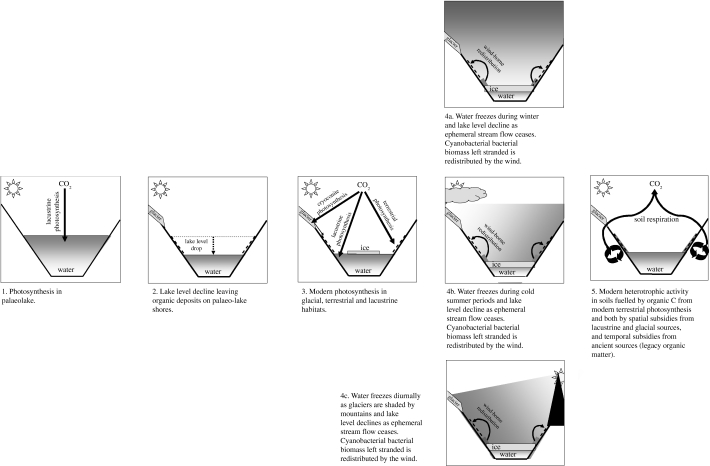
The Dry Valleys contain hotspots of productivity, which can act as point sources of resources. The relatively large standing biomass in lakes, most notably as cyanobacteria mats, and imbalances between primary production and consumption in the absence of macro-consumers, make lakes obvious sources of organic resources (Elberling et al. in press). Productivity in lakes in Dry Valleys is in the range 10–100 g C m−2 yr−1 (Vincent 1988). Transfer of only a modest fraction of this to the land surface could be sufficient to sustain the measured rates of soil respiration (Moorhead et al. 2003; Elberling et al. in press). Rapid turnover of organic matter in Dry Valley soils is also consistent with regular inputs of relatively labile organic residues (Hopkins et al. 2005, in press; Elberling et al. in press). Aeolian transport of lacustrine microbial mats, endolithic communities and organisms (Wilson 1965; Parker et al. 1982; Nienow & Friedmann 1993; Greenfield 1998; Moorhead et al. 2003; Nkem et al. 2006) supports the hypothesis that particulate (modern) matter is transported from productive sites (lakes) to low-productivity sites (soils). Increases in soil respiration along transects towards a lake and the decreasing organic C concentration with soil depth (Elberling et al. in press) provide indirect evidence of redistribution of lacustrine detritus from wet sites at lake edges to surrounding drier soils (Elberling et al. in press; Hopkins et al. in press). The mechanism proposed by Elberling et al. (in press) relies on lacustrine organic detritus washing up at the lake edge, where it is dried and dispersed by the wind (figure 3). Seasonal, diurnal and/or weather-dependent fluctuations in lake level resulting from periods when feeder streams stop flowing and glacier surfaces freeze may all contribute to wash-up and stranding of lacustrine detritus.
6. Conclusions
Information about C-transformation processes and rates in Antarctic soils and the contributions they make to C cycling in Dry Valley ecosystems is relatively limited. Furthermore, there are insufficient data to assess comprehensively the controls on productivity and the contributions they may make to heterotrophic activity in soils of the Dry Valleys. However, all processes discussed above are in operation in at least some Dry Valleys and their relative magnitudes vary between valleys. We propose the scheme summarized in figure 4 as a conceptual model, bringing together current observations on temporal and spatial subsidies, in situ productivity and redistribution of lacustrine detritus. This scheme attempts to unify different aspects of C cycling in the Dry Valleys, namely legacy versus contemporary organic C sources and terrestrial versus lacustrine organic C sources, in a manner that illustrates that they are not mutually exclusive. The challenge for understanding the landscape ecology, productivity and biodiversity of Antarctic Dry Valleys is to quantify the relative magnitude of each process, the causes of variation in magnitude of processes between valleys, and their sensitivity to environmental change.
Figure 4.
Conceptual summary of the sources and transfers of organic resources in the Antarctic Dry Valleys.
