Abstract
Vertical jumping was used to assess muscle mechanical output in bonobos and comparisons were drawn to human jumping. Jump height, defined as the vertical displacement of the body centre of mass during the airborne phase, was determined for three bonobos of varying age and sex. All bonobos reached jump heights above 0.7 m, which greatly exceeds typical human maximal performance (0.3–0.4 m). Jumps by one male bonobo (34 kg) and one human male (61.5 kg) were analysed using an inverse dynamics approach. Despite the difference in size, the mechanical output delivered by the bonobo and the human jumper during the push-off was similar: about 450 J, with a peak power output close to 3000 W. In the bonobo, most of the mechanical output was generated at the hips. To account for the mechanical output, the muscles actuating the bonobo's hips (directly and indirectly) must deliver muscle-mass-specific power and work output of 615 W kg−1 and 92 J kg−1, respectively. This was twice the output expected on the basis of muscle mass specific work and power in other jumping animals but seems physiologically possible. We suggest that the difference is due to a higher specific force (force per unit of cross-sectional area) in the bonobo.
Keywords: functional morphology, kinematics, ground reaction force, inverse dynamics, specific work
1. Introduction
Renowned researchers who have worked intensively with living chimpanzees (Pan troglodytes) or bonobos (Pan paniscus) have stated repeatedly that these animals are amazingly strong. Gardner & Gardner (1969) wrote, ‘Chimpanzees are also very strong animals; a full grown specimen is likely to weigh more than 120 pounds (55 kilograms) and is estimated to be from three to five times as strong as a man, pound-for-pound’. Savage-Rumbaugh et al. (1998) wrote, ‘Kanzi [a bonobo, M.S.], as an adult, measures up to his name; he is bold and brave; he is also large (165 pounds) and very strong—five times stronger than a 165 pound human male in excellent physical shape’. Jane Goodall, in an interview with Jay Ingram on Discovery Channel Canada (broadcasted 4 September 2001), said that an adult male chimpanzee in the wild ‘would be at least six times stronger than a normal male’. She explained that ‘there are many examples where you see them manipulating big branches in a way that shows their strength’. Anecdotic accounts of the strength of chimpanzees and bonobos are abundant. Classic studies on in vivo isometric strength in captive chimpanzees have been conducted by Bauman (1923, 1926) and Finch (1943). Bauman set up a dynamometer, which was previously used to test leg and back strength in college students, outside the chimpanzee cage at the New York Zoological Park. He reported that a female chimpanzee ‘Suzette’, a former circus attraction, ‘sprang to the rope and, bracing both feet against the bars, pulled back with both hands upon the rope, making a pull on the latter that recorded 1260 lb upon the dial of the recording device’. Further on, he wrote: ‘An average college student of Suzette's weight, 135 pounds, can pull in an approximately similar position and manner but 332 pounds, while one out of every hundred students can thus pull 500 pounds’. The performance of a male chimpanzee ‘Boma’ reported in the same study was equally impressive. In the experiment of Finch (1943), chimpanzee performance was less phenomenal than in Bauman's study, but it still took a 190-lb man to beat a 107-lb chimpanzee in a rope-pulling task.
Puzzlingly, neither bonobos nor chimpanzees seem to be overly muscular compared with healthy, active humans, especially from the waist down (Bauman 1926; Thorpe et al. 1999; Payne 2001). Several hypotheses for the superior strength of apes have been suggested, ranging from ‘continuity and amount of exercise’ (Yerkes 1943) to ‘different leverage’ or ‘greater dovetailing of muscle cells’ (Edwards 1963). But mostly, it is conjectured that ape muscle is intrinsically superior to human muscle (Goodall (interview); Bauman 1926; Edwards 1963; Savage-Rumbaugh et al. 1998). In fact, Edwards (1963) wrote, ‘To evaluate the above hypotheses, tests are being conducted at Holloman AFB [Air Force Base, M.S.] to compare chimpanzees and humans in a near-immobilizing chair, testing muscle groups individually’. This study, which consisted of a well-controlled elbow flexion task, where subjects pulled on a rope and lifted a weight, was documented as a US Air Force report (Edwards 1965): ‘The outpulling by the largest chimpanzee of a human weight-lifter fully 2.5 times as large in body-weight seems especially noteworthy’. Not only did the chimpanzees show superior strength, they also showed superior endurance: ‘near-maximal pulls of the chimpanzees were made in much more rapid succession than those of the humans without apparent reductions in the scores achieved’. However, neither muscle moment arms nor muscle physiological cross-sectional areas were measured in this study, so it is not possible to link the force measured externally to the force delivered by the muscles.
