Abstract
In many taxa, reproductive performance increases throughout the lifespan and this may occur in part because older adults invest more in reproduction. The mechanisms that facilitate an increase in reproductive performance with age, however, are poorly understood. In response to stressors, vertebrates release glucocorticoids, which enhance survival but concurrently shift investment away from reproduction. Consequently, when the value of current reproduction is high relative to the value of future reproduction and survival, as it is in older adults, life history theory predicts that the stress response should be suppressed. In this study, we tested the hypothesis that older parents would respond less strongly to a stressor in a natural, breeding population of common terns (Sterna hirundo). Common terns are long-lived seabirds and reproductive performance is known to increase throughout the lifespan of this species. As predicted, the maximum level of glucocorticoids released in response to handling stress decreased significantly with age. We suggest that suppression of the stress response may be an important physiological mechanism that facilitates an increase in reproductive performance with age.
Keywords: age, common tern (Sterna hirundo), corticosterone, life history evolution, parental care, stress response
1. Introduction
The trade-off between investment in current reproduction versus future reproduction and survival is a central tenet of life history theory (Roff 1992, 2002; Stearns 1992). Although this trade-off is well documented, the physiological mechanisms underlying it are poorly understood (Sinervo & Svensson 1998; Ketterson & Nolan 1999; Williams 2001). Hormonal mechanisms commonly mediate suites of morphological, physiological and behavioural traits (Sinervo & Svensson 1998; Ketterson & Nolan 1999; Williams 2001). Therefore, a greater knowledge of how these mechanisms regulate the phenotypic expression and integration of life history traits is essential for understanding trade-offs and predicting the evolution of life histories (Sinervo & Svensson 1998; Ketterson & Nolan 1999; Ricklefs & Wikelski 2002; Barnes & Partridge 2003).
One hormonal mechanism that may be important in mediating the trade-off between investment in current reproduction versus future reproduction and survival is the stress response (Salmon et al. 2001; Ricklefs & Wikelski 2002). In vertebrates, stressors such as severe weather, reduced food availability or the presence of predators stimulate the hypothalamic–pituitary–adrenal (HPA) axis to induce a rise in glucocorticoid (CORT) levels (Sapolsky et al. 2000). Short-term elevations of CORT levels in response to stressors enhance survival by stimulating gluconeogenesis (Sapolsky et al. 2000) and foraging behaviour (Wingfield 2003), but simultaneously interfere with reproduction by inhibiting mating (Wingfield & Sapolsky 2003) and parental care behaviour (Silverin 1987; Wingfield et al. 1995; Kitaysky et al. 2001; Wingfield 2003).
When the value of current reproduction is high relative to the value of future reproduction and survival, the stress response is expected to be suppressed to ensure that critical resources are not diverted away from reproduction (Wingfield et al. 1995, 2000; Silverin et al. 1997; Meddle et al. 2003). For example, the stress response is attenuated when breeding opportunities are limited (Wingfield et al. 1992, 1994, 1995; Romero et al. 1997, 1998; Silverin 1997), during the breeding stages when parents are investing the most in offspring (Meddle et al. 2003) and in the sex that contributes more to parental care (Wingfield et al. 1995; O'Reilly & Wingfield 2001).
As organisms age, the value of current reproduction relative to the value of future reproduction and survival is expected to increase as the number of future reproductive opportunities declines (Clutton-Brock 1991; Roff 1992, 2002; Stearns 1992). Consequently, older adults are predicted to invest more in reproduction at the cost of future reproduction and survival. Age-related changes in reproductive investment are inherently difficult to measure (Clutton-Brock 1991). However, consistent with the prediction that reproductive investment should increase with age, reproductive performance has been shown to increase throughout the lifespan of many organisms (Clutton-Brock 1991; Roff 1992, 2002; Stearns 1992).
Given the foregoing evidence that the stress response is often suppressed when the value of current reproduction is high, the integration of theory and mechanism would predict that older adults should respond less strongly to stressors during reproduction than younger adults (Clutton-Brock 1991; Roff 1992, 2002; Stearns 1992). A few studies of non-breeding animals provide tentative support for this hypothesis. Older female mice produce lower CORT levels in response to stressors (Stein-Behrens & Sapolsky 1992) and older age-classes of turtles respond less strongly to stressors than younger age-classes (Jessop & Hamann 2005). In addition, the stress response is suppressed with age in some human studies (reviewed in Otte et al. 2005). Attenuation of the stress response may be a common physiological mechanism that facilitates an increase in reproductive performance with age. However, no study to date has addressed this question in a natural population during reproduction.