Acknowledgments
We are grateful to multiple collaborators for useful discussions that have helped shape the ideas presented here including B. J. Adams, J. E. Barrett, P. Broady, T. G. A. Green, I. Hogg, K. K. Newsham, R. A. Virginia, D. H. Wall, D. A. Wardle and D. D. Wynn-Williams (deceased). We also wish to acknowledge support from Antarctica New Zealand, UK Natural Environment Research Council, the Royal Society and the Transantarctic Association
References
- Adams, B. J., Bardgett, R. D., Ayres, E., Wall, D. H., Aislabie, J., Bamforth, S., Bargagli, R., Cary, C., Cavacini, P., Connell, L., Convey, P., Fell, J. W., Frati, F., Hogg, I. D., Newsham, K. K., O'Donnell, A., Russell, N., Seppelt, R. D. & Stevens, M. I. In press. Diversity and distribution of Victoria Land biota. Soil Biol. Biochem (doi:10.1016/j.soilbio.2006.04.030)
- Alfinito S, Fumanti B, Cavacini P. Epiphytic algae on mosses from northern Victoria Land, Antarctica. Nova Hedwigia. 1998;66:473–480. [Google Scholar]
- Bargagli R, Smith R.I.L, Martella L, Monaci F, Sanchez-Hernandez J.C, Ugolini F.C. Solution geochemistry and behaviour of major and trace elements during summer in a moss community at Edmonson Point, Victoria Land, Antarctica. Antarctic Sci. 1999;11:3–12. [Google Scholar]
- Barrett J.E, Virginia R.A, Wall D.H. Trends in resin and KCl-extractable soil nitrogen across landscape gradients in Taylor Valley, Antarctica. Ecosystems. 2002;5:289–299. doi:10.1007/s10021-001-0072-6 [Google Scholar]
- Barrett J.E, Virginia R.A, Wall D.H, Parsons A.N, Powers L.E, Burkins M.B. Variation in biogeochemistry and soil biodiversity across spatial scales in a polar desert ecosystem. Ecology. 2004;85:3105–3118. [Google Scholar]
- Barrett J.E, Virginia R.A, Parsons A.N, Wall D.H. Potential soil organic matter turnover in Taylor Valley, Antarctica. Arctic Antarctic Alpine Res. 2005;37:108–117. [Google Scholar]
- Barrett, J. E., Virginia, R. A., Hopkins, D. W., Aislabie, J., Bargagli, R., Bockheim, J. G., Campbell, I. B., Lyons, W. B., Moorhead, D., Nkem, J., Sletten, R. S., Steltzer, H., Wall, D. H. & Wallenstein, M. In press. Terrestrial ecosystem processes of Victoria Land, Antarctica. Soil Biol. Biochem (doi:10.1016/j.soilbio.2006.04.041)
- Beyer L, Bockheim J.G, Campbell I.B, Claridge G.G.C. Genesis, properties and sensitivity of Antarctic Gelisols. Antarctic Sci. 1999;11:387–398. [Google Scholar]
- Bockheim J.G. Properties and classification of cold desert soils from Antarctica. Soil Sci. Soc. Am. J. 1997;61:224–231. [Google Scholar]
- Bonani G, Friemann E.I, Ocampo-Friedmann R, McKay C.P, Woelfli W. Preliminary report of radiocarbon dating of cryptoendolithic microorganisms. Polarforschung. 1988;58:199–200. [PubMed] [Google Scholar]
- British Antarctic Survey. British Antarctic Survey; Cambridge, UK: 2004. Antarctica, 1 : 10 000 000 scale map. BAS (Misc) 11. [Google Scholar]
- Burkins M.B, Virginia R.A, Chamberlain C.P, Wall D.H. Origin and distribution of soil organic matter in Taylor Valley, Antarctica. Ecology. 2000;81:2377–2391. doi:10.2307/177461 [Google Scholar]
- Burkins M.B, Virginia R.A, Wall D.H. Organic carbon cycling in Taylor Valley, Antarctica: quantifying soil reservoirs and soil respiration. Global Change Biol. 2002;7:113–125. doi:10.1046/j.1365-2486.2001.00393.x [Google Scholar]
- Campbell I.B, Claridge G.G.C. Developments in Soil Science. vol. 16. Elsevier; Amsterdam, The Netherlands: 1987. Antarctica: soils, weathering processes and environment. 368 pp. [Google Scholar]
