Abstract
Radiations of large clades often accompany rapid morphological diversification. Evolutionary biologists debate the impact of external restrictions imposed by ecology, and intrinsic constraints imposed by development and genetics, on the rate at which morphological innovations are gained. These issues are particularly interesting for groups such as tetrapods, which evolved novel body plans relative to their piscine ancestors and which also invaded new ecosystems following terrestrialization. Prior studies have addressed these issues by looking at either ranges of morphological variation or rates of character change. Here, we address a related but distinct issue: the numbers of characters that freely vary within a clade. We modify techniques similar to those used by ecologists to infer species richnesses to estimate the number of potentially varying characters given the distributions of changes implied by a model phylogeny. Our results suggest both increasing constraints/restrictions and episodes of ‘character release’ (i.e. increasing the number of potentially varying characters). In particular, we show that stem lissamphibians had a restricted character space relative to that of stem amniotes, and that stem amniotes both had restrictions on some parts of character space but also invaded new character space that had been unavailable to stem tetrapods.
Keywords: Palaeozoic, lissamphibians, amniotes, constraints, ecological restrictions, character release
1. Introduction
Numerous major radiations are marked by rapid diversification of new morphotypes (see Wagner (2001) for a review). Two related but distinct aspects of such radiations are the relative rates at which characters change and the range of morphologies generated. In particular, the early stages of some radiations appear to be marked by variation among characters that are either invariant or only infrequently variant within major clades (e.g. Campbell & Marshall 1987; Coates & Clack 1990). To date, studies have assessed whether the range of total variation in morphospace differs over time (e.g. Foote 1992, 1994) or among different groups within a larger clade (Foote 1993; Stockmeyer Lofgren et al. 2003). In addition, studies suggest that there are finite numbers of recognizably distinct characters and character states available to a clade (Wagner 2000). However, no studies to date have combined these approaches to assess whether the range of potentially varying characters differs over time or among clades.
Here, we test whether the range of characters available to Palaeozoic tetrapods remained constant. Palaeozoic tetrapods are a useful group for such a study for several reasons. First, studies indicate that characters now static or rarely variant within large clades are variant among early tetrapods, with some early species possessing conditions not observed in any major clades (Coates & Clack 1990; Coates et al. 2002; Shubin et al. 2004). If such characters have become ‘canalized’ (see Waddington 1959) or functionally restricted since the early phases of tetrapod evolution, then evolution essentially has removed those characters (or conditions thereof) from the pool of potentially varying morphologies. Second, tetrapods include two clades—the Lissamphibia and the Amniota—that radiated extensively after the divergence of limbed vertebrates. On one hand, one can think of these subsequent radiations ‘rerunning the clock’ (e.g. Gould 2002): if there was canalization prior to the diversifications of the two clades, then we do not expect to see the same range of character evolution in either stem amniotes or stem lissamphibians as we see in all tetrapods. Moreover, because amniotes and lissamphibians are sister taxa, they represent rerunning the clock twice in parallel. However, the amniote radiation coincides with the invasion of new ecospace. If the invasion of new ecospace reduced ecological restrictions on character evolution, then we expect to see ‘character release’ (see Grant 1972) and an increase in the available character space in the amniote clade.
Another reason why Palaeozoic tetrapods are useful for this study is that workers have conducted extensive phylogenetic analyses on early tetrapods (e.g. Ruta et al. 2003; Ruta & Coates in press). This provides both a large (by palaeontological standards) morphological dataset and a phylogenetic model for assessing how frequently characters change over time and across phylogeny. Moreover, analyses of these data suggest that rates of character change decreased over time (Ruta et al. 2006). Increasing constraints and/or restrictions both predict this pattern, as this essentially involves a drastic decrease in the rate of change for some characters.
