Abstract
The Cape Floristic Region is exceptionally species-rich both for its area and latitude, and this diversity is highly unevenly distributed among genera. The modern flora is hypothesized to result largely from recent (post-Oligocene) speciation, and it has long been speculated that particular species-poor lineages pre-date this burst of speciation. Here, we employ molecular phylogenetic data in combination with fossil calibrations to estimate the minimum duration of Cape occupation by 14 unrelated putative relicts. Estimates vary widely between lineages (7–101 Myr ago), and when compared with the estimated timing of onset of the modern flora's radiation, it is clear that many, but possibly not all, of these lineages pre-date its establishment. Statistical comparisons of diversities with lineage age show that low species diversity of many of the putative relicts results from a lower rate of diversification than in dated Cape radiations. In other putative relicts, however, we cannot reject the possibility that they diversify at the same underlying rate as the radiations, but have been present in the Cape for insufficient time to accumulate higher diversity. Although the extremes in diversity of currently dated Cape lineages fall outside expectations under a constant underlying diversification rate, sampling of all Cape lineages would be required to reject this null hypothesis.
Keywords: Cape flora, palaeoendemics, relict lineages, phylogeny, divergence times
1. Introduction
In many groups of organisms, species richness increases with decreasing latitude (Fischer 1960; Pianka 1966; Brown 1988). While this trend is broadly true in plants, Mediterranean latitudes are frequently richer than expected (Linder 2003; Kier et al. 2005). The Cape Floristic Region (CFR) is exceptional even for such latitudes, being much richer than expected for a Mediterranean region of its size (Cowling et al. 1996), and on a par with many tropical rainforests (Linder 2003). The CFR is also notable for the taxonomic distribution of its diversity, a great proportion of which can be attributed to a small number of unusually large genera, while other genera are comparatively species-poor. Ongoing molecular phylogenetic studies in combination with limited fossil evidence are broadly consistent with the prediction that the high species richness of the CFR has resulted from intense speciation of a limited number of ancestral lineages within relatively recent times (probably since Mid–Late Miocene; Linder 2003).
However, a comprehensive understanding of the origins of high species richness in the Cape is inextricably tied to the origins of its taxonomic skew. As for any flora, such a skew could result from stochasticity in the distribution of species diversity across lineages, or from the particular sequence of historical events in the Cape which favoured massive speciation in some lineages but not others. Before we can attempt to disentangle these two possibilities, we need a better understanding of the relative timescales in which lineages of different size have been present in the Cape. Have some Cape lineages failed to speciate over a timescale in which massive speciation has occurred in others, or could diversity be closely related to duration of Cape occupancy?
Over the years of botanical study in the Cape, a similar set of monotypic or little-speciated Cape lineages have been repeatedly presented as likely relicts of the environments pre-dating the modern Cape flora. Linder (2003) and Goldblatt & Manning (2000, 2002) suggest a relictual status for such lineages on the basis of their small size, taxonomic isolation, and frequently high levels of biogeographic isolation (i.e. that they are endemic to the Cape and that their closest relatives are more likely to occur on other continents than in the same or neighbouring regions of southern Africa: Goldblatt & Manning's (2000, 2002) ‘palaeoendemics’). These authors, along with Levyns (1962, 1964) and Linder et al. (1992), further suggest that the habitats to which these lineages are confined—including stream margins, forest patches and fire-protected outcrops—may be relictual; unlike most natural habitats in the modern Cape they are buffered from summer drought and regular fire. Many of these lineages have long been highlighted as belonging to groups confined to the southern continents (Levyns 1962, 1964; Goldblatt 1978), potentially dating back to Gondwanan break-up, and formed a component of Marloth's (1929) ‘old Cape element’.
With the exception of three lineages (Podocarpus, Widdringtonia and Gunnera), none of these putatively relictual lineages have a fossil record in southern Africa, and therefore their relictual status in the Cape, namely their presence prior to the establishment of the modern flora, has remained a matter of speculation. Further, in the absence of a time frame for their presence in the Cape, it remains unclear whether their species-poverty results from a lower rate of diversification than in the species-rich Cape lineages, or whether they might be diversifying at a similar rate but have experienced insufficient time to accumulate higher diversity.
Here, we make use of available molecular phylogenetic data covering 14 of the putative relict Cape lineages of Levyns (1962, 1964), Goldblatt & Manning (2000) and Linder (2003) (from the genera Brabejum, Cunonia, Curtisia, Gunnera, Ilex, Lachnostylis, Metrosideros, Ocotea, Platylophus, Podocarpus, Prionium, Roridula, Smelophyllum and Widdringtonia), in combination with earliest fossil records of related forms outside Africa, to estimate the minimum duration of presence in the Cape of the putative relicts using relaxed clock methods. We first make use of these estimates to test the relictual status of these lineages by comparing their estimated antiquity in the Cape with our best surrogate for the timing of establishment of the modern flora (Late Miocene origin of the Benguela current, ca 10 Myr ago; Linder 2003). Second, to evaluate the scenarios of insufficient time versus low diversification rate in explaining low diversity, we test whether the species diversities within the putative relict lineages depart from expectations for their age based on the range of high diversification rates obtained from dated Cape radiations. Third, to investigate whether diversification rate variation in the Cape departs from stochastic expectations under a constant underlying rate, we compare the extremes of diversity in dated Cape lineages with confidence intervals of expectations for their age under different constant rates.