- Campbell I.B, Claridge G.G.C. Soil temperature, moisture and salinity patterns in Transantarctic Mountain cold desert ecosystems. In: Davidson W, Howard-Williams C, Broady P, editors. Antarctic ecosystems: models for wider ecological understanding. New Zealand Natural Sciences, Canterbury University; Christchurch, New Zealand: 2000. pp. 233–240. [Google Scholar]
- Cathey D.D, Parker B.C, Simmons G.M, Yongue W.H, Van Brunt M.R. The microfauna or algal mats and artificial substrates in southern Victoria Land lakes of Antarctica. Hydrobiologia. 1981;85:3–15. doi:10.1007/BF00011340 [Google Scholar]
- Claridge G.G. Seal tracks in the Taylor Dry Valley. Nature. 1961;190:559. [Google Scholar]
- Conovitz P.A, MacDonald L.H, McKnight D.M. Spatial and temporal active dynamics along three glacial melt-water stream in the McMurdo Dry Valleys, Antarctica. Arctic Antarctic Alpine Res. 2006;38:42–53. [Google Scholar]
- Cooke R.U, Warren A, Goudie A. Desert geomorphology. University College London Press; London, UK: 1993. [Google Scholar]
- Coutright E.M, Wall D.H, Virginia D.A. Determining habitat suitability for soil invertebrates in an extreme environment: the McMurdo Dry Valleys, Antarctica. Antarctic Sci. 2001;13:9–17. doi:10.1017/S0954102001000037 [Google Scholar]
- Denton G.H, Sugden D.E, Marchant D.R, Hall B.L, Wilch T.I. East Antarctic ice sheet sensitivity to Pliocene climatic change from a Dry Valleys perspective. Geografiska Annaler. 1993;75A:155–204. [Google Scholar]
- Doran P.T, et al. Antarctic climate cooling and terrestrial ecosystem response. Nature. 2002;415:517–520. doi: 10.1038/nature710. doi:10.1038/nature710 [DOI] [PubMed] [Google Scholar]
- Dort W. Mummified seals of southern Victoria Land. Antarctic J. US. 1971;6:210–211. [Google Scholar]
- Dort W. The mummified seals of Southern Victoria Land, Antarctica. In: Parker B.C, editor. Terrestrial biology III: Antarctic research series 30. American Geophysical Union; Washington, DC: 1981. pp. 123–154. [Google Scholar]
- Elberling B. Seasonal trends of soil CO2 dynamics in a soil subject to freezing. J. Hydrol. 2003;276:159–175. doi:10.1016/S0022-1694(03)00067-2 [Google Scholar]
- Elberling, B., Gregorich, E. G., Hopkins, D. W., Sparrow, A. D., Novis, P. & Greenfield, L. G. In press. Distribution and dynamics of soil organic matter in an Antarctic dry valley. Soil Biol. Biochem (doi:10.1016/j.soilbio.2005.12.011) [DOI] [PMC free article] [PubMed]
- Freckman D.H, Virginia R.A. Low-diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. [Google Scholar]
- Friedmann E.I. Endolithic microorganisms in the Antarctic cold desert. Science. 1982;215:1045–1053. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- Friedmann E.I, Ocampo R. Endolithic blue-green algae in the Dry Valleys: primary producers in the Antarctic desert ecosystem. Science. 1976;193:1247–1249. doi: 10.1126/science.193.4259.1247. [DOI] [PubMed] [Google Scholar]
- Friedmann E.I, Kappen L, Meyer M.A, Nienow J.A. Long-term productivity in the crypoendolithic microbial community of the Ross Desert, Antarctica. Microbial Ecol. 1993;25:51–69. doi: 10.1007/BF00182129. doi:10.1007/BF00182129 [DOI] [PubMed] [Google Scholar]
- Gooseff M.N, Barrett J.E, Doran P.T, Fountain A.G, Lyon W.B, Parsons A.N, Porazinska D.L, Virgina R.A, Wall D.H. Snow-patch influence on soil, biogeochemical processes and invertebrate distribution in the McMurdo Dry Valleys, Antarctica. Arctic Antarctic Alpine Res. 2003;35:91–99. [Google Scholar]