2. Material and methods
(a) Tetrapod characters and phylogeny
We analyse 92 Palaeozoic tetrapod species using 339 skeletal characters (see Ruta & Coates in press). These include 51 stem amniotes and 22 stem lissamphibians as well as 19 stem tetrapods. Hereafter, we use ‘lissamphibian’ and ‘amniotes’ to denote the Palaeozoic stem members of those clades. In the case of amniotes, we also stress that it is not known when non-skeletal specializations evolved: thus, our definition probably includes taxa that lacked an amnion.
We contrast three portions of tetrapod phylogeny: lissamphibians, amniotes and basal limbed tetrapods (i.e. tetrapods excluding amniotes and amphibians). We estimate the available character spaces for each clade or paraclade, as well as the more inclusive clades (lissamphibians+amniotes and all Palaeozoic limbed tetrapods) in order to assess whether the range of easily available characters differed between early and derived tetrapods and/or among different tetrapod clades and paraclades.
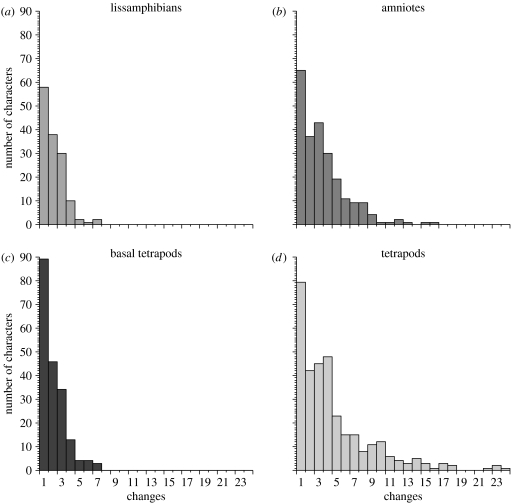
The data used to evaluate character space size are the number of characters that change 1, 2, 3, etc. times in each portion of phylogeny (figure 1). This requires a model phylogeny. For that, we use the same tree that Ruta et al. (2006) used to evaluate rates (see Ruta & Coates (in press) for phylogenetic analyses). We emphasize that other parsimony trees in Ruta & Coates's (in press) work have the same implications for this study. We address the possible effects of phylogenetic error below.
Figure 1.
Numbers of characters changing 1, 2, 3, etc. times on different portions of tetrapod phylogeny (a–c) and on the whole phylogeny (d).
(b) Estimating available character space
The number of characters that might vary in a clade is unknown. We recognize variable characters only if two or more conditions (states) are observed in a clade. Thus, it is possible (and usually probable) that we have not observed two or more states for some characters simply because those characters failed to change, either among the taxa that we have sampled or even ever, even though they could have changed at any time.
A preliminary way to evaluate whether different portions of a phylogeny have different numbers of available characters is to examine an exhaustion curve of characters (Wagner 2000). This allows one to see if similar numbers of changes affect similar numbers of characters in two different groups. However, this does not permit direct tests of the null hypothesis that the same range of characters is available to all portions of the clade.
The use of exhaustion curves to assess available character spaces is similar to the use of rarefaction to assess relative numbers of species in different communities (Sanders 1968). Ultimately, the question that we need to assess concerns the number of entities (characters or species) that fall behind the ‘veil’ line (Preston 1948). The latter represents the number of entities that are unobserved. Several approaches exist for estimating the number of unobserved entities (Efron & Thisted 1976; Chao 1984). The important data for such tests are the number of entities observed once, twice, thrice, etc. In the case of characters, an ‘observation’ is an inferred change (figure 1): this represents an opportunity to observe characters just as a specimen offers an opportunity to see a species.
In addition to the number of entities, another parameter now is important: the relative frequency at which we sample those entities. If the probability of encountering each entity is equal (e.g. all characters have the same probability of change), then we expect a different distribution of entities seen once, twice, etc. from what we expect if the probability of encountering entities varies (see Hurlbert 1971; Kosnik & Wagner 2006).