2. Material and methods
(a) Sampling
We used DNA sequences available for 14 of the 22 putatively relictual species-poor (1–4 species) Cape lineages proposed by Levyns (1962, 1964), Goldblatt & Manning (2000) and Linder (2003) (see electronic supplementary material A) to reconstruct phylogenetic relationships already published for groups containing putative relicts and their closest relatives. Fossil calibration points were applied to each tree, and the minimum duration of Cape presence of putative relicts was estimated from the timing of divergence of these lineages from their closest non-Cape relatives. Recent studies suggest that levels of taxon sampling can significantly affect dating estimates, and that minimal sampling of taxa leads to greater distortion in dating estimates than does minimal sampling of genes (Linder et al. 2005; Pirie et al. 2005; B. Warren & J. Hawkins 2004, unpublished data). Where a choice of genes was available, we therefore picked the most species-rich datasets, which were not necessarily the most gene-rich datasets (see electronic supplementary material A).
(b) Phylogeny construction
Each dataset excluding outgroups was analysed in Modeltest (Posada & Crandall 1998) to determine the substitution model best describing the data. Bayesian analyses were performed using Markov Chain Monte Carlo (MCMC) methods as implemented in the software MrBayes v. 2.01 (Huelsenbeck et al. 2001). For each analysis, four chains were run simultaneously for two million generations starting from random initial trees, and sampled every 10 generations. Variation in the maximum-likelihood (ML) scores in this sample was examined graphically, and sample points collected prior to stationarity were eliminated (‘burn-in’). Consensus phylogenies and the posterior probabilities of nodes were determined from the remaining trees.
(c) Fossil calibration
Each phylogeny was calibrated using the earliest fossils that we could find associated either with the family to which the putative relict belongs, or to genera within that family (see electronic supplementary material A), and conforming with at least one of two criteria: (i) critical discussion of the characters used to assign the fossil to the extant taxon or node in the phylogeny of extant forms has been published, and (ii) personal communications from palaeobotanists knowledgeable about the group in question confirm assignment of the fossil to the extant taxon. We avoided using any secondary calibrations obtained from dating other phylogenies. Where fossils are recorded from an undated position within a formation of known age bounds, we assigned the fossil with the minimum age of the formation following the International Commission on Stratigraphy (Gradstein et al. 2004), since this provides the most conservative minimum estimate of the fossil's age. Likewise, in the absence of evidence to the contrary, we assigned each fossil to the stem node leading to the taxon in question, since this produces a more conservative estimate of minimum divergence times than does crown node calibration. Where such nodes gained less than 95% Bayesian branch support we considered them to be unsupported and used the closest supported node basal to the stem node in question, thereby dating the polytomy from which the taxon emanates. Fossils always provide only a minimum estimate of when a taxon existed. However, under relaxed clocks, rates frequently appear to change too readily across a tree in the absence of an upper constraint on divergence times (Pérez-Losada et al. 2004; Ho et al. 2005). Therefore, we compromised by fixing the age of the basal-most calibration point(s) to their minimal age(s), and constraining all nested calibration points by their minimal ages (see electronic supplementary material A). Such an approach allows information from multiple calibration points to be incorporated into the dating estimate, while also setting an upper bound on minimum age estimates.
(d) Bayesian relaxed-clock analysis
Bayesian methods that relax a strict molecular clock were used to estimate divergence times as implemented in the software Multidivtime (Thorne et al. 1998; Thorne & Kishino 2002). This approach employs a probabilistic model to estimate the change in evolutionary rate over time and uses the MCMC procedure to derive the posterior distribution of rates and time. Steps involved are detailed in an online manual (Rutschmann 2004). Following Thorne & Kishino (2002), indels were treated as missing data. First, the ML branch lengths of the rooted evolutionary tree were estimated in the program Estbranches (Thorne et al. 1998) along with a variance–covariance matrix of branch length estimates. F84+G model is the most parameter-rich model currently available in Estbranches, and parameter values were estimated using the Baseml program in the PAML package (Yang 1997). In both of these programs, we approximated the gamma shape parameter (G) with five rate categories. Second, the program Multidivtime was used to approximate the prior and posterior distributions of substitution rates and divergence times along with their standard deviations and 95% credibility intervals via MCMC.
Three priors other than calibration points were estimated for each phylogeny as follows: first, the mean of the prior distribution of the time separating the ingroup root from the present (rttm) was set to our best prior estimate of this time based on available fossil information. Second, the mean of the prior distribution for the rate of molecular evolution at the ingroup root node (rtrate) was set to the ML branch length obtained from ingroup root to tips when a molecular clock was enforced on the Bayesian consensus topology, divided by rttm. Third, the mean for the prior distribution for the Brownian motion parameter ‘nu’ (brownmean), which determines the permitted rate change between nodes, was set to 1.5 divided by rttm, following the manual's recommendation that this value multiplied by rttm should be about 1–2. For each of these three parameters, the corresponding standard deviation of the prior distribution (rttmsd, rtratesd and brownsd, respectively) was set to equal the mean in order to reflect our lack of knowledge regarding prior distributions. Finally, for the parameter bigtime, a number which should be higher than the time between the tips and root of each phylogeny in the user's most liberal imagination, a date of 300 Myr ago (end of Carboniferous) was used for all angiosperm phylogenies, and 420 Myr ago (end of Silurian) for both gymnosperm phylogenies. After an initial burnin period of 100 000 generations, the Markov chain was run for 999 901 generations with a sample frequency of one sample every 100 generations, in order to generate 10 000 samples. The analysis was first used to approximate the prior distribution of rates and divergence times, and then repeated at least twice from different random initial seed numbers to approximate posteriors and check for convergence.
(e) Diversification rates
We followed the methodology and justifications of Magallón & Sanderson (2001) for calculating diversification rates for both stem and crown groups, and comparing diversification rates under upper and lower bounds of relative extinction rate of 0.9 and zero, respectively. For most of the putative relict lineages, the absence of diversification precluded direct calculation of diversification rate. However, we followed Magallón & Sanderson's (2001) protocol for calculating the 95% confidence interval for the expected number of species in a clade that diversifies at a given rate for each interval of time from its origin onwards. Rates used were the two extremes of rate variation calculated for published Cape radiations, and the published overall rate of the angiosperms. Standing diversities of Cape clades were compared with the 95% confidence interval of expected diversity of a clade of the same age under the rate in question (all formulae provided in electronic supplementary material B).
