Abstract
Tracks of marine animals in the wild, now increasingly acquired by electronic tagging of individuals, are of prime interest not only to identify habitats and high-risk areas, but also to gain detailed information about the behaviour of these animals. Using recent satellite-derived current estimates and leatherback turtle (Dermochelys coriacea) tracking data, we demonstrate that oceanic currents, usually neglected when analysing tracking data, can substantially distort the observed trajectories. Consequently, this will affect several important results deduced from the analysis of tracking data, such as the evaluation of the orientation skills and the energy budget of animals or the identification of foraging areas. We conclude that currents should be systematically taken into account to ensure the unbiased interpretation of tracking data, which now play a major role in marine conservation biology.
Keywords: biologging, travelling versus foraging behaviour, impact of ocean currents, marine animal behaviour, satellite oceanography, wild-life tracking
1. Introduction
Understanding how marine animals use the oceanic environment and its constraints is crucial for the development of sound management strategies for marine ecosystems that are threatened by climate change (Parmesan & Yohe 2003; Schmittner 2005) and direct anthropogenic pressure (Myers & Worm 2003; Lewison et al. 2004a). This could not be achieved without the detailed observation of free-ranging organisms, which is now possible through the electronic tagging of individuals (Block 2005). In the last few years, this biologging approach has been widely used for various marine species, greatly enhancing our knowledge of physiology, habitat use and movements of long-distance migrants (McConnell et al. 1992; Bost et al. 1997; Lutcavage et al. 1999; Costa & Sinervo 2004; Block et al. 2005). Biologging also provides us with unique observations, which are helpful in developing effective marine ecosystem conservation measures. In particular, electronic tracking provides evidence that the foraging hot spots frequented by pelagic fish and their associated fishing fleets are also exploited by endangered species, such as sea turtles (Spotila et al. 1996). Hence, the probability of incidentally catching endangered species is greatly increased in these areas (Ferraroli et al. 2004; Hays et al. 2004a; Lewison et al. 2004b). Accurately locating these zones and understanding how animals use these critical areas is indispensable if we want to define how, where and when fishery management procedures must be applied, so that we can ensure the sustainable exploitation of commercial species while minimizing the by-catch of endangered species (Lewison et al. 2004a,b).
Identifying when and where marine animals forage is therefore crucial and such information can be deduced from the recorded trajectories of tagged animals (Sibert et al. 1999; Benhamou 2004; Newlands et al. 2004; Gutenkunst et al. submitted). Other information that can be deduced from track analyses includes the identification of migration corridors (Morreale et al. 1996), dispersion patterns (e.g. Block et al. 2005), environmental preferences (e.g. Polovina et al. 2004) and navigation strategies (e.g. Akesson et al. 2003).
While the influence of ocean currents on recorded trajectories has been detected and qualitatively assessed (Luschi et al. 1998, 2003a,b; Hays et al. 1999; Polovina et al. 2000), their impact on the results of track analyses has never been quantitatively assessed. The lack of detailed current estimates along the trajectories of aquatic species has prevented such an assessment, while the effects of wind on migrating birds are well documented (e.g. Richardson 1990; Weimerskirch et al. 2000). Recent progress in satellite oceanography (Rio & Hernandez 2003, 2004) allows the synoptic estimation of surface currents. We use these current estimates here to conduct the first quantitative evaluation of the impact of oceanic currents on the trajectories of marine animals and investigate the consequences of the results deduced from tracking analysis.
2. Material and methods
(a) Separating swimming and drifting
The trajectories of marine animals reflect the combined effects of the animal's voluntary motion (swimming) and its transportation by oceanic currents (drift). More precisely, trajectories are time-series of the animal's location [X(t0), X(t1), …, X(tN)] at the sea surface. The observed velocity of the animal over ground (Vg) is the time derivative of X. In practice, Vg is estimated by computing the distances, in the x and y directions, between the two consecutive locations and then dividing these distances by the time elapsed. This velocity is the sum of the animal's swimming velocity (Vs) and the velocity of the fluid in which the animal moves, i.e. the velocity of the current (Vc):
| 2.1 |
Tracks are thus simple linear combinations of the animal's own motion and the motion of the surrounding fluid:
| 2.2 |
Depending on whether the animal, or the ocean, is quiet or active, the relative importance of these two components on the observed motion can be highly variable and affect most results regarding the animal's behaviour inferred from track analyses.