We consider three general models of relative frequencies of change: identical, exponential, and lognormal. The identical hypothesis is the simplest one. Here, the probability of any character i being the one that changes (fi) is simply 1/C, where C is the hypothesized number of characters. The exponential hypothesis assumes that rates vary such that fi=m×fi−1 for all characters, with:
| 2.1 |
This assumes that characters have a uniform distribution of ‘half-lives.’ Finally, the lognormal hypothesis assumes a normal distribution of half-lives. Each rate class changes m times more frequently than the prior rate class, with the number of characters in each rate class proportional to the area under a normal curve. Thus, the identical hypothesis has a single parameter (number of characters) whereas the exponential and lognormal hypotheses require two parameters (number of characters and a parameter describing the shift in rates of change).
Given a hypothesis of C characters each with some fi, the expected number of entities observed n times (On) given N total observations is:
| 2.2 |
Here, On is the number of characters changing n times and N is the total number of changes. Dewdney (1998) showed that a Poisson distribution adequately predicts the probability of the observed On given an expected On. Thus, the likelihood of any particular model of change given the ‘observed’ On is:
| 2.3 |
where λ is the expected number of species observed n times (E[On|N changes]).
Finally, we are not interested only in estimating the most likely sizes of character spaces. We also want to test the null hypothesis that two portions of a larger phylogeny have the same sized character spaces. Thus, we determine likelihood curves for two-units of support (i.e. log-likelihoods up to two less than the most likely hypothesis; Edwards 1992). When contrasting two hypotheses of same general model (e.g. two lognormal distributions), then the null hypothesis is a special case of the test hypothesis, where C1=C2 and (if applicable m1=m2). In such cases, twice the expected difference in log-likelihoods follows a chi-squared distribution in which the degrees of freedom equal the number of differing parameters (Sokal & Rohlf 1981). We now can use the log-likelihood ratio test to reject the idea of a single character space system in favour of the idea that there are two. Suppose that we wish to test the hypothesis that both lissamphibian and amniote character spaces represent the same lognormal distribution versus the hypothesis that they represent two distinct lognormal distributions. Here, there are two parameters that can differ, C and m. We reject the null hypothesis if the deviation from expectation is 6.0 or greater, which means that we would reject the null hypothesis (m1=m2 and C1=C2) if the log-likelihood of the test hypothesis (C1≠C2, m1≠m2) is greater than 3.0.
Alternatively, we can find the optimal m for each C under the exponential or lognormal hypotheses. Because we really are interested only in C, we then go from three parameters (C1=C2, m1≠m2) to four parameters (C1≠C2, m1≠m2). Now, we reject the null hypothesis if the difference in log-likelihoods exceeds 1.98.
Comparing a subclade with a more inclusive clade complicates matters. Suppose that we wish to evaluate whether amniotes have the same character space as do lissamphibians+amniotes. Because amniotes themselves contribute to the lissamphiban+amniote character space, these do not represent independent comparisons. As a result, the expected difference in log-likelihoods should be less than that predicted by a chi-squared distribution. In this case, the log-likelihood ratio test is highly conservative.
3. Results
(a) Character exhaustion
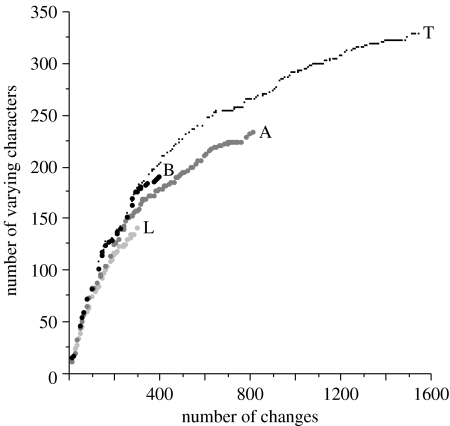
Tetrapod clades and paraclades show clear signs of exhausting available character space at different rates (figure 2). Lissamphibians exhaust character space most rapidly: that is, lissamphibians distribute the same number of changes over fewer characters, suggesting a greater number of static characters. Amniotes exhaust the character space less rapidly, whereas basal tetrapods derive the greatest number of characters for a given number of steps.