3. Results
Log likelihood ratio tests rejected the null hypothesis of rate constancy for all phylogenies (p<0.01 in all cases), both with and without the deletion of indels. As in calibration, we treat nodes gaining less than 95% Bayesian branch support as unsupported, and refer to divergence estimates of the closest supported node basal to the Cape–non-Cape divergence (figure 1; electronic supplementary material C). Supported nodes separating Roridula gorgonias and Curtisia dentata-Grubbia lineages from their closest non-Cape relatives correspond to nodes of calibration, and minimum age estimates for these divergences are therefore defined by the fossil dates. For all other nodes of interest, Bayesian relaxed-clock analyses were used to estimate divergence times (figure 2). Smelophyllum was the only dataset for which convergence was not met after 999 901 generations. Consequently, the Markov chain was run twice for 4 999 901 generations for this dataset, following a burnin of 500 000 generations. For all lineages, we quote the mean of the two independent runs, since estimates differ by no more than 3.5% between runs.
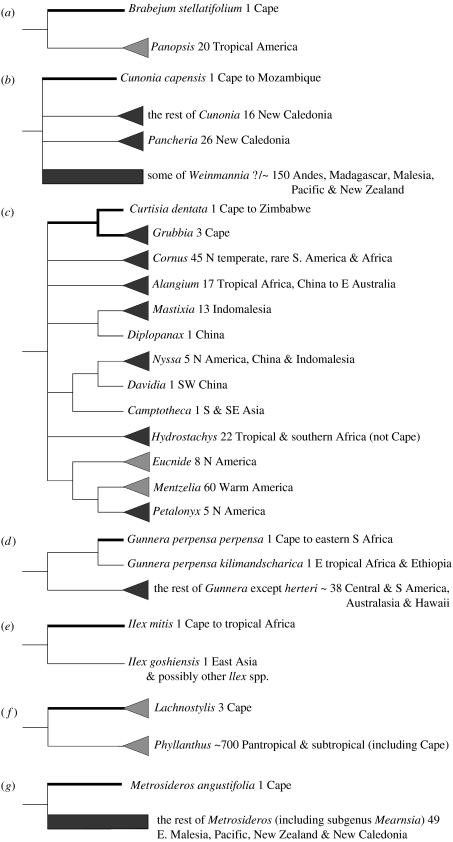
Figure 1.
Stylized representation of Cape–non-Cape nodes for which divergence time minima were estimated. The rest of each phylogeny, including nodes of calibration, is omitted. Putative relict Cape lineages are represented by thick branches emanating from the nodes being dated, while their non-Cape (or partially non-Cape) relatives are represented by thin branches. ‘Cape’ refers to the area covered by Goldblatt & Manning (2000). Dark triangles, the subset of available samples for the group supports its monophyly; dark rectangles, the subset of available samples for the group demonstrates that it is non-monophyletic, and that two or more sections emanate from the proximal node; light triangles, the group is only represented by one sample, and there is therefore currently no molecular phylogenetic evidence for or against its monophyly. (a) Brabejum; (b) Cunonia; (c) Curtisia-Grubbia; (d) Gunnera; (e) Ilex; (f) Lachnostylis; (g) Metrosideros; (h) Ocotea; (i) Platylophus; (j) Podocarpus; (k) Prionium; (l) Roridula; (m) Smelophyllum and (n) Widdringtonia.
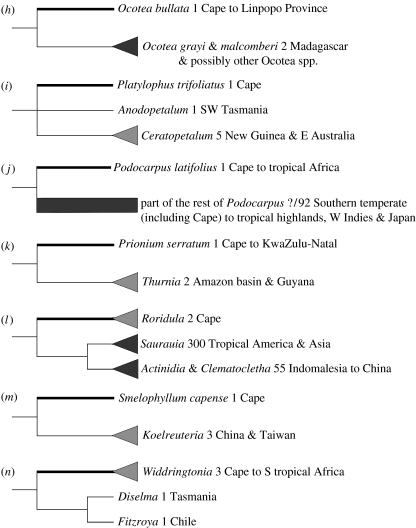
Figure 2.
Estimates of divergence minima for putative relict lineages in relation to the estimated timing of establishment of the Benguela upwelling system. Values illustrated are the mean of two independent runs of the Markov chain. Thin lines, lower credibility intervals; asterisks, first appearances in southern African fossil record; horizontal arrows, variation in published minimum estimates for the timing of onset of radiation in Cape clades with medium to high species diversity, resulting from differences in sampling, calibration and dating methods. The Phylica phylogeny was directly calibrated using the estimated timing of origin of volcanic islands, and therefore yields a maximum estimate of the timing of onset of radiation (Richardson et al. 2001). Other Cape radiations (Ehrharta, Indigofera, Moraea, Pelargonium and Restionaceae) were secondarily calibrated using divergence estimates obtained from other dated phylogenies, themselves calibrated with fossils, thereby yielding minimum estimates for the onset of radiation (Goldblatt et al. 2002; Schrire et al. 2003; Verboom et al. 2003; Linder & Hardy 2004; Bakker et al. 2005; Mummenhoff et al. 2005).
Estimates for the minimum timing of divergence of the sequenced putative relict Cape lineages from their closest non-Cape relatives vary greatly (7–101 Myr ago, figure 2), but pre-date the establishment of the Benguela upwelling system (ca 10 Myr ago) in all but two (Gunnera and Ocotea) of the 14 lineages. However, lower credibility intervals overlap with the Benguela upwelling system in six lineages (Prionium, Ocotea, Brabejum, Smelophyllum, Gunnera and Ilex; figure 2).