(b) Processing satellite tracking data
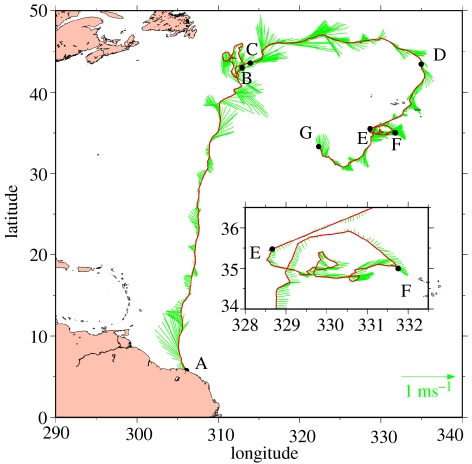
To quantify the impact of ocean currents on a wide range of oceanic conditions, we analyse a very long (11 635 km, 295 days) trajectory of an Argos-tracked female leatherback turtle (Dermochelys coriacea), hereafter referred to as T8 (figure 1). T8 left French Guyana, South America on 29 June 2000 and circulated through most of the North Atlantic Ocean, crossing major current systems and quiet oceanic areas, before transmission stopped on 20 April 2001.
Figure 1.
Trajectory of Argos-tracked leatherback turtle T8 (red line) with superimposed surface current vectors (green). This track is resampled at 3 h intervals and a current vector is plotted every 12 h. Each vector has its origin on the track. Black dots labelled A–G are used for track segmentation. T8 reaches these milestones at the following dates: A (departure), 29 June 2000; B, 10 September 2000; C, 14 October 2000; D, 14 December 2000; E, 17 January 2001; F, 22 February 2001; G (end of data transmission), 20 April 2001. A zoom showing details of the E–F segment is inserted.
The trajectory of T8 was edited as follows. All Argos locations implying an apparent velocity above 10 km h−1 were discarded (Eckert 2002). The track was then smoothed and resampled at 3 h intervals using simple local linear regression with a time window of 2 days. Such smoothing is needed to filter out most of the Argos location error, which acts as high-frequency noise that would subsequently be amplified in the velocity-derivation process. The associated resampling is a standard procedure allowing the computation of homogeneous velocities (Vg) over constant time-intervals. With 3 h intervals, the mean distance between successive resampled positions is close to 5 km. This spatial resolution provides a more than sufficient sampling of the mesoscale variations of the ocean current field (see §2c).
(c) Estimating currents from satellite observations
Strictly speaking, the velocity of the current (Vc) should be estimated at the depth of the tracked animal. This is not a simple task as currents are not easily monitored over vast oceanic areas, except for surface currents which can be estimated using satellite observations (e.g. Ducet et al. 2000). Fortunately, leatherback turtles mostly dive in the near-surface epipelagic zone (Hays et al. 2004b) where the current velocity generally differs little from its surface value (see §4). Satellite-derived surface current estimates can thus be used to approximate Vc. They are obtained as the sum of the mean (Vm) and anomaly (Va) of the surface geostrophic current plus the surface Ekman current (Ve):
| 2.3 |
The mean geostrophic velocity is provided by Rio & Hernandez (2004) on a regular 1×1° grid. The geostrophic relation is used to deduce Va from gridded fields of sea-level anomalies (SLA) measured by the radar altimeters onboard the TOPEX/POSEIDON and ERS-2 satellites. Weekly SLA fields are obtained from AVISO (http://www.aviso.oceanobs.com) on a 1/3×1/3° Mercator grid. The Ekman component of the current (Ve) is computed as a function of the surface wind stress using the Rio & Hernandez (2003) model. Daily wind stresses, derived from QuickSCAT scatterometer measurements, are obtained from CERSAT (http://www.ifremer.fr/cersat) on a regular 25×25 km grid. Then, based on these gridded velocity fields, the three components of the surface current are linearly interpolated (in space and time) at each position of the resampled track.