Figure 2.
Exhaustion of available characters by portions of the tetrapod clade versus each other and the entire clade (T). L, lissamphibians; A, amniotes; and B, basal tetrapods excluding lissamphibians and amniotes.
(b) Estimates of available character space
For all five examinations, a lognormal model describes the distribution of character changes significantly better than either the uniform or exponential models (table 1). For lissamphibians, amniotes and basal tetrapods, the log-likelihoods of the best lognormals are less than 2.0 and greater than the log-likelihoods of the best exponentials. Thus, we would not reject the latter out of hand for any of the three partitions. However, the lognormal is appreciably better for the entire clade as well as for lissamphibians+amniotes (table 1). Thus, we will assume a lognormal distribution when testing character space size. Finally, in all cases the tests suggest that many more characters were available that could change than we actually observe changing.
Table 1.
Best hypotheses for three models of character spaces. (‘n’ gives the number of taxa in each clade. ‘Ch’ gives either the observed number of variable characters, or the most-likely number given the best character space hypothesis. For the lognormal, ‘m’ gives the magnitude of increase in frequency of change as one increases an octave (see Preston 1948). For the exponential, ‘m’ gives how many times more frequently character X changes than character X+1.)
| taxon | observed | lognormal | exponential | uniform | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Ch | Ch | m | ln L | Ch | m | ln L | Ch | ln L | |
| basal tetrapods | 19 | 193 | 279 | 1.7668778 | −19.1 | 314 | 1.00855294 | −19.3 | 236 | −48.9 |
| lissamphibia | 22 | 130 | 183 | 1.4112231 | −17.4 | 183 | 1.00668109 | −18.5 | 170 | −73.3 |
| amniotes | 54 | 234 | 274 | 1.9714190 | −35.0 | 290 | 1.00973399 | −36.3 | 241 | −163.2 |
| lissamphibians+amniotes | 76 | 281 | 332 | 2.3024773 | −44.9 | 332 | 1.01009661 | −50.2 | 286 | −183.2 |
| all tetrapods | 95 | 329 | 380 | 2.4424095 | −58.6 | 404 | 1.00928947 | −62.0 | 332 | −286.5 |
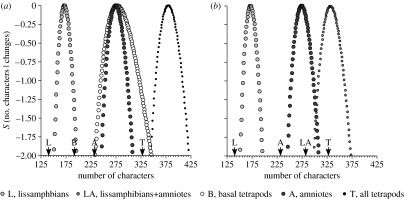
The support curve for lissamphibian character space does not overlap at all with the support curves for either amniote or basal tetrapod character spaces (figure 3a; note that support curves show the difference between the log-likelihood of the most likely hypothesis and the hypothesis in question). This indicates that the character space for lissamphibians is significantly smaller than that of basal tetrapods or amniotes (figure 3a). The minimum difference in log-likelihoods between the three- and four-parameter hypotheses in both cases is greater than 4.0 (p<0.005), and the actual differences in likelihood obviously are considerably greater than this, given how rapidly the curves fall off. Indeed, we can emphatically reject the idea that Palaeozoic lissamphibians have as many total characters as Palaeozoic amniotes had observed characters (figure 3a). However, there is no indication that the character spaces for basal tetrapods or amniotes were significantly different from one another in the Palaeozoic.
Figure 3.
Support for hypotheses of character space size for different portions of the tetrapod tree. Arrows denote observed numbers of varying characters (see table 1). S (support) gives the differences between the log-likelihood for that number of characters and the log-likelihood of the most likely hypothesis. Note that the likelihood assumes a different lognormal distribution for each number of characters on each curve; (a) provides a contrast for the crown clades (L and A) and basal amniotes (B) or all tetrapods (T); (b) provides a contrast for the crown clades with the total crown clade (LA).