In this study, we tested the hypothesis that the stress response is suppressed with age in a wild population of common terns (Sterna hirundo). Common terns are long-lived seabirds and reproductive performance increases throughout the lifespan of this species (Nisbet et al. 2002). To measure the stress response we used a standardized handling stress protocol (Wingfield et al. 1995). We predicted that there would be a negative relationship between age and maximum CORT levels produced in response to handling stress. We tested this prediction in breeding adults across the entire range of the reproductive lifespan (3–28 years).
2. Material and methods
(a) Study site and species
Field research was conducted between May and July 2004, on Bird Island, in Buzzards Bay, MA, USA (41°40′ N, 70°43′ W; for a detailed description of this study site see Nisbet et al. (1984)). Common terns have been studied at this site for more than 30 years and about 37% of the adults are of known age (Nisbet et al. 2002). Male and female terns provide extensive care for offspring and have similar life history strategies (Nisbet 2002); hence known-aged adults of both sexes were included in this study.
(b) Measuring the stress response
Nests were located and marked during the laying period. To minimize potential variation in the stress response due to reproductive stage and time of day, all adults were sampled between days 9 and 15 of the incubation period between 06.30 and 12.00. Adults were captured on the nest using walk-in treadle traps and blood samples were collected at three time intervals (0–3, 10, and 30 min after capture). All initial blood samples were collected within 3 min and the time required to collect these samples (hereafter bleed time) was recorded. After the initial blood sample was drawn, adults were placed in individual holding tubes and subsequent blood samples were collected from the alar vein after 10 and 30 min. This standardized handling stress protocol has been effectively used to determine the stress response in numerous field studies (Wingfield et al. 1995; Meddle et al. 2003).
Adults were weighed and measured before release. Sexes were determined based on head length (Nisbet 2002); birds in the zone of overlap were treated as indeterminate. Blood samples were kept on ice for less than 6 h before they were centrifuged and separated. Plasma was stored at −20 °C until further analysis.
(c) CORT assay
Plasma CORT levels (corticosterone, the primary avian glucocorticoid) were measured using standard radioimmunoassay techniques (Wingfield & Farner 1975; Ketterson et al. 1991). Samples were extracted with 4 ml of diethyl ether, evaporated under nitrogen gas and resuspended in phosphate buffer. Samples were then assayed in duplicate, and assay values were corrected for plasma volumes and individual recoveries after extraction. Average intra and inter-assay coefficients of variation were 13 and 19%, respectively. CORT levels are reported as ng ml−1.
(d) Statistical analysis
Stress response data were obtained for 69 birds of known age. We analysed differences among individuals in baseline and maximum CORT levels using multiple regression analyses. Baseline CORT levels refer to the initial sample collected within the first 3 min of capture. Maximum CORT levels refer to the highest stress-induced CORT levels (either the 10 or 30 min sample). CORT levels were log-transformed to improve normality. Our regression models included the effects of bleed time, body mass, date (the date the bird was captured), and age on baseline and maximum CORT levels. Bleed time was included in the analysis because it has the potential to influence baseline CORT levels (Romero & Reed 2005). Body mass and date were included in the analysis because they are expected to affect both baseline and maximum CORT levels independently of age (Wingfield et al. 1995; Kitaysky et al. 1999; Adams et al. 2005). Recapture history, defined as the number of prior years in which a bird had been previously captured one or more times, was included in the analysis to test for possible effects of acclimation (Sapolsky et al. 2000; Romero 2004). In our regression models, we used a backward elimination process and excluded independent variables with p>0.1. We also used a partial correlation analysis to examine the relationship between age and body mass. All statistical analyses were performed in SPSS (13.0 for Windows).
Sex could be accurately assigned for 37 adults (17 female, 20 male) and t-tests were used to examine the effects of sex on baseline and maximum CORT levels. There was no significant effect of sex on either measure (p>0.16); consequently, sex was not included as a factor in subsequent analyses.