This surface velocity reconstruction technique was recently evaluated by Pascual et al. (2006), who compared the velocities using this technique to a large set (over 600 000 measurements) of surface velocity observations from the Global Drifter Program (http://www.aoml.noaa.gov/phod/dac). Velocity estimates prove to be essentially unbiased with a mean error below 1 cm s−1 for both the meridional and the zonal component of the velocity vector. In energetic areas (with root-mean-square velocities above 20 cm s−1), estimated velocities explain 73.3% of the drifter zonal velocity variance and 66.4% of the drifter meridional velocity variance. Most of the unexplained variance appears to lie in high-frequency signals, not resolved by altimetric observations (Le Traon & Dibarboure 2002). The comparison with drifter observations excluded shallow coastal areas, where significant deviations from geostrophic equilibrium are known to occur, and the Equatorial band, where both the geostrophic and Ekman approximations break down. In our case, these limitations only impact the first 4 days of tracking after which T8 leaves the Guiana shelf and enters the open waters of the North Atlantic Ocean. The reconstructed currents thus provide realistic surface velocity estimates along almost the entire T8 track. Still, as pointed out earlier, surface velocity estimates are only used as a proxy for the current velocity at the depth of the tracked animal. This approximation is justified here as recent observations of the open-ocean diving behaviour of leatherback turtles indicate that adult leatherbacks, like T8, generally spend over 50% of their time near the surface (typically between 0 and 50 m) and rarely dive below 200 m (Hays et al. 2004b; James et al. 2005). These animals thus mostly occupy the upper oceanic layers where the vertical variations of the current are essentially the vertical variations of its Ekman component. This relatively small current component (mean velocity of the Ekman current below 3 cm s−1 for a mean total current velocity of 19 cm s−1 along T8 track) is maximum at the surface and decreases with depth. An animal regularly diving from the surface to modest depths shall thus experience an average current speed differing from the surface current value by only a few centimetres per second. More important deviations from the surface current will only be experienced in rare circumstances when the turtle dives down to several hundred metres (Hays et al. 2004b) below the core of major surface currents or mesoscale features.
(d) Defining a current-corrected track
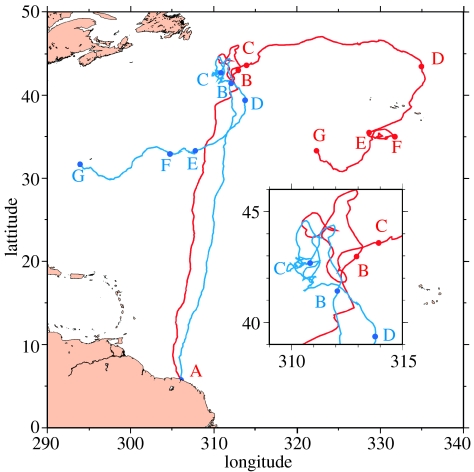
At first sight, the relationship between the apparent movement of T8 and the current direction (figure 1) is not at all obvious. It is better analysed comparing (figure 2) the observed track with the current-corrected track Xcc(t), i.e. the trajectory that the animal would have followed in a motionless ocean, swimming exactly the way she swam:
| 2.4 |
Figure 2.
Comparison of the observed (red) and current-corrected (blue) track of T8. Milestones are positioned on the current-corrected track at the same dates as on the observed track. A zoom showing details of the B–C segment is inserted. Note that the current-corrected track displays the animal's own motion (Vs) integrated in time but, taken alone, a position along that track bears no direct interpretation (and could very well be on the continent!).
In practice, this current-corrected track is readily computed using the available tracking and current data:
| 2.5 |
where Δt is the period at which the observed track has been resampled and N is the total number of locations in this track.