We can strongly reject the idea that either lissamphibians or amniotes had character spaces as large as that available to all tetrapods: despite the lack of independence, the whole clade has significantly more observed characters than we can hypothesize for either lissamphibians or amniotes (figure 3a). We can also reject the idea that basal tetrapods had a character space as big as that of all tetrapods: even if the two datasets were independent, we would reject the hypothesis (Δln L=3.67; p=0.007). Finally, we can also reject the idea that amniotes have a character space as large as that of the lissamphibian+amniote clade (figure 3b): even if we assumed that the two datasets were independent, the difference in log-likelihood between the best three-parameter and four-parameter hypotheses (5.75) is significant (p=0.016).
4. Discussion
Palaeozoic tetrapods show evidence of both increasing constraints/restrictions and character release. Lissamphibians provide the most obvious example of constraints/restrictions, as their available character space is significantly smaller than the character spaces of either their sister taxon (amniotes) or their ancestors (basal tetrapods). Frequently, we cannot distinguish between increasing ecological restrictions and increasing intrinsic constraints (Wagner 1995). However, lissamphibians probably retained the general ecological habits of basal tetrapods (see, e.g. Romer 1966). Given that they represent a monophyletic radiation into ‘primitive’ ecospace, especially with regard to amphibious larvae, and given that lissamphibians soon were the only tetrapods in that morphospace, lissamphibians should not have been any more restricted by ecology than basal tetrapods. This strongly suggests that intrinsic constraints of some sort account for the lissamphibian pattern. However, it bears stressing that lissamphibians were not evolving solely within existing character space: because amniote character space is smaller than that of lissamphibians+amniotes, it necessarily follows that some lissamphibian+amniote characters belong solely to lissamphibians.
Amniotes probably show both increasing constraint/restriction and character release. Although, basal tetrapods and amniotes show character spaces of a similar size, neither character space is as large as that of the whole tetrapod clade. The simplest explanation for this is that the main difference between stem tetrapod character space and total Palaeozoic tetrapod character space is new characters that amniotes add to tetrapod space. A likely cause for this character release is the invasion of completely terrestrial habitats that was made possible the evolution of the amnion itself. Both synapsids and diapsids appear by the Late Carboniferous (Reisz 1997), which provides a strong indication that that an amnion (and thus fully terrestrial habits) had evolved by that time. Amniotes can and do occupy a much wider variety of environments and ecological habits than do modern amphibians, which likely retain primitive ecologies similar to those of basal tetrapods. Thus, the second phase of terrestrialization also would have involved colonization of new ecospace, which we expect to allow for greater morphological variation (Valentine 1980). However, other aspects of the tetrapod skeleton that varied among basal tetrapods must have become constrained/restricted among amniotes: otherwise amniote character space would equal (or nearly equal) that of tetrapods as a whole. Constraints and/or restrictions might account for this pattern: static characters within amniotes might already have become canalized or truly terrestrial habitats might have placed restrictions on skeletal evolution.
If relaxed ecological restrictions did induce character release among amniotes, then it is very possible that expanding this study to include post-Palaeozoic tetrapods should show additional increases in the character spaces of both lissamphibians and amniotes following the vacating of ecospace by the end-Permian extinction. Similar patterns have been observed for both echinoderms (Foote 1996; Ciampaglio 2002) and brachiopods (Ciampaglio 2004) after mass extinctions. Contrasting shifts in character space accompanying major shifts in ecology with those seen within clades during ‘background’ times (sensu Jablonski 1986) might offer means of estimating general rates at which characters are ‘gained’ and ‘lost’ to clades as well as offer some clarification of the circumstances needed for rapid morphological diversification.
Finally, our results suggest that much potential morphological variation among tetrapods was lost by the extinction of all non-lissamphibian and non-amniote tetrapods. The observed character space for basal tetrapods is much less than the most likely space, and it is clear that many of these characters should not have been available to either lissamphibians or amniotes. This is an issue pertinent to modern concerns about biological diversity: if, as implied here, the evolutionary potential of taxa varies across phylogeny, then there is an additional argument favouring strategies that focus on preserving breadth of phylogeny rather than just numbers of species (see, e.g. Faith 2002).