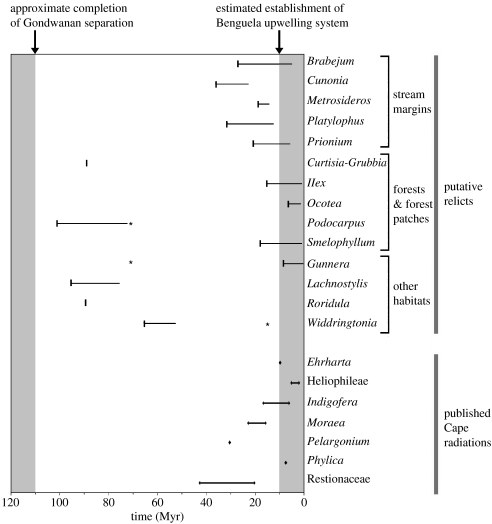
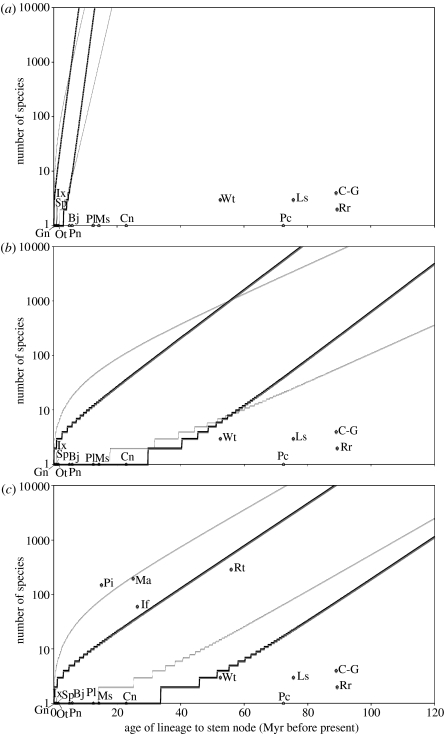
With the exception of Gunnera, Ilex, Smelophyllum and Ocotea, based on our lower credibility intervals of dating estimates, all putative relicts fall below the 95% confidence interval of expected diversity (with relative extinction rates, ϵ, of both 0 and 0.9) for their age based on the highest rate of diversification calculated for a Cape radiation (1.0004 under ϵ=0.0 and 0.5799 under ϵ=0.9 for the Heliophileae; table 1, figure 3a). Under the lowest rate of diversification calculated for a Cape radiation (0.1014 under ϵ=0.0 and 0.0607 under ϵ=0.9 for the Restio-clade of the African Restionaceae; table 1) Gunnera, Ilex, Smelophyllum, Ocotea, Brabejum, Prionium, Platylophus and Metrosideros all fall within the 95% confidence interval of expected diversity for their age when ϵ=0.0 and Cunonia also falls within the interval when ϵ=0.9. The remaining putative relicts (Lachnostylis, Podocarpus, Curtisia-Grubbia, Roridula and Widdringtonia) all fall below the confidence interval in both cases (figure 3b). When diversities of dated large and small Cape lineages are compared with expectations under different constant rates of diversification, no underlying rate is able to accommodate all lineages within the 95% confidence interval of expected diversity. High rates yield outliers with diversities falling below the 95% confidence interval, low rates yield outliers with diversities falling above the interval, and intermediate rates (such as that estimated for the angiosperms as a whole; Magallón & Sanderson 2001) yield outliers falling both above and below the interval (figure 3c). The minimum number of outliers that must be accommodated under any underlying rate is four.
Table 1.
Per-lineage diversification rate estimates for dated Cape radiations. (Where Cape clades are believed to be nested within a taxon, the name given to the corresponding section in the source publication is quoted. Use of available stem group or crown group ages for each clade is indicated. Rates of diversification were estimated for both upper and lower published estimates of divergence times; Richardson et al. 2001; Goldblatt et al. 2002; Schrire et al. 2003; Linder & Hardy 2004; Bakker et al. 2005; Mummenhoff et al. 2005.)
| Cape clade | dated node | upper divergence time estimate | lower divergence time estimate | ||
|---|---|---|---|---|---|
| ϵ=0.0 | ϵ=0.9 | ϵ=0.0 | ϵ=0.9 | ||
| Heliophileae | crown | 0.6854 | 0.3973 | 1.0004 | 0.5799 |
| Indigofera section: ‘Cape clade’ | stem | 0.1554 | 0.0733 | 0.1987 | 0.0937 |
| Indigofera section: ‘Cape clade’ | crown | 0.2001 | 0.1106 | 0.5631 | 0.3112 |
| Moraea | stem | 0.2111 | 0.1208 | 0.2111 | 0.1208 |
| Moraea | crown | 0.1993 | 0.1291 | 0.2939 | 0.1903 |
| Pelargonium section: ‘winter rainfall clade’ | crown | 0.2070 | 0.1343 | 0.2876 | 0.1867 |
| Phylica | stem | 0.3340 | 0.1844 | 0.4176 | 0.2305 |
| Phylica | crown | 0.5397 | 0.3394 | 0.6168 | 0.3878 |
| Restionaceae section: ‘Restio-clade’ | stem | 0.1014 | 0.0607 | 0.1297 | 0.0777 |
| Restionaceae section: ‘Restio-clade’ | crown | 0.1161 | 0.0781 | 0.2487 | 0.1672 |
Figure 3.