3. Results
(a) New evidence for strong compass sense
Between departure (A) and 34° N, over more than 3250 km, the observed track is remarkably straight, and the current-corrected track is even slightly straighter. Then, between 34° N and milestone B (another 1000 km), the observed trajectory displays several marked heading changes, while the current intensifies as T8 crosses the Gulf Stream system. Interestingly, unlike the observed track, the current-corrected track (figure 2) remains remarkably straight in this highly dynamic area. This reveals an interesting navigation strategy in which T8 does not change her swimming direction to compensate for the current drift but, on the contrary, maintains a steady heading while crossing strong currents pushing at cross-angles. This behaviour, not detectable without current correction, is an additional proof of the strong compass sense previously detected in leatherback turtles (Lohmann & Lohmann 1993).
(b) Swimming and drifting: impact on energy budget
After a period of apparent erratic motion (between B and C), T8 started moving east in the frontal area between the subtropical and subpolar gyres (between C and D), where she is pushed by the powerful flow of the Gulf Stream Extension and then the North Atlantic Current with estimated velocities often above 0.5 m s−1, peaking at 0.95 m s−1. Current impact on the track is remarkable: the current-corrected track reveals that T8 has little active motion towards the east. The distance actually swam by T8 between C and D (length of the current-corrected track) is only 1196 km, while the distance travelled is 2144 km. Thus, almost half of the observed displacement is due to the current drift. Since the work done by the animal is essentially proportional to the distance swum, any energy budget using the distance computed along the observed track would be grossly wrong. Segment C–D is obviously the track segment where the current impact is the largest. Over the other segments, the difference between the current-corrected track length and the observed track length remains between 5 and 20% of the latter.
(c) Navigation strategy in the presence of strong currents
In the last recorded part of the trajectory (D–G), T8 appears to progressively come back to the western part of the Atlantic Basin, with the conspicuous exception of the E–F segment. But, the current-corrected track (figure 2) reveals that, even if apparent motion from E to F is towards the east, T8 actually kept swimming towards west between these two points. More precisely, computed velocities indicate that, while T8 was swimming at 0.11 m s−1 (mean of the westward component of her swimming velocity), the Azores current, centred around 35° N, pushed her faster to the east (mean of the eastward component of the current velocity: 0.20 m s−1). The convoluted shape of the E–F segment (figure 1) indicates that T8 was actually foraging, while slowly swimming against the current, in the very productive Azores front. After this feeding event, the T8 trajectory becomes straighter as she steadily heads northwest (right after F). By doing so, T8 gets out of the narrow core of the Azores current and starts actually moving west again in a region where her motion is no longer opposed by a strong eastward flow. She then heads to the south, encountering the Azores current again. This time, she crosses it completely and then keeps moving to the west. Further work is needed to understand the exact navigation mechanisms used to shape such a complex trajectory, but our analysis shows that this can only be done with detailed knowledge of the currents.
(d) Diagnosing travelling or foraging behaviour in the presence of currents
Going one step further, new current data allow us to revisit more elaborate track analyses aiming at the identification of the two main movement behaviours, i.e. travelling versus food prospecting. These two motion types are characterized by long-range directed displacements and erratic motions, respectively. Based on this simple characterization, several techniques (e.g. Benhamou 2004; Newlands et al. 2004) have been developed to automatically identify track segments where either travelling (directed motion) or foraging (erratic motion) dominates. Such analyses are extremely useful when no other behavioural information is available. They provide important information on feeding ground locations, migration areas and switching frequency between travelling and foraging behaviour (Newlands et al. 2004). Track analyses are also useful when combined with complementary biologging measurements (e.g. dive profiles) to obtain more precise descriptions of the animal's movement and behaviour in relation to its environment (e.g. Georges et al. 2000; LeBoeuf et al. 2000; Hays et al. 2004b; James et al. 2005; Sale et al. 2006).
It is apparent that this movement characterization (erratic or directed) concerns the animal's own motion (Vs) and not the combined motion of the animal and surrounding fluid (Vs+Vc). In other words, such track analyses should be performed on current-corrected tracks, not on observed tracks as usually done.