- Gooseff M.N, McKnight D.M, Runkel R.L, Duff J.H. Denitrification and hydrologic transient storage in a glacial meltwater stream, McMurdo Dry Valleys, Antarctica. Limnol. Oceanogr. 2004;49:1884–1895. [Google Scholar]
- Green T.G.A, Seppelt R.D, Schwarz A.-M. J. Epilithic lichens on the floor of the Taylor Valley, Ross Dependency, Antarctica. Lichenologist. 1992;24:57–61. [Google Scholar]
- Green T.G.A, Schroeter B, Kappen L, Seppelt R.D, Maseyk K. An assessment of the relationship between chlorophyll a fluorescence and CO2 gas exchange from field measurements on a moss and lichen. Planta. 1998;206:611–618. doi:10.1007/s004250050439 [Google Scholar]
- Greenfield L.G. Precipitation nitrogen at Signy Island and continental Cape Bird, Antarctica. Polar Biol. 1992;11:649–653. doi:10.1007/BF00237961 [Google Scholar]
- Greenfield L.G. Nitrogen in soil Nostoc mats: foams, release and implications for nutrient cycling in Antarctica. NZ J. Nat. Sci. 1998;23:101–107. [Google Scholar]
- Gregorich E. G., Hopkins D. W., Elberling B., Sparrow A. D., Novis P., Greenfield L. G. & Rochette, P. In press. Emission of CO2, CH4 and N2O from lakeshore soils in an Antarctic dry valley. Soil Biol. Biochem (doi:10.1016/j.soilbio.2006.01.015)
- Hall B.L, Denton G.H. Holocene history of the Wilson Piedmont Glacier along the southern Scott Coast, Antarctica. Holocene. 2002;12:619–627. doi:10.1191/0959683602hl572rp [Google Scholar]
- Hall B.L, Denton G.H. Surficial geology and geomorphology of eastern and central Wright Valley. Geomorphology. 2005;64:25–65. doi:10.1016/j.geomorph.2004.05.002 [Google Scholar]
- Hall B.L, Denton G.H, Overtuf B. Glacial Lake Wright, a high-level Antarctic lake during the LGM and early Holocene. Antarctic Sci. 2001;13:53–60. doi:10.1017/S0954102001000086 [Google Scholar]
- Hall B.L, Denton G.H, Overtuf B, Hendy C.H. Glacial Lake Victoria, a high-level Antarctic lake inferred from lacustrine deposits in Victoria Valley. J. Quat. Sci. 2002;17:697–706. doi:10.1002/jqs.691 [Google Scholar]
- Hawes I, Schwarz A.M. Photosynthesis in an extreme shade environment: benthic microbial mats from Lake Hoare, a permanently ice-covered Antarctic lake. J. Phycol. 1999;35:448–459. doi:10.1046/j.1529-8817.1999.3530448.x [Google Scholar]
- Higgins S.M, Denton G.H, Hendy C.H. Glacial geomorphology of Bonney drift, Taylor Valley, Antarctica. Geografiska Annaler Series A—Phys. Geography. 2000;82A:365–389. doi:10.1111/1468-0459.00129 [Google Scholar]
- Hopkins D.W, Gregorich E.G. Carbon as a substrate for soil organisms. In: Bardgett R.D, Usher M.B, Hopkins D.W, editors. Biodiversity and function in soils. British Ecological Society Ecological Reviews. Cambridge University Press; Cambridge, UK: 2005. pp. 57–79. [Google Scholar]
- Hopkins D.W, Elberling B, Greenfield L.G, Gregorich E.G, Novis P, O'Donnell A.G, Sparrow A.D. Soil micro-organisms in Antarctic Dry Valleys: resource supply and utilization. In: Gadd G.M, Semple K.T, Lappin-Scott H.M, editors. Micro-organisms and earth systems—advances in geomicrobiology. SGM symposium. vol. 65. Cambridge University Press; Cambridge, UK: 2005. pp. 71–84. [Google Scholar]
- Hopkins, D. W., Sparrow, A. D., Elberling, B. Gregorich, E. G., Novis, P., Greenfield, L. G. & Tilston, E. L. In press. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic dry valley. Soil Biol. Biochem (doi:10.1016/j.soilbio.2006.01.012)
- Janetschek H. Environments and ecology of terrestrial arthropods in the high Antarctic. In: Holdgate M.W, editor. Antarctic ecology. Scientific committee on Antarctic research. Academic Press; London, UK: 1970. pp. 871–885. [Google Scholar]