Confidence intervals of expected diversity according to age of stem group. Cape lineages are mapped according to their standing diversity and age of the (stem) node separating them from their closest non-Cape relative. For all dates obtained from this study, ages plotted are lower credibility intervals of divergence minima. Bj, Brabejum; Cn, Cunonia; C-G, Curtisia-Grubbia; Gn, Gunnera; Ix, Ilex; If, Indigofera; Ls, Lachnostylis; Ma, Moraea; Ms, Metrosideros; Ot, Ocotea; Pi, Phylica; Pl, Platylophus; Pc, Podocarpus; Pn, Prionium; Rt, Restionaceae; Rr, Roridula; Sp, Smelophyllum; Wt, Widdringtonia. (a) The 95% confidence interval of expected diversity through time of a lineage which diversifies at the highest rate estimated for the Heliophileae, the highest rate calculated for a dated Cape radiation, in the absence of extinction (ϵ=0.0, r0.0=1.0004; thick lines) and under a high relative extinction rate (ϵ=0.9, r0.9=0.5799; thin lines). Putative relict lineages are mapped. (b) The 95% confidence interval of expected diversity through time of a lineage which diversifies at the lowest rate estimated for the Restio-clade of the African Restionaceae, the lowest rate calculated for a dated Cape radiation, in the absence of extinction (ϵ=0.0, r0.0=0.1014; thick lines) and under a high relative extinction rate (ϵ=0.9, r0.9=0.0607; thin lines). Putative relict lineages are mapped. (c) The 95% confidence interval of expected diversity through time of a lineage which diversifies with a rate equal to that of the angiosperms as a whole in the absence of extinction (ϵ=0.0, r0.0=0.0893; thick lines) and under a high relative extinction rate (ϵ=0.9, r0.9=0.0767; thin lines). All Cape plant lineages with dated stem nodes (both radiations and putative relicts) are mapped.
4. Discussion
(a) Timing of arrival of putative relicts in the Cape relative to the establishment of the modern flora
Our estimates of the timing of divergence of putative relict Cape lineages from their closest non-Cape relatives must be interpreted as minimum bound estimates for the duration of Cape occupancy. First, such nodes need not necessarily represent colonization of the Cape. In many cases, lineages may have been present in the Cape for a much longer period, but have periodically dispersed out of it. Nonetheless, barring unknown range changes and extinction events, such nodes represent the most recent divergence event that can be associated with Cape colonization, and therefore provide our most conservative estimate of duration of Cape occupation. Second, the methods used to calibrate the tree also derive minimum estimates of divergence times. When fossils are associated to an extant plant genus or family, we can conclude that this taxon was present at the time the fossil was formed, but it tells us nothing about the timing of origin of the taxon. In addition, we have deliberately been conservative in our relaxed-clock calibrations, using minimum estimates for the age of fossils, and calibrating on stem rather than crown nodes.
The most striking aspect of divergence minima for the putative relicts is the wide spread in estimated age, from the Mid-Cretaceous through to the present. Some lineages are likely to have a Cape history dating back to Gondwanan separation, others may have arrived by dispersal in the Late Cretaceous and Early Tertiary, while still others could have arrived after the establishment of the ‘modern flora’. In southern Africa, there are few confidently dated fossil sites for most of the Tertiary, and the timing of establishment of the modern flora remains equivocal. A frequently posited trigger for the radiation of the modern flora is climatic change that may have been associated with the establishment of the Benguela upwelling system at around 10 Myr ago (Siesser 1980; Linder 2003). Divergence estimates exceed 10 Myr ago for all lineages except Gunnera and Ocotea. However, the lower credibility intervals for the divergence minima in four other lineages (Prionium, Smelophyllum, Brabejum and Ilex) postdate 10 Myr ago , preventing us from ruling out recent (less than 10 Myr ago) Cape colonization in these cases.
Comparisons of divergence minima with published relaxed-clock-based minimum estimates of the onset of radiation for numerous Cape clades (figure 2) presents an even more varied picture. Many relict lineage divergences from non-Cape sisters pre-date all the dated Cape radiations, some lie between the oldest and youngest Cape radiations, and, when lower credibility intervals are considered, some could conceivably postdate all radiations. Therefore, several of the lineages are clearly relicts of the flora existing in the Cape prior to the establishment of the modern flora, regardless of how we estimate its timing of establishment, while for others such relict status remains likely even if a more recent origin cannot be ruled out.
In Podocarpus and Widdringtonia, divergence minima based on non-African relaxed clock calibrations (101 and 65 Myr ago, respectively) pre-date their first appearance in the sparse southern African fossil record (ca 71 and 15 Myr ago, respectively; Coetzee & Rogers 1982; Scholtz 1985), allowing some confidence in the results. Further, in Widdringtonia, fossils dating to ca 95 Myr ago resembling the extant genus are known from North America (McIver 2001). Our estimate of the minimum duration of Cape occupation of this genus is therefore consistent with McIver's (2001) hypothesis that Widdringtonia originated in the Early Cretaceous floras of Laurasia and later migrated to Africa. By contrast, in Gunnera, our minimum estimate of the divergence time of the South African subspecies from the East tropical African subspecies based on one sample of each population (8.5 Myr ago) postdates its likely first appearance in the southern African fossil record (ca 71 Myr ago; Scholtz 1985). However, whether these two samples can be treated as representative of independent Cape and non-Cape lineages remains open to question. The alternative is that there is only a single independent Gunnera lineage in Africa, and that the divergence between the two samples is part of a continuum of genetic variation across the continent. Our minimum estimate of the divergence of the African species from its closest relative is much greater (50 Myr ago), and more consistent with the incidence of southern African fossils. Wider African Gunnera sampling would be required to address this issue.
While relict lineages typical of forest patches and Afromontane forests show a wide range of ages, divergence minima for those typical of stream margins (Prionium, Metrosideros, Brabejum, Platylophus and Cunonia) all fall within the Eocene, Oligocene and earliest Miocene. No southern African fossils are available for this period, but a summer-wet climatic regime is post-dicted for the Eocene and Early Miocene (Zachos et al. 2001; Linder 2003). These observations are consistent with Linder's (2003) hypothesis that the extensive summer-droughted fire-prone habitats of the modern Cape environment are more recent than the moister fire-protected habitats.