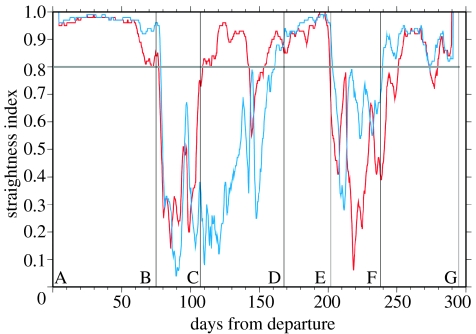
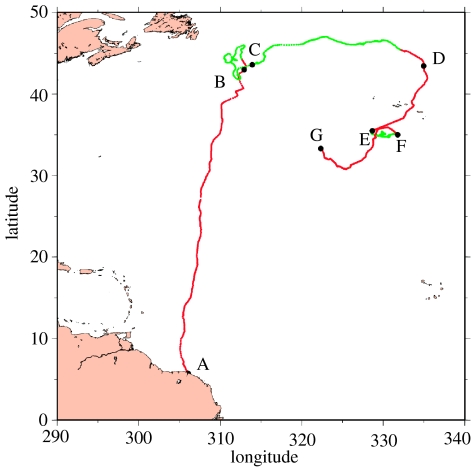
To show the differences between the two approaches we analysed the impact of the current correction on the straightness index S of the T8 track. This index, defined by the ratio of the beeline distance between two points on the track and the actual length of the track between these two points (Batschelet 1981), is often used to distinguish between travelling and foraging behaviours in track analyses (Benhamou 2004). If the animal travels straight from one point to the other, S=1, whereas S→0 in the case of erratic motion. We computed the value of S to the middle of 4 day long periods for both the actual and the current-corrected tracks. This period of 4 days was chosen to detect travelling or foraging events extending over several days as the original sampling frequency of data, and their subsequent smoothing, prevents detailed analysis at shorter periods. Both the original and the current-corrected straightness indices showed a bimodal distribution, with S=0.8 threshold value separating both modes. Accordingly, we used S=0.8 for separating travelling (S>0.8) from foraging (S<0.8) behaviour, for both the original and the current-corrected straightness indices (figure 3). Figure 3 shows that current correction neither modifies the ‘travelling diagnosis’ on segments A–B, D–E and F–G, nor the ‘foraging diagnosis’ on B–C and F–G. But on segment C–D, the uncorrected index points at a dominant travelling behaviour (S>0.8 during 51 days of this 61-day period), whereas the current-corrected value of S clearly indicates a foraging behaviour, except for the last 7 days of that period. The difference in the diagnosis is huge as the current-corrected index suggests that, between C and D, T8 foraged during 54 days along a 1807 km track segment (figure 4). On the contrary, the uncorrected index only points at a 10-day, 191 km long foraging episode. The diagnosis of a much longer foraging period obtained with the current-correction is otherwise supported by the fact that the concerned track segment between C and D lies in the transition zone between the subtropical and subpolar gyres where food abounds (Polovina et al. 2001). In addition, T8 was particularly slow along that C–D segment (mean swimming velocity: 0.26 m s−1), suggesting that she was indeed searching and/or processing food.
Figure 3.
Straightness index (S) computed along the observed (red) and current-corrected (blue) tracks. S is computed over 4 day long segments and the computed value is attributed to the central time of each segment. This yields values of S every 3 h, except for the first and last 2 days of the tracking period. Travelling is diagnosed for S>0.8 and foraging for S<0.8.
Figure 4.
Localization of the travelling (red) and foraging (green) segments on the observed track, based on the value of the current-corrected straightness index. All fixes corresponding to S>0.8 are plotted in red. Fixes with S<0.8 are plotted in green.
4. Discussion
T8's track is remarkable as it displays various types of animal movement patterns occurring in various ocean regions with widely different dynamics. Analysis of over 10 other postnesting trajectories of leatherback turtles tracked from French Guyana (Ferraroli et al. 2004) provides similar results, even if no single other trajectory is as complete and as informative as T8's track. In all trajectories, currents prove to commonly add sinuosity to long-range directed track segments confirming that leatherback turtles tend to maintain a more stable heading than indicated by the observed tracks. Their compass sense is truly remarkable, even in the presence of strong currents. The other tracks also provide examples of (probably) foraging turtles either swimming with the current (like in the C–D segment), or actively swimming against it (like in the E–F segment). The reason for such different navigation strategies relative to the current needs to be further analysed with support from complementary diving data.