- Johnston C.G, Vestal J.R. Photosynthetic carbon incorporations and turnover in Antarctic cryptoendolithic microbial communities: are they the slowest growing communities on earth? Appl. Environ. Microbiol. 1991;57:2308–2311. doi: 10.1128/aem.57.8.2308-2311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L, Schroeter B, Green T.G.A, Seppelt R.D. Chlorophyll a fluorescence and CO2 exchange of Umbilicaria aprina under extreme light stress in the cold. Oecologia. 1998;113:325–331. doi: 10.1007/s004420050383. doi:10.1007/s004420050383 [DOI] [PubMed] [Google Scholar]
- Kennedy A.D. Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arctic Alpine Res. 1993;25:308–315. doi:10.2307/1551914 [Google Scholar]
- Kirschbaum M.U.F. Will changes in soil organic carbon act as a positive or negative feedback on global warming. Biogeochemistry. 2000;48:21–51. doi:10.1023/A:1006238902976 [Google Scholar]
- Legrand M, Ducroz F, Wagenbach D, Mulvaney R, Hall J. Ammonium in coastal Antarctic aerosol and snow: role of polar ocean and penguin emissions. J. Geophys. Res.-Atm. 1998;103:11 043–11 056. doi:10.1029/97JD01976 [Google Scholar]
- Lewis-Smith R.I. Oases as centres of high plant diversity and dispersal in Antarctica. In: Lyons W.B, Howard-Williams C, Hawes I, editors. Ecosystem processes in Antarctic ice-free landscapes. A.A. Balkema; Rotterdam, The Netherlands: 1997. pp. 119–128. [Google Scholar]
- Ludwig J.A, Whitford W.G. Short-term water and energy flow in arid ecosystems. In: Goodall D.W, Perry R.A, Perry K.M.W, editors. Arid-land ecosystems: structure, functioning and management. vol. 2. Cambridge University Press; New York, NY: 1981. pp. 271–299. [Google Scholar]
- Lyons W.B, Welch K.A, Casey A.E, Wall D.H, Virginia R.A, Fountain A.G, Doran P.T, Csatho B, Tremper C. Groundwater seeps in the Taylor Valley, Antarctica: an example of a decadal subsurface melt event. Ann. Glaciol. 2005;40:200–2006. [Google Scholar]
- Mabin, M. G. C. 1986 Radiocarbon dating of “Heroic Era” penguin and seal remains from Antarctica. In 1986 San Antonio Annual Meeting Abstracts with Programs, vol. 18, pp. 678. Boulder, CO: Geological Society of America.
- Marshall W.A. Aerial transport of keratinaceous substrate and distribution of the fungus Geomyces pannorum in Antarctic soils. Microbial Ecol. 1998;36:212–219. doi: 10.1007/s002489900108. doi:10.1007/s002489900108 [DOI] [PubMed] [Google Scholar]
- McKnight D.M, Niyogi D.K, Alger A.S, Bomblies A, Conovitz P.A, Tate C.M. Dry valley streams in Antarctica: Ecosystems waiting for water. Bioscience. 1999;49:985–995. doi:10.2307/1313732 [Google Scholar]
- Moorhead D.L, Barrett J.E, Virginia R.A, Wall D.H, Porazinska D. Organic matter and soil biota of upland wetlands in Taylor Valley, Antarctica. Polar Biol. 2003;26:567–576. doi:10.1007/s00300-003-0524-x [Google Scholar]
- Nkem, J. H., Wall, D. H., Virginia, R. A., Barrett, J. E., Broos, E., Porazinska, D. L. & Adams, B. L. 2006 Wind dispersal of soil invertebrates in the McMurdo Dry Valleys, Antarctica. Polar Biol 29, 346-352. (doi:10.1007/s00300-005-0061-x)
- Nienow J.A, Friedmann E.I. Terresrial lithophytic (rock) communities. In: Friedmann E.I, editor. Antarctic microbiology. Wiley; New York, NY: 1993. pp. 181–196. [Google Scholar]
- Noy-Meir I. Desert ecosystems: environment and producers. Annu. Rev. Ecol. Syst. 1973;4:25–51. doi:10.1146/annurev.es.04.110173.000325 [Google Scholar]