5. Conclusions
Palaeozoic tetrapod evolution suggests both patterns of increasing constraint on morphological variation and the release of characters because of the invasion of new habitats. Amniotes in particular appear to have expanded tetrapod character space, possibly as a result of acquiring fully terrestrial habitats, whereas lissamphibians appear to have become restricted within tetrapod character space. However, lissamphibians also acquire a relatively small number of new characters, and amniotes clearly were restricted relative to the character space available to basal tetrapods. Thus, instead of an ‘either/or’ situation, it appears that some character systems shut down while others become active over the history of highly diverse clades.
Acknowledgments
P.J.W.'s contributions were funded by NSF Grant EAR-0207874. M.R.'s research was funded by a John Caldwell Meeker Research Fellowship at the Department of Geology, Field Museum of Natural History, Chicago. M.I.C.'s work was funded by an award from the Faculty Research Fund, Pritzker School of Medicine, University of Chicago. We thank M. Friedman for reminding us of the term ‘character release.’ Two anonymous reviewers provided helpful comments. Software for conducting these analyses is available at http://pjw3.fmnh.org/data.html.
Footnotes
Author for correspondence on rates analysis (pwagner@fieldmuseum.org).
References
- Campbell K.S.W, Marshall C.R. Rates of evolution among Paleozoic echinoderms. In: Campbell K.S.W, Day M.F, editors. Rates of evolution. Allen & Unwin Press; London, UK: 1987. pp. 61–100. [Google Scholar]
- Chao A. Nonparametric-estimation of the number of classes in a population. Scand. J. Stat. 1984;11:265–270. [Google Scholar]
- Ciampaglio C.N. Determining the role that ecological and developmental constraints play in controlling disparity: examples from the crinoid and blastozoan fossil record. Evol. Dev. 2002;4:170–188. doi: 10.1046/j.1525-142x.2002.02001.x. doi:10.1046/j.1525-142X.2002.02001.x [DOI] [PubMed] [Google Scholar]
- Ciampaglio C.N. Measuring changes in articulate brachiopod morphology before and after the Permian mass extinction event: do developmental constraints limit morphological innovation? Evol. Dev. 2004;6:260–274. doi: 10.1111/j.1525-142X.2004.04031.x. doi:10.1111/j.1525-142X.2004.04031.x [DOI] [PubMed] [Google Scholar]
- Coates M.I, Clack J.A. Polydactyly in the earliest known tetrapod limbs. Nature. 1990;347:66–69. doi:10.1038/347066a0 [Google Scholar]
- Coates M.I, Jeffery J.E, Ruta M. Fins to limbs: what the fossils say. Evol. Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. doi:10.1046/j.1525-142X.2002.02026.x [DOI] [PubMed] [Google Scholar]
- Dewdney A.K. A general theory of the sampling process with applications to the ‘Veil Line’. Theor. Popul. Biol. 1998;54:294–302. doi: 10.1006/tpbi.1997.1370. doi:10.1006/tpbi.1997.1370 [DOI] [PubMed] [Google Scholar]
- Edwards A.W.F. Johns Hopkins University Press; Baltimore, MD: 1992. Likelihood—expanded edition. [Google Scholar]
- Efron B, Thisted R. Estimating the number of unseen species: how many words did Shakespeare know? Biometrika. 1976;63:435–447. [Google Scholar]
- Faith D.P. Quantifying biodiversity: a phylogenetic perspective. Conserv. Biol. 2002;16:248–252. doi: 10.1046/j.1523-1739.2002.00503.x. doi:10.1046/j.1523-1739.2002.00503.x [DOI] [PubMed] [Google Scholar]
- Foote M. Paleozoic record of morphological diversity in blastozoan echinoderms. Proc. Natl Acad. Sci., USA. 1992;89:7325–7329. doi: 10.1073/pnas.89.16.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote M. Discordance and concordance between morphological and taxonomic diversity. Paleobiology. 1993;19:185–204. [Google Scholar]