Altogether, our results reveal that currents have a highly variable but rarely negligible impact on marine animals' tracks. Ignoring current effects can thus be misleading when trying to infer animal behaviour from tracking data. Additionally, precise information on the currents significantly modifies, and moreover improves, our interpretation of observed trajectories. We thus conclude that currents should be systematically taken into account to warrant unbiased analysis of marine animals' tracking data. Surface currents, derived from satellite observations, are readily available to analyse tracks from (mostly) epipelagic animals. For deeper-diving animals, realistic current estimates at all depths should soon be available from operational global ocean models.
Marine animals faster than leatherback turtles will clearly be less impacted by currents, but oceanographic information will remain important for interpreting their actual behaviour. An accurate determination of the currents will indeed be needed to understand how travelling animals shape their trajectories as a function of currents. This might help unravel some of the remaining mysteries of animal navigation.
Currents will be even more important to analyse foraging behaviour. Indeed, foraging typically occurs in rich, dynamically active areas where currents tend to be faster while feeding animals tend to slow down. In such areas, the balance between oceanic movements and animal motion will be subtle. Current correction will thus be critically needed to properly assess foraging habitat and the time budget of individuals. This shall lead to a more accurate estimation of food requirements, and ultimately to a better quantification of the way animal populations impact on trophic resources and respond to changes in food availability, throughout marine ecosystems.
Acknowledgements
We thank the customary chiefs and inhabitants of Awala-Yalimapo (French Guyana) and the Amana Natural Reserve for their support in the field, and Ph. Schaeffer for assistance with data visualization. This study was carried out under CNRS institutional license (B67 482 18) and adhered to the legal requirements of the country in which the work was carried out, and all institutional guidelines.
Footnotes
These authors contributed equally to this work.
References
- Akesson S, Broderick A.C, Godley B.J, Lushi P, Papi F, Hays G.C. Navigation by green turtles; which strategy do displaced adults use to find Ascension Island? Oïkos. 2003;103:363–372. [Google Scholar]
- Batschelet E. Academic Press; London, UK: 1981. Circular statistics in biology. [Google Scholar]
- Benhamou S. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity or fractal dimension? J. Theor. Biol. 2004;229:209–220. doi: 10.1016/j.jtbi.2004.03.016. doi:10.1016/j.jtbi.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Block B. Physiological ecology in the 21st century: advancements in biologging science. Integr. Comput. Biol. 2005;43:305–320. doi: 10.1093/icb/45.2.305. doi:10.1093/icb/45.2.305 [DOI] [PubMed] [Google Scholar]
- Block B.A, Teo S.L.H, Walli A, Boustany A, Stokesbury M.J.W, Farwell C.J, Weng K.C, Dewar H, Williams T.D. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434:1121–1127. doi: 10.1038/nature03463. doi:10.1038/nature03463 [DOI] [PubMed] [Google Scholar]
- Bost C.A, Georges J.-Y, Guinet C, Cherel Y, Pütz K, Charrassin J.B, Handrich Y, Zorn T, Lage J, Le Maho Y. Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar. Ecol. Prog. Ser. 1997;150:21–33. [Google Scholar]
- Costa D.P, Sinervo B. Field physiology: physiological insights from animals in nature. Annu. Rev. Physiol. 2004;66:209–238. doi: 10.1146/annurev.physiol.66.032102.114245. doi:10.1146/annurev.physiol.66.032102.114245 [DOI] [PubMed] [Google Scholar]