3. Results
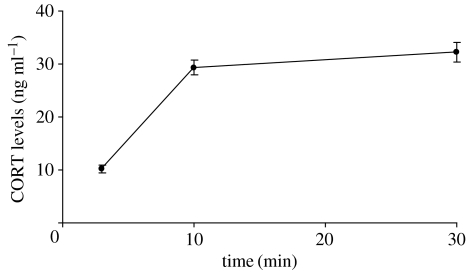
A repeated-measures analysis of variance that included time as a fixed factor verified that standardized handling stress significantly elevated CORT levels (figure 1) (F2,67=136.09, p<0.001, means±s.e.: 10.16±0.70 ng ml−1 time 0–3 min, 28.74±1.38 ng ml−1 time 10 min, 31.86±1.87 ng ml−1 time 30 min). Baseline CORT levels ranged from 2.17 to 32.14 ng ml−1. Maximum CORT levels ranged from 14.32 to 85.02 ng ml−1 (mean±s.e.: 34.25±1.76 ng ml−1).
Figure 1.
Changes in plasma CORT levels (mean±s.e.m.) in response to standardized handling stress over 30 min (n=69).
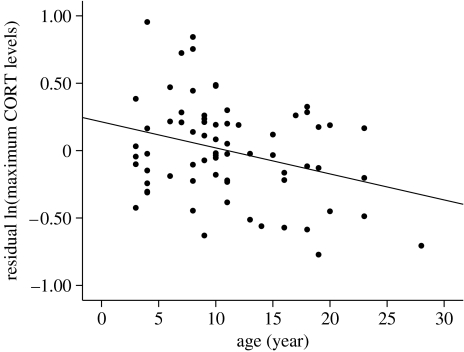
There was a significant negative relationship between maximum CORT levels and age (figure 2), body mass, and date; and no significant effect of bleed time or recapture history on maximum CORT levels (table 1). No tern included in this study had been captured in more than five previous years and almost half of the terns had not been handled since they were originally banded as chicks.
Figure 2.
The relationship between age and residual natural log-transformed maximum CORT levels. Plotted values are residuals from a multiple regression that included body mass and date (n=69).
Table 1.
Effects of independent variables on maximum and baseline CORT levels. (Independent variables with p<0.1 were included in the final multiple regression analyses and are signified in bold. β, standardized partial regression coefficient from an analysis of log-transformed CORT (ng ml−1); b, unstandardized partial regression coefficient from an analysis of untransformed CORT (ng ml−1). All significance testing was conducted on log-transformed data.)
| maximum CORT | baseline CORT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | d.f. | β | b | p | F | d.f. | β | b | p | |
| independent variables | ||||||||||
| age (yr) | 9.53 | 1,65 | −0.38 | −0.85 | 0.003 | <0.01 | 1,63 | −0.01 | −0.05 | 0.940 |
| body mass (g) | 17.66 | 1,65 | −0.53 | −0.80 | <0.001 | 11.51 | 1,65 | −0.38 | −0.28 | 0.001 |
| date | 4.67 | 1,65 | −0.30 | −0.50 | 0.034 | 8.93 | 1,65 | −0.33 | −0.31 | 0.004 |
| bleed time (s) | 0.03 | 1,63 | −0.02 | −0.02 | 0.856 | 20.17 | 1,65 | 0.44 | 0.07 | <0.001 |
| recapture history | 0.03 | 1,63 | −0.03 | −0.04 | 0.863 | 0.236 | 1,63 | 0.07 | 0.03 | 0.629 |
Baseline CORT levels significantly increased with bleed time and significantly decreased with body mass and date (table 1). However, there was no relationship between baseline CORT levels and age or recapture history (table 1). All statistical tests were performed on log-transformed CORT levels; however, in order to provide a more interpretable description of the influence of these parameters on maximum and baseline CORT levels, untransformed CORT levels were also analysed and untransformed partial regression coefficients are reported in table 1.
Finally, body mass, a measure of condition in terns, decreased with age while controlling for date (partial r66=−0.238, p=0.050).
4. Discussion
In this study, we found that stress-induced maximum CORT levels decreased significantly with age in common terns. To our knowledge, this is the first study to demonstrate that older parents respond less strongly to a stressor in a natural population. In other bird species, elevated CORT levels have been shown to suppress brooding (Kitaysky et al. 2001) and offspring feeding rates (Silverin 1987), as well as lead to higher levels of nest abandonment (Silverin 1988; Love et al. 2004). Assuming that terns would be similarly affected, our finding that the stress response declines with age suggests that older adults should be less likely to reduce parental care in response to stressors than younger adults. Older terns have previously been shown to raise a greater number of offspring to independence than younger terns (Nisbet et al. 2002). Taken together, these results suggest that attenuation of the stress response may act as a mechanism to facilitate an increase in reproductive performance with age.