- Pannewitz S, Green T.G.A, Maysek K, Schlensog M, Seppelt R, Sancho L.G, Turk R, Schroeter B. Photosynthetic responses of three common mosses from continental Antarctica. Antarctic Sci. 2005;17:341–352. doi:10.1017/S0954102005002774 [Google Scholar]
- Pannewitz S, Green T.G.A, Schlensog M, Seppelt R, Sancho L.G, Schroeter B. Photosynthesis performance of Xanthoria mawsonii C.W. Dodge in coastal habitats, Ross Sea region, continental Antarctica. Lichenologist. 2006;38:67–81. doi:10.1017/S0024282905005384 [Google Scholar]
- Parker B.C, Simmons G.M, Wharton R.A, Jr, Seaburg K.G, Love F.G. Removal of organic and inorganic matter from Antarctic lakes by aerial escape of blue-green algal mats. J. Phycol. 1982;18:72–78. doi:10.1111/j.0022-3646.1982.00072.x [Google Scholar]
- Parsons A.N, Barrett J.E, Wall D.H, Virginia R.A. Soil carbon dioxide flux in Antarctic Dry Valley ecosystems. Ecosystems. 2004;7:286–295. doi:10.1007/s10021-003-0132-1 [Google Scholar]
- Péwé T.L. Multiple glaciation in McMurdo sound region, Antarctica—a progress report. J. Geol. 1960;68:498–514. [Google Scholar]
- Péwé T.L, Rivard N.R, Llano G.A. Mummified seals in the McMurdo sound region, Antarctica. Science. 1959;130:716. doi: 10.1126/science.130.3377.716. [DOI] [PubMed] [Google Scholar]
- Pocock T, Lachance M.A, Proschold T, Priscu J, Kim S.S, Huner N.P.A. Identification of a psychrophilic green alga from Lake Bonney, Antarctica: Chlamydomonas raudensis Ettl. (UWO 241) Chlorophyceae. J. Phycol. 2004;40:1138–1148. doi:10.1111/j.1529-8817.2004.04060.x [Google Scholar]
- Schlesinger W.H, Pippen J.S, Wallenstein M.D, Hofmockel K.S, Klepeis D.M, Mahall B.E. Community composition and photosynthesis by Photoautotrophs under quartz pebbles, southern Mojave desert. Ecology. 2003;84:3222–3231. [Google Scholar]
- Stevens M.I, Hogg I.D. Expanded distributional records of Collembola and Acari in southern Victoria Land. Antarctica. Pedobiologia. 2002;46:485–495. doi:10.1078/0031-4056-00154 [Google Scholar]
- Schwarz A.M.J, Green T.G.A, Seppelt R.D. Terrestrial vegetation at Canada Glacier, Southern Victoria Land, Antarctica. Polar Biol. 1992;12:397–404. doi:10.1007/BF00243110 [Google Scholar]
- Taylor T.G. The physiography of the McMurdo Sound and Granite Harbour region. British (Terra Nova) Antarctic Expedition 1910–13. Harrison; London, UK: 1922. [Google Scholar]
- Treonis A.M, Wall D.H, Virginia R.A. Invertebrate biodiversity in Antarctic dry valley soils and sediments. Ecosystems. 1999;2:482–492. doi:10.1007/s100219900096 [Google Scholar]
- Treonis A.M, Wall D.H, Virginia R.A. Field and microcosm studies of decomposition and soil biota in a cold desert soil. Ecosystems. 2002;5:159–170. doi:10.1007/s10021-001-0062-8 [Google Scholar]
- Vincent W.F. Microbial ecosystems of Antarctica. Cambridge University Press; Cambridge, UK: 1988. p. 304. [Google Scholar]
- Virginia R.A, Wall D.H. How soils structure communities in the Antarctic Dry Valleys. Bioscience. 1999;49:973–983. doi:10.2307/1313731 [Google Scholar]
- Wall D.H. Biodiversity and ecosystem functioning in terrestrial habitats of Antarctica. Antarctic Sci. 2005;17:523–531. doi:10.1017/S0954102005002944 [Google Scholar]
- Webb P.N, Leckie R.M. Complete seal carcass at Lake Miers, Miers Valley. Antarctic J. US. 1977;12:11–12. [Google Scholar]
- Wilson A.T. Escape of algae from frozen lakes and ponds. Science. 1965;46:376. [Google Scholar]
- Wilson E.O. The future of life. Alfred A. Knopf.; New York, NY: 2002. [Google Scholar]