- Foote M. Morphological disparity in Ordovician–Devonian crinoids and the early saturation of morphological space. Paleobiology. 1994;20:320–344. [Google Scholar]
- Foote M. Ecological controls on the evolutionary recovery of Post-Paleozoic crinoids. Science. 1996;274:1492–1495. doi: 10.1126/science.274.5292.1492. doi:10.1126/science.274.5292.1492 [DOI] [PubMed] [Google Scholar]
- Gould S.J. Belknap Press; Cambridge, UK: 2002. The structure of evolutionary theory. [Google Scholar]
- Grant P.R. Convergent and divergent character displacement. Biol. J. Linn. Soc. 1972;4:39–68. [Google Scholar]
- Hurlbert S.H. The nonconcept of species diversity: a critique and alternative parameters. Ecology. 1971;52:577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- Jablonski D. Background and mass extinctions: the alteration of macroevolutionary regimes. Science. 1986;231:129–133. doi: 10.1126/science.231.4734.129. [DOI] [PubMed] [Google Scholar]
- Kosnik M.A, Wagner P.J. The effect of species-abundance distribution shape on the number of sampled taxa. Evol. Ecol. Res. 2006;8:1–17. [Google Scholar]
- Preston W.H. The commonness and rarity of species. Ecology. 1948;29:254–283. [Google Scholar]
- Reisz R.R. The origin and early evolutionary history of amniotes. Trend. Ecol. Evol. 1997;12:218–222. doi: 10.1016/s0169-5347(97)01060-4. doi:10.1016/S0169-5347(97)01060-4 [DOI] [PubMed] [Google Scholar]
- Romer A.S. 3rd edn. The University of Chicago Press; Chicago, IL: 1966. Vertebrate paleontology. [Google Scholar]
- Ruta, M. & Coates, M. I. 2006 Dates, nodes, and character conflict: addressing the amphibian origin problem. J. Syst. Paleontol.4
- Ruta M, Coates M.I, Quicke D.L.J. Early tetrapod relationships revisited. Biol. Rev. 2003;78:251–345. doi: 10.1017/s1464793102006103. doi:10.1017/S1464793102006103 [DOI] [PubMed] [Google Scholar]
- Ruta, M., Wagner, P. J. & Coates, M. I. 2006 Evolutionary patterns in early tetrapods. I. Rapid initial diversification followed by decrease in rates of character change. Proc. R. Soc. B273 (doi:10.1098/rspb.2006.3577) [DOI] [PMC free article] [PubMed]
- Sanders H.L. Marine benthic diversity: a comparative study. Am. Nat. 1968;102:243–282. doi:10.1086/282541 [Google Scholar]
- Shubin N.H, Daeschler E.B, Coates M.I. The early evolution of the tetrapod humerus. Science. 2004;304:90–93. doi: 10.1126/science.1094295. doi:10.1126/science.1094295 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. W. H. Freeman; New York, NY: 1981. Biometry. [Google Scholar]
- Stockmeyer Lofgren A, Plotnick R.E, Wagner P.J. Morphological diversity of Carboniferous arthropods and insights on disparity patterns of the Phanerozoic. Paleobiology. 2003;29:350–369. [Google Scholar]
- Valentine J.W. Determinants of diversity in higher taxonomic categories. Paleobiology. 1980;6:444–450. [Google Scholar]
- Waddington C.H. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183:1654–1655. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wagner P.J. Testing evolutionary constraint hypotheses with early Paleozoic gastropods. Paleobiology. 1995;21:248–272. [Google Scholar]
- Wagner P.J. Exhaustion of cladistic character states among fossil taxa. Evolution. 2000;54:365–386. doi: 10.1111/j.0014-3820.2000.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Wagner P.J. Constraints on the evolution of form. In: Briggs D.E.G, Crowther P.R, editors. Palaeobiology II. Blackwell; Oxford, UK: 2001. pp. 154–159. [Google Scholar]