In addition to a suppression of the stress response with age, we also found that both baseline and maximum CORT levels declined with increasing body mass and date. Heavier adults are predicted to respond less strongly to stressors because they have greater potential energy reserves (Wingfield et al. 1995) and our results are consistent with previous studies on other long-lived seabirds (Kitaysky et al. 1999; Adams et al. 2005). However, our finding that the stress response decreased across the breeding season is novel. In contrast, the stress response increased with date in black-legged kittiwakes (Rissa tridactyla), another long-lived seabird (Kitaysky et al. 1999). However, that study did not control for variation in the stress response due to reproductive stage.
A decline in the stress response with age is consistent with other studies that have found that the stress response is suppressed when the value of current reproduction is high relative to the value of future reproduction and survival (Wingfield et al. 1992, 1994, 1995; Silverin et al. 1997). Furthermore, it suggests that the stress response may be a flexible mechanism that enables adults to adaptively modify reproductive investment with age. However, because this study was cross-sectional in design, other factors may also have contributed to the negative relationship we found between the stress response and age.
One possibility is that the stress response decreases with age due to senescent declines of the HPA axis. However, senescence of the HPA axis would be expected to lead to age-related changes in baseline CORT levels (Stein-Behrens & Sapolsky 1992), which were not detected in terns. This suggests that there were no age-related declines in adrenal function and that negative feedback mechanisms of the HPA axis have not become impaired (Stein-Behrens & Sapolsky 1992).
Another possibility is that the stress response is suppressed in older terns because they have had more experience with capture and handling. However, we found no relationship between the number of years in which a tern had been captured and either baseline or maximum CORT levels. This suggests that terns have not become acclimated to the specific capture, restraint, and blood sampling protocol used in this study and that they instead respond to it as a novel stressor. However, older terns may also respond less strongly to other commonly encountered stressors, such as reduced food availability, inclement weather and predators because they have become acclimated to these stressors (Sapolsky et al. 2000; Romero 2004).
It is also possible that the stress response decreases with age because individuals that respond less strongly to stressors have been favoured by selection and are therefore more likely to be represented in older age classes. However, the stress response is thought to be an adaptive mechanism that enhances survival by initiating a suite of physiological and behavioural changes that increase an organism's ability to cope with and evade stressful stimuli and hence exposure to chronically elevated CORT levels (Wingfield et al. 1995; Ricklefs & Wikelski 2002). Consequently, individuals with low stress responses may be expected to be less likely to survive in the face of stressors, not more (Wingfield et al. 1995, 2000; Ricklefs & Wikelski 2002). Furthermore, if the relationship we found between the stress response and age was the result of previous selection events, we might have expected older terns to be in better condition. Body mass is thought to be a good measure of condition in terns because heavier terns have greater potential energy reserves (Wendeln & Becker 1999). We found that body mass decreased with age when controlling for date. If body mass is a good measure of condition in terns, this suggests that older terns are not simply in better condition.
In summary, this is the first study to demonstrate that the stress response decreases with age during reproduction in a natural population. Attenuation of the stress response may be an important mechanism that facilitates an increase in reproductive performance with age. Additional studies in other systems will elucidate whether modulation of the stress response is a common life history adaptation that allows adults to flexibly adjust reproductive investment throughout their lifespan. To assess the potential roles of senescence, previous experience, and selection in shaping the relationship between age and the stress response it will be necessary to examine longitudinal stress response data in a wide array of species that differ in lifespan.
Future research should also examine the mechanisms underlying the attenuation of the stress response with age. Currently, there is little information about how the stress response is modulated (Romero et al. 1998; Wingfield & Sapolsky 2003) and age-related modifications of the stress response may be occurring at multiple levels of the HPA axis. In addition, future studies should investigate whether there are other downstream changes that facilitate a decreased response to stressors with age. For example, CORT binds to corticosteroid binding globulins (CBG) in the plasma (Breuner & Orchinik 2002), limiting the amount of free CORT that is able to activate intracellular receptors (Breuner et al. 2003). In addition to producing lower levels of CORT in response to stressors, older individuals may further modulate the stress response by increasing CBG levels, thereby reducing the amount of free CORT that enters the cell. There may also be age-related changes occurring at the level of the receptor that influence sensitivity to elevated CORT levels. In some organisms, elevated CORT levels do not interfere with reproduction presumably because CORT receptors have been downregulated (Stein-Behrens & Sapolsky 1992). Older adults may have fewer CORT receptors thereby further reducing the possibility that stress will interfere with reproduction.