- Ducet N, Le Traon P.Y, Reverdin G. Global high-resolution mapping of ocean circulation from TOPEX/Poseidon and ERS-1 and -2. J. Geophys. Res. 2000;105:19 477–19 498. doi:10.1029/2000JC900063 [Google Scholar]
- Eckert S.A. Swim speed and movement patterns of gravid leatherback sea turtles (Dermochelys coriacea) at St. Croix, US Virgin Island. J. Exp. Biol. 2002;205:3689–3697. doi: 10.1242/jeb.205.23.3689. [DOI] [PubMed] [Google Scholar]
- Ferraroli S, Georges J.Y, Gaspar P, Le Maho Y. Where leatherback turtles meet fisheries. Nature. 2004;429:521–522. doi: 10.1038/429521a. doi:10.1038/429521a [DOI] [PubMed] [Google Scholar]
- Georges J.Y, Bonadonna F, Guinet C. Foraging habitat and diving activity of lactating subantarctic fur seals in relation to sea surface temperatures on Amsterdam Island. Mar. Ecol. Prog. Ser. 2000;196:291–304. [Google Scholar]
- Gutenkunst, R., Newlands, N., Lutcavage, M. & Edelstein-Keshet, L. Submitted. Inferring resource distributions from Atlantic bluefin tuna movements: an analysis based on net displacement and length of track. J. Theor. Biol [DOI] [PubMed]
- Hays G.C, Luschi P, Papi F, del Seppia C, Marsh R. Changes in behaviour during the interesting period and postnesting migration for Ascension Island green turtles. Mar. Ecol. Prog. Ser. 1999;189:263–273. [Google Scholar]
- Hays G.C, Houghton J.D.R, Myers A.E. Pan-Atlantic leatherback turtle movements. Nature. 2004a;429:522. doi: 10.1038/429522a. doi:10.1038/429522a [DOI] [PubMed] [Google Scholar]
- Hays G.C, Houghton J.D.R, Isaac C, King R.S, Lloyd C, Lovell P. First records of oceanic dive profiles for leatherback turtles (Dermochelys coriacea) indicate behavioural plasticity associated with long distance migration. Anim. Behav. 2004b;67:733–743. doi:10.1016/j.anbehav.2003.08.011 [Google Scholar]
- James M.C, Myers R.A, Ottensmeyer C.A. Behaviour of leatherback sea turtles, Dermochelys coriacea, during the migratory cycle. Proc. R. Soc. B. 2005;272:1547–1555. doi: 10.1098/rspb.2005.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Traon P.Y, Dibarboure G. Velocity mapping capabilities of present and future altimeter missions: the role of high frequency signals. J. Atmos. Oceanic Technol. 2002;19:2077–2088. doi:10.1175/1520-0426(2002)019<2077:VMCOPA>2.0.CO;2 [Google Scholar]
- LeBoeuf B.J, Crocker D.E, Costa D.P, Blackwell S.B, Webb P.M, Houser D.S. Foraging ecology of northern elephant seals. Ecol. Monogr. 2000;70:353–382. doi:10.2307/2657207 [Google Scholar]
- Lewison R.L, Crowder L.B, Read A.J, Freeman S.A. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 2004a;19:598–604. doi:10.1016/j.tree.2004.09.004 [Google Scholar]
- Lewison R.L, Freeman S.A, Crowder L.B. Quantifying the effects of fisheries on threatened species: the impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecol. Lett. 2004b;7:221–231. doi:10.1111/j.1461-0248.2004.00573.x [Google Scholar]
- Lohmann K.J, Lohmann C.M.F. A light-independent magnetic compass in the leatherback sea turtle. Biol. Bull. 1993;185:149–151. doi: 10.2307/1542138. [DOI] [PubMed] [Google Scholar]
- Luschi P, Hays G.C, Del Seppia C, Marsh R, Papi F. The navigational feats of green sea turtles migrating from Ascension Island investigated by satellite telemetry. Proc. R. Soc. B. 1998;265:2279–2284. doi: 10.1098/rspb.1998.0571. doi:10.1098/rspb.1998.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschi P, Hays G.C, Papi F. A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos. 2003a;103:293–302. doi:10.1034/j.1600-0706.2003.12123.x [Google Scholar]