(b) The origins of low species richness in putative relict lineages
Two alternative scenarios could explain the lack of species diversity in the putative relict lineages, neither of which can be refuted on the basis of their known distributions and taxonomy.
Insufficient time. Such lineages exhibit the same rate of diversification as the larger Cape radiations, but have only arrived in the Cape recently. The factors driving speciation in other lineages have therefore had insufficient time to result in speciation.
Insufficient rate of net diversification. Such lineages have been present in the Cape for at least as long as the species-rich lineages, but have low diversity in the modern flora as a result of a lower rate of diversification; the factors driving speciation in other lineages may not have taken effect, or there may have been speciation followed by high levels of extinction. This low rate of net diversification may result from one or a number of factors, including character differences between lineages and differences in environment experienced.
Based on our lower credibility intervals of divergence minima and the range of diversification rates calculated for available Cape ‘radiations’, we are unable to reject scenario (i) for Gunnera, Ilex, Smelophyllum, Ocotea, Brabejum, Prionium, Platylophus, Metrosideros and Cunonia. However, their lack of departure from expectations under high rates of diversification could result from underestimation of their length of Cape occupancy. For the other putative relicts—Widdringtonia, Lachnostylis, Podocarpus, Curtisia-Grubbia and Roridula—we are able to reject scenario (i) on the basis of our lower estimates of their length of Cape occupation.
Clearly, exactly which lineages fall inside and outside confidence intervals of expected diversity depends on the range of diversification rates available for comparison. The range of rates used here (table 1) come from some of the most species-rich clades in the Cape. However, it seems likely that for most if not all rates picked from more diverse (more than three species) Cape lineages for comparison, the broader conclusion that we cannot reject scenario (i) for at least some putative relicts will hold; Gunnera, Ilex, Smelophyllum and Ocotea fall within the 95% confidence interval of expected diversity even under the highest estimated diversification rate for a plant radiation, 1.75 Myr−1 (Klak et al. 2004; based on the ice plant radiation in southern Africa, beyond the Cape sensu Goldblatt & Manning 2000).
Given that our dated Cape radiations and putative relicts cannot all be accommodated within the confidence interval of expected diversities under any single rate of diversification, on the basis of the dated lineages presented here, we can provisionally reject the possibility that putative relicts and ‘radiations’ fall within a stochastic distribution of diversities in which the underlying diversification rate is constant. However, as Magallón & Sanderson (2001) note, care must be taken in inferring that particular datapoints are outliers, since if the datapoints were a random sample from the assumed distribution, 5% of them should fall outside the confidence intervals just by chance. In this case (figure 3c), the minimum number of outliers that must be accommodated under any underlying rate is four, constituting 22% of the datapoints. Nonetheless, it is difficult to predict what the percentage of outliers would be if all Cape lineages were dated. In addition, whether or not the distribution of diversification rates across Cape lineages differs significantly from the distribution across other floras remains open to question. Addressing these questions would require a phylogenetic framework and date estimates for all the seed plant lineages that have invaded the Cape. Given the problems associated with sampling in dating estimates (Linder et al. 2005; Pirie et al. 2005; B. Warren & J. Hawkins 2004, unpublished data), individual dating of species-level phylogenies is much more likely to yield meaningful estimates of the length of Cape occupation by all Cape lineages than is the dating of a single heavily under-sampled phylogeny. While we are still a long way from achieving species-level sampling of all Cape plant lineages, recent interest in DNA barcoding along with the existing high level of phylogenetic sampling of the Cape flora makes it seem probable that this goal will eventually be reached, possibly sooner in the Cape than in other floristic regions.
Whatever the contribution of stochasticity, low diversification rates and species richness of relictual lineages in the modern flora may result from a lack of speciation, a high level of extinction, or a combination of the two. On biogeographic grounds, a significant level of extinction seems likely in some of the lineages under study, but less likely in others. Regardless of whether distributions reflect vicariance or dispersal, and whether we consider past or present continental configurations, modern distributions of some of the oldest lineages are difficult to explain without invoking extinction or major range-contraction. Widdringtonia consists of three species, of which two are confined to the Cape and one extends north to southern tropical Africa. The genus is sister (100% Bayesian branch support) to a lineage containing Diselma and Fitzroya, both of which are monotypic genera, confined to Tasmania and southern Chile, respectively. That extinction has played a role in this disjunct distribution is supported by the presence of North American Widdringtonia-like species in the fossil record, now extinct (McIver 2001). In some of the other lineages—Prionium, Platylophus and Smelophyllum—similar range disjunctions of modern relatives make past extinction events seem probable, even if fossil confirmation is unavailable, while in others—Gunnera and Ocotea—related lineages are much less geographically isolated (figure 1). Linder et al. (1992) suggest that rapid climatic deterioration at the end on the Tertiary from a tropical to summer dry Mediterranean-type climate may have driven widespread extinction prior to the establishment of the modern flora.
A low rate of recent speciation is also a probable factor contributing to low species diversity within relict lineages. Why recent speciation should be high in some Cape lineages but not others could relate to stochasticity, differences in environmental forces experienced, or intrinsic differences between lineages. Differences in environmental pressures experienced by the putative relicts and the diverse lineages might result from habitat differences. Several theories have been proposed to explain high diversity in the Cape, including important roles for fire (Cowling 1987), topographic gradients (Linder et al. 1992) and Pleistocene climatic cycles (Midgley et al. 2001). It has long been suggested that putative relicts occupy ‘relictual’ habitats (stream margins, forest patches and fire-protected outcrops), buffered from the rigours of fire and summer drought that characterize the fynbos. On the one hand, it may be perceived that such buffering would have shielded the relict lineages from the forces driving speciation in the fynbos. On the other hand, fragmentation and isolation of habitats with associated allopatric speciation are key components of these speciation hypotheses, and might be expected to have had as strong an influence on the ‘relictual’ habitats as any. A more detailed landscape-scale understanding of the historical influence of fire and climatic change would be required to resolve the issue.