Acknowledgements
We thank J. Lepire, V. Apanius, E. Bridge, J. Hatch, M. Haussmann, J. Spendelow and J. Tims for assistance in the field, C. Mostello and other staff of the Massachusetts Division of Fisheries and Wildlife for logistical support, the Town of Marion for permission to work at Bird Island, J. Jawor for laboratory assistance, J. Atwell, E. D. Brodie III, J. Grindstaff, J. McGlothlin, T. Ord, and three anonymous reviewers for critical comments on earlier drafts of the manuscript and B. Gross, N. Kane, L. Romero, R. Sapolsky, S. Schaack, B. Van Roo and J. Wingfield for useful discussions. This study was funded by the Center for the Integrative Study of Animal Behaviour, the Society for Integrative and Comparative Biology, the Animal Behaviour Society, and the American Ornithologists' Union.
References
- Adams N.J, Cockrem J.F, Taylor G.A, Candy E.J, Bridges J. Corticosterone responses of grey-faced petrels (Pterodroma macroptera gouldi) are higher during incubation than during other breeding stages. Physiol. Biochem. Zool. 2005;78:69–77. doi: 10.1086/423740. doi:10.1086/423740 [DOI] [PubMed] [Google Scholar]
- Barnes A, Partridge L. Costing reproduction. Anim. Behav. 2003;66:199–204. doi:10.1006/anbe.2003.2122 [Google Scholar]
- Breuner C, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. doi:10.1677/joe.0.1750099 [DOI] [PubMed] [Google Scholar]
- Breuner C, Orchinik M, Hahn T, Meddle S, Moore I, Owen-Ashley N, Sperry T, Wingfield J.C. Differential mechanisms for regulation of the stress response across latitudinal gradients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R594–R600. doi: 10.1152/ajpregu.00748.2002. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Jessop T.S, Hamann M. Interplay between age class, sex, and stress response in green turtles (Chelonia mydas) Aust. J. Zool. 2005;2:131–136. doi:10.1071/ZO04061 [Google Scholar]
- Ketterson E.D, Nolan V., Jr Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 1999;154:S4–S25. doi: 10.1086/303280. doi:10.1086/303280 [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr., Wolf L, Ziengenfus C, Dufty A.M, Ball G.F, Johnsen T.S. Testosterone and avian life-histories: the effects of experimentally elevated testosterone on corticosterone and body mass in dark-eyed juncos. Horm. Behav. 1991;25:489–503. doi: 10.1016/0018-506x(91)90016-b. doi:10.1016/0018-506X(91)90016-B [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Wingfield J.C, Piatt J.F. Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes. Funct. Ecol. 1999;13:577–584. doi:10.1046/j.1365-2435.1999.00352.x [Google Scholar]
- Kitaysky A.S, Wingfield J.C, Piatt J.F. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001;12:619–625. doi:10.1093/beheco/12.5.619 [Google Scholar]
- Love O.P, Breuner C.W, Vézina F, Williams T.D. Mediation of corticosterone-induced reproductive conflict. Horm. Behav. 2004;46:59–65. doi: 10.1016/j.yhbeh.2004.02.001. doi:10.1016/j.yhbeh.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Meddle S, Owen-Ashley N, Richardson M, Wingfield J.C. Modulation of the hypothalamic–pituitary–adrenal axis of an arctic-breeding polygynandrous songbird, the Smith's longspur (Calcarius pictus) Proc. R. Soc. B. 2003;270:1849–1856. doi: 10.1098/rspb.2003.2455. doi:10.1098/rspb.2003.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet I.C.T. Common tern. In: Poole A, Gill F, editors. The birds of North America. Vol. 618. The Birds of North America Inc.; Philadelphia, PA: 2002. pp. 1–40. [Google Scholar]
- Nisbet I.C.T, Winchell J.M, Heise A.E. Influence of age on the breeding biology of common terns. Colon. Waterbird. 1984;7:117–126. doi:10.2307/1521090 [Google Scholar]
- Nisbet I.C.T, Apanius V, Friar M.S. Breeding performance of very old common terns. J. Field Ornithol. 2002;73:117–240. [Google Scholar]
- O'Reilly K.M, Wingfield J.C. Ecological factors underlying the adrenocortical response to capture stress in arctic-breeding shorebirds. Gen. Comp. Endocrinol. 2001;124:1–11. doi: 10.1006/gcen.2001.7676. doi:10.1006/gcen.2001.7676 [DOI] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan T.C, Marmar C.R, Yaffe K, Mohr D.C. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. doi:10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Ricklefs R, Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Roff D. Chapman & Hall; New York: 1992. The evolution of life-histories: theory and analysis. [Google Scholar]