- Luschi P, Sale A, Mencacci R, Hugues G.R, Lutjeharms J.R.E, Papi F. Current transport of leatherback sea turtles (Dermochelys coriacea) in the ocean. Proc. R. Soc. B. 2003b;270:S129–S132. doi: 10.1098/rsbl.2003.0036. doi:10.1098/rsbl.2003.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcavage M.E, Brill R.W, Skomal G.B, Chase B.C, Howey P.W. Results of pop-up satellite tagging of spawning size class fish in the Gulf of Maine: do North Atlantic bluefin tuna span in the mid-Atlantic? Can. J. Fish. Aquat. Sci. 1999;56:173–177. doi:10.1139/cjfas-56-2-173 [Google Scholar]
- McConnell B.J, Chambers C, Fedak M.A. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarctic Sci. 1992;4:393–398. [Google Scholar]
- Morreale S.J, Standore E.A, Spotila J.R, Paladino F.V. Migration corridor for sea turtles. Nature. 1996;348:319–320. doi:10.1038/384319a0 [Google Scholar]
- Myers R.A, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. doi:10.1038/nature01610 [DOI] [PubMed] [Google Scholar]
- Newlands N.K, Lutcavage M.E, Picker T.J. Analysis of foraging movements of Atlantic bluefin tuna (Thunnus thynnus): individuals switch between two modes of search behaviour. Popul. Ecol. 2004;46:39–53. doi:10.1007/sl10144-004-0169-9 [Google Scholar]
- Parmesan C, Yohe H. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Pascual A, Faugère Y, Larnicol G, Le Traon P.Y. Improved description of the ocean mesoscale variability by combining four satellite altimeter missions. Geophys. Res. Lett. 2006;33:L02611. doi:10.1029/2005GL024633 [Google Scholar]
- Polovina J.J, Kobayashi D.R, Parker D.M, Seki M.P, Balazs G.H. Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish Oceanogr. 2000;9:71–82. doi:10.1046/j.1365-2419.2000.00123.x [Google Scholar]
- Polovina J.J, Howell E, Kobayashi D.R, Seki M.P. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 2001;49:469–483. doi:10.1016/S0079-6611(01)00036-2 [Google Scholar]
- Polovina J.J, Balazs G.H, Howell E.A, Parker D.M, Seki M.P, Dutton P.H. Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish Oceanogr. 2004;13:36–51. doi:10.1046/j.1365-2419.2003.00270.x [Google Scholar]
- Richardson W.J. Wind and orientation of migrating birds: a review. Cell. Mol. Life Sci. 1990;46:416–425. doi: 10.1007/978-3-0348-7208-9_11. [DOI] [PubMed] [Google Scholar]
- Rio M.-H, Hernandez F. High-frequency response of wind-driven currents measured by drifting buoys and altimetry over the world ocean. J. Geophys. Res. 2003;108:3283. doi:10.1029/2002JC001655 [Google Scholar]
- Rio M.-H, Hernandez F. A mean dynamic topography computed over the world ocean from altimetry, in situ measurements, and a geoid model. J. Geophys. Res. 2004;109:C12032. doi:10.1029/2003JC002226 [Google Scholar]
- Sale A, Luschi P, Mencacci R, Lambardi P, Hugues G.R, Hays G.C, Benvenuti S, Papi F. Long-term monitoring of leatherback turtle diving behaviour during oceanic movements. J. Exp. Mar. Biol. Ecol. 2006;328:197–210. doi:10.1016/j.jembe.2005.07.006 [Google Scholar]
- Schmittner A. Decline of the marine ecosystem caused by a reduction in the Atlantic overturning circulation. Nature. 2005;434:628–633. doi: 10.1038/nature03476. doi:10.1038/nature03476 [DOI] [PubMed] [Google Scholar]
- Sibert J.R, Hampton J, Fournier D.A, Bills P.J. An advection-diffusion–reaction model for the estimation of fish movement parameters from tagging data, with application to skipjack tuna (Katsuwonus pelamis) Can. J. Fish Aquat. Sci. 1999;56:925–938. doi:10.1139/cjfas-56-6-925 [Google Scholar]
- Spotila J.R, Dunham A.E, Leslie A.J, Steyermark A.C, Plotkin P.T, Paladino F.V. Worldwide population decline of Dermochelys coriacea: are leatherback turtles going extinct? Chelonian Conserv. Biol. 1996;2:209–222. [Google Scholar]
- Weimerskirch H, Guionnet T, Martin J, Shaffer S.A, Costa D.P. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. Biol. Sci. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. doi:10.1098/rspb.2000.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]