An alternative to differences in environmental pressures experienced between lineages is the possibility that factors intrinsic to the relictual lineages render them a lower ‘speciation potential’ than in other lineages. For example, it is conceivable that the relict lineages are less labile with respect to topographic, edaphic and pollinator-driven adaptations than their species-rich counterparts. Such stasis in relictual lineages could either be a by-product of a long duration of presence in relictual habitats and an associated high degree of specialization, or alternatively, simply be a result of phylogenetic ‘clumping’ in the lability of these characters across the tree of life. A useful test of the latter proposition would be to determine whether there is a significant correlation between the species-richness of Cape lineages and their non-Cape sisters.
5. Conclusions
Future palaeobotanical study in the Cape is vital to test the robustness of inferences made here on the basis of currently available data. In addition, as molecular phylogenetic coverage of Cape lineages increases, it would be desirable to determine whether minimum durations of Cape occupancy estimated here are typical of all species-poor lineages (1–4 species) of the Cape, or whether other criteria used to identify putative relicts under consideration here (taxonomic and biogeographic isolation) are important estimators of relict status. Further, the utility of taxonomic isolation in estimating relictual status is likely to be dependent on the uniformity of the rate of morphological evolution across lineages. The greater the variation in rate, the poorer the correlation expected between taxonomic isolation and lineage age. If species-level phylogenies covering the entire Cape flora become available in the future, it is possible that numerous other relict lineages pre-dating the modern flora will be identified. The Cape has already become one of the best-known species-rich floras phylogenetically. Determining the origins of the phylogenetic distribution of species richness in a flora as compact and diverse as the Cape would serve as a useful model for understanding diversification patterns giving rise to modern floras worldwide.
Acknowledgments
We thank two anonymous reviewers for valuable comments on an earlier version of this work. J. Thorne and Z. Yang provided much support in the use of their programs. We also thank K. Bremer, A. Chanderbali, C. Fan, P. Gadek, C. Quinn, M. Simmons, L. Wanntorp and S. Wright for supplying alignments used in their published phylogenies. B.H.W. was supported by a grant from the Leverhulme Trust.
Supplementary Material
Sequence datasets and fossils used in phylogeny construction and calibration.
Formulae used in the calculation of diversification rates and 95% confidence intervals of expected diversity according to the age of a group.
Dated phylogenies, including nodes of calibration.
References
- Bakker F.T, Culham A, Marais E.M, Gibby M. Nested radiation in Cape Pelargonium. In: Bakker F.T, Chatrou L.W, Gravendeel B, Pelser P.B, editors. Plant species-level systematics: new perspectives on pattern and process. vol. 143. A. R. G. Gantner Verlag; Ruggell, Liechtenstein: 2005. pp. 75–100. [Google Scholar]
- Brown J.H. Species diversity. In: Myers A.A, Giller P.S, editors. Analytical biogeography: an integrated approach to the study of animal and plant distributions. Chapman & Hall; London, UK: 1988. pp. 57–89. [Google Scholar]
- Coetzee J.A, Rogers J. Palynological and lithological evidence for the Miocene palaeoenvironment in the Saldanha region (South Africa) Palaeogeogr. Palaeoclimatol. Palaeoecol. 1982;39:71–85. doi:10.1016/0031-0182(82)90073-6 [Google Scholar]
- Cowling R.M. Fire and its role in coexistence and speciation in Gondwanan shrublands. S. Afr. J. Sci. 1987;83:106–112. [Google Scholar]
- Cowling R.M, Rundel P.W, Lamont B.B, Arroyo M.K, Arianoutsou M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996;11:362–366. doi: 10.1016/0169-5347(96)10044-6. doi:10.1016/0169-5347(96)10044-6 [DOI] [PubMed] [Google Scholar]
- Fischer A.G. Latitudinal variations in organic diversity. Evolution. 1960;14:64–81. [Google Scholar]
- Goldblatt P. An analysis of the flora of southern Africa, its characteristics, relationships, and origins. Ann. Mo. Bot. Gard. 1978;65:369–436. [Google Scholar]
- Goldblatt P, Manning J. National Botanical Institute of South Africa and Missouri Botanical Garden; Missouri: 2000. Cape plants—a conspectus of the Cape flora of South Africa. pp. 229-230. [Google Scholar]
- Goldblatt P, Manning J.C. Plant diversity of the Cape Region of southern Africa. Ann. Mo. Bot. Gard. 2002;89:281–302. [Google Scholar]
- Goldblatt P, Savolainen V, Porteous O, Sostaric I, Powell M, Reeves G, Manning J.C, Barraclough T.G, Chase M.W. Radiation in the Cape flora and the phylogeny of peacock irises Moraea (Iridaceae) based on four plastid DNA regions. Mol. Phylogenet. Evol. 2002;25:341–360. doi: 10.1016/s1055-7903(02)00235-x. doi:10.1016/S1055-7903(02)00235-X [DOI] [PubMed] [Google Scholar]
- Gradstein F.M, et al. Cambridge University Press; Cambridge, UK: 2004. A geologic time scale 2004. [Google Scholar]
- Ho S.Y.W, Phillips M.J, Drummond A.J, Cooper A. Accuracy of rate estimation under relaxed-clock models with a critical focus on the early metazoan radiation. Mol. Biol. Evol. 2005;22:1355–1363. doi: 10.1093/molbev/msi125. doi:10.1093/molbev/msi125 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P, Ronquist F, Nielsen R, Bollback J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. doi:10.1126/science.1065889 [DOI] [PubMed] [Google Scholar]