- Roff D. Sinauer; Sunderland, MA: 2002. Life-history evolution. [Google Scholar]
- Romero L.M. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. doi:10.1016/j.tree.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Romero L.M, Reed J.M. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol. A. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Romero L.M, Ramenofsky M, Wingfield J.C. Season and migration alters the corticosterone response to capture and handling in an Arctic migrant, the white-crowned sparrow (Zonotrichia leucophrys gambelii) Comp. Biochem. Physiol. B. 1997;116:171–177. doi: 10.1016/s0742-8413(96)00208-3. [DOI] [PubMed] [Google Scholar]
- Romero L.M, Soma K, Wingfield J.C. Hypothalamic–pituitary–adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;274:R1338–R1344. doi: 10.1152/ajpregu.1998.274.5.R1338. [DOI] [PubMed] [Google Scholar]
- Salmon A.B, Marx D.B, Harshman L.G. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution. 2001;55:1600–1608. doi: 10.1111/j.0014-3820.2001.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Silverin B. Corticosterone-binding proteins and behavioural effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 1987;64:67–74. doi: 10.1016/0016-6480(86)90029-8. doi:10.1016/0016-6480(86)90029-8 [DOI] [PubMed] [Google Scholar]
- Silverin B. Behavioural and hormonal responses of the pied flycatcher to environmental stressors. Anim. Behav. 1988;55:1411–1420. doi: 10.1006/anbe.1997.0717. doi:10.1006/anbe.1997.0717 [DOI] [PubMed] [Google Scholar]
- Silverin B, Arvidsson B, Wingfield J.C. The adrenocortical responses to stress in breeding Willow Warblers (Phylloscopus trochilus) in Sweden: effects of latitude and gender. Funct. Ecol. 1997;11:376–384. doi:10.1046/j.1365-2435.1997.00097.x [Google Scholar]
- Sinervo B, Svensson E. Mechanistic and selective causes of life-history trade-offs and plasticity. Oikos. 1998;83:432–442. [Google Scholar]
- Stearns S. Oxford University Press; New York: 1992. The evolution of life-histories. [Google Scholar]
- Stein-Behrens B, Sapolsky R.M. Stress, glucocorticoids, and aging. Aging Clin. Exp. Res. 1992;4:197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- Wendeln H, Becker P.H. Effects of parental quality and effort on the reproduction of common terns. J. Anim. Ecol. 1999;68:205–214. doi:10.1046/j.1365-2656.1999.00276.x [Google Scholar]
- Williams T. Experimental manipulation of female reproduction reveals an intraspecific egg size–clutch size trade-off. Proc. R. Soc. B. 2001;268:423–428. doi: 10.1098/rspb.2000.1374. doi:10.1098/rspb.2000.1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C. Control of behavioural strategies for capricious environments. Anim. Behav. 2003;66:807–816. doi:10.1006/anbe.2003.2298 [Google Scholar]
- Wingfield J.C, Farner D. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Sapolsky R.M. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Vleck C.M, Moore M.C. Seasonal changes of the adrenalcortical-response to stress in birds of the Sonoran Desert. J. Exp. Zool. 1992;264:419–428. doi: 10.1002/jez.1402640407. doi:10.1002/jez.1402640407 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Deviche P, Sharbaugh S, Astheimer L, Holberton R, Suydam R, Hunt K. Seasonal changes of the adrenalcortical responses to stress in redpolls (Acanthis flammea), in Alaska. J. Exp. Zool. 1994;270:372–380. doi:10.1002/jez.1402700406 [Google Scholar]
- Wingfield J.C, O'Reilly K, Astheimer L. Ecological bases of the modulation of adrenocortical responses to stress in Arctic birds. Am. Zool. 1995;35:285–294. [Google Scholar]
- Wingfield J.C, Jacobs J, Tramontin A, Perfito N, Meddle S, Maney D, Soma K. Toward an ecological basis of hormone–behaviour interactions in reproduction of birds. In: Wallen K, Schneider J, editors. Reproduction in context. The MIT Press; Cambridge, MA: 2000. pp. 85–128. [Google Scholar]