- Kier G, Mutke J, Dinerstein E, Ricketts T.H, Küper W, Kreft H, Barthlott W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005;32:1107–1116. doi:10.1111/j.1365-2699.2005.01272.x [Google Scholar]
- Klak C, Reeves G, Hedderson T. Unmatched tempo of evolution in southern African semi-desert ice plants. Nature. 2004;427:63–65. doi: 10.1038/nature02243. doi:10.1038/nature02243 [DOI] [PubMed] [Google Scholar]
- Levyns M.R. Possible Antarctic elements in the South African flora. S. Afr. J. Sci. 1962;58:237–241. [Google Scholar]
- Levyns M.R. Migrations and origin of the Cape flora. Trans. R. Soc. S. Afr. 1964;37:85–107. [Google Scholar]
- Linder H.P. The radiation of the Cape flora, southern Africa. Biol. Rev. 2003;78:597–638. doi: 10.1017/s1464793103006171. doi:10.1017/S1464793103006171 [DOI] [PubMed] [Google Scholar]
- Linder H.P, Hardy C.R. Evolution of the species-rich Cape flora. Phil. Trans. R. Soc. B. 2004;359:1623–1632. doi: 10.1098/rstb.2004.1534. doi:10.1098/rstb.2004.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder H.P, Meadows M.E, Cowling R.M. History of the Cape flora. In: Cowling R.M, editor. The ecology of fynbos. Oxford University Press; Cape Town, South Africa: 1992. [Google Scholar]
- Linder H.P, Hardy C.R, Rutschmann F. Taxon sampling effects in molecular clock dating: an example from the African Restionaceae. Mol. Phylogenet. Evol. 2005;35:569–582. doi: 10.1016/j.ympev.2004.12.006. doi:10.1016/j.ympev.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Magallón S, Sanderson M.J. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Marloth R. Remarks on the realm of the Cape flora. S. Afr. J. Sci. 1929;26:154–159. [Google Scholar]
- McIver E.E. Cretaceous Widdringtonia Endl. (Cupressaceae) from North America. Int. J. Plant Sci. 2001;162:937–961. doi:10.1086/320776 [Google Scholar]
- Midgley G.F, Hannah L, Roberts R, McDonald D.J, Allsopp J. Have Pleistocene climatic cycles influenced species richness in the greater Cape Mediterranean Region? J. Mediterr. Ecol. 2001;2:137–144. [Google Scholar]
- Mummenhoff K, Al-Shehbaz I.A, Bakker F.T, Linder H.P, Mühlhausen A. Phylogeny, morphological evolution, and speciation of endemic Brassicaceae genera in the Cape flora of southern Africa. Ann. Mo. Bot. Gard. 2005;92:400–424. [Google Scholar]
- Pérez-Losada M, Høeg J.T, Crandall K.A. Unraveling the evolutionary radiation of the Thoracican barnacles using molecular and morphological evidence: a comparison of several divergence time estimation approaches. Syst. Biol. 2004;53:244–264. doi: 10.1080/10635150490423458. doi:10.1080/10635150490423458 [DOI] [PubMed] [Google Scholar]
- Pianka E.R. Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 1966;100:33–46. doi:10.1086/282398 [Google Scholar]
- Pirie M.D, Chartrou L.W, Erkens R.H.J, Maas J.W, van der Niet T, Mols J.B, Richardson J.E. Phylogeny reconstruction and molecular dating in four Neotropical genera of Annonaceae: the effect of taxon sampling in age estimations. In: Bakker F.T, Chartrou B, Gravendeel B, Pelser P.B, editors. Plant species-level systematics: new perspectives on pattern and process. vol. 143. A.R.G. Gantner Verlag; Ruggell, Liechtenstein: 2005. pp. 149–174. [Google Scholar]
- Posada D, Crandall K.A. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Richardson J.E, Welts F.M, Fay M.F, Cronk Q.C.B, Linder H.P, Reeves G, Chase M.W. Rapid and recent origin of species richness in the Cape flora of South Africa. Nature. 2001;412:181–183. doi: 10.1038/35084067. doi:10.1038/35084067 [DOI] [PubMed] [Google Scholar]
- Rutschmann F. University of Zurich; Switzerland: 2004. Bayesian molecular dating using PAML/multidivtime: a step-by-step manual. pp. 1–14. [Google Scholar]
- Scholtz A. The palynology of the upper lacustrine sediments of the Arnot Pipe, Banke, Namaqualand. Ann. S. Afr. Mus. 1985;95:1–109. [Google Scholar]
- Schrire B.D, Lavin M, Barker N.P, Cortes-Burns H, von Senger I, Kim J.H. Towards a phylogeny of Indigofera (Leguminosae-Papilionoideae): identification of major clades and relative ages. In: Klitgaard B.B, Bruneau A, editors. Higher level systematics. vol. 10. Royal Botanic Gardens; Kew, UK: 2003. pp. 269–302. [Google Scholar]
- Siesser W.G. Late Miocene origin of the Benguela upwelling system off northern Namibia. Science. 1980;208:283–285. doi: 10.1126/science.208.4441.283. [DOI] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H. Divergence time and evolutionary rate estimation with multilocus data. Syst. Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. doi:10.1080/10635150290102456 [DOI] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H, Painter I.S. Estimating the rate of evolution of the rate of molecular evolution. Mol. Biol. Evol. 1998;15:1647–1657. doi: 10.1093/oxfordjournals.molbev.a025892. [DOI] [PubMed] [Google Scholar]
- Verboom G.A, Linder H.P, Stock W.D. Phylogenetics of the grass genus Ehrharta Thunb.: evidence for radiation in the summer-arid zone of the South African Cape. Evolution. 2003;57:1008–1021. doi: 10.1111/j.0014-3820.2003.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence datasets and fossils used in phylogeny construction and calibration.
Formulae used in the calculation of diversification rates and 95% confidence intervals of expected diversity according to the age of a group.
Dated phylogenies, including nodes of calibration.