For several reasons it is important to establish whether there is a difference in the intrinsic muscle properties between humans and chimpanzees/bonobos. First, the quest to understand the evolution of human bipedalism has recently culminated in forward dynamic musculoskeletal models of optimal australopithecine gait (Nagano et al. 2005; Sellers et al. 2005). While skeletal properties of Australopithecus can be deduced from fossil remains, muscle properties are not known. Obviously, the muscles are a critical feature of these models. Any knowledge about muscle properties from other hominids can be applied to enhance the purely human-based estimates that are currently used in the models. Second, bonobos are genetically closely related to us. If they were found to possess superior muscle properties it would become an interesting endeavour to unravel the basis of this difference, with possible applications in medical research on muscle disorders.
In the present study, vertical squat jumping is used to compare in vivo skeletal muscle properties in the bonobo and human. The bonobo is a highly endangered species, hardly accessible to any kind of experimental intervention. Jumping performance, however, can be quantified with minimal disturbance of the animals' well-being and sheds light on the dynamic capabilities of its muscles: the work that is generated by the muscles during the push-off closely matches the potential energy gained relative to the deepest crouch during the push-off. Unlike muscle force, the work output of muscle during a single shortening contraction depends critically on muscle volume and not on other anatomical details such as ‘leverage’ or dovetailing, an advantage of using a dynamic task instead of an isometric one. Knowledge of bonobo morphology and musculature that has been acquired in cadaver studies (Zihlman 1984; Payne et al. in press a) will be placed in a functional context, and comparisons will be drawn to human jumping.
2. Material and methods
(a) Experimental set-up
Several bonobos, residing at the Wild Animal Park Planckendael (Belgium), were motivated to jump to a piece of fruit or the spot of light projected by a laser pointer (the animals had previously been trained to touch the light spot wherever the caretaker pointed it). No jumping-specific training was provided. The jumps, which were executed in the outdoor enclosure or in the indoor night-time enclosure, were recorded on high-speed video (100 or 250 Hz). A fully instrumented measurement setup called the ‘catwalk’ was in place in the outdoor enclosure. This setup contained a force plate (AMTI BP400-1000, size: 1×0.4 m; sample frequency: 1000 Hz) mounted on a concrete base with a pedobarometric plate (RSScan footscan plate, size: 1×0.4 m; sample frequency: 50 Hz) on top. For jumps performed on the measurement platform, ground reaction forces and moments in vertical (z), lateral (x) and fore–aft (y) direction as well as the pressures exerted by each foot during push-off could be recorded. Further, the setup contained an additional camera (50 Hz) and a reference grid perpendicular to the axis of the camera for the extraction of sagittal plane kinematics from the video recording (figure 1). See D'Aout et al. (2001) for a detailed description of the catwalk.
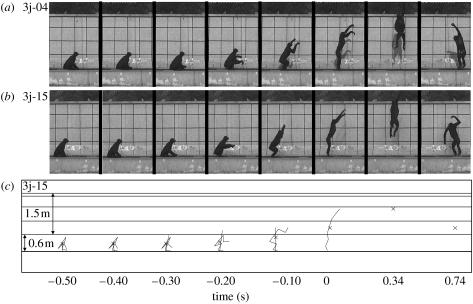
Figure 1.
(a), (b) Selected frames of jumps 3j-04 and 3j-15 showing the 0.5 s before toe-off (frames at 0.1-s intervals), toe-off, apex, landing. (c) Stick figures with ground reaction force vector and COM position (×) for jump 3j-15 at the same instants in time.
(b) Subjects
From a social group of eight bonobos, three individuals complied with the experiment: an adult male ‘Kidogo’ (age, 20 years; mass, 34 kg), a subadult male ‘Vifijo’ (age, 11 years; mass, 38 kg) and a subadult female ‘Djanoa’ (age, 10 years; mass, unknown). Only the first subject (Kidogo) performed jumps in the outdoor enclosure.
(c) Data processing
In the current study only jumps without run-up were considered, because in those jumps no horizontal kinetic energy can be transferred to raise the body centre of mass (COM), so all energy is delivered by the muscles during the push-off. Jump height, defined as vertical displacement of the COM during the airborne phase (from toe-off to the highest position), was estimated according to the flight time method (Bosco et al. 1983) for all bonobos. Flight time was determined from the high-speed video recordings.
In the outdoor enclosure, the ground reaction force and its point of application were recorded for the entire push-off for three jumps performed by one subject (Kidogo, jumps 3j-04, 3j-05 and 3j-15). For those jumps, jump height was also calculated from vertical take-off velocity of the COM, as obtained through time-integration of the vertical acceleration of COM, calculated from the vertical ground reaction force. Further, the ground reaction forces were used to calculate the change and rate of change in COM energy during the push-off (Henry et al. 2005). Since the sagittal plane kinematics could be extracted from the video recordings, all three push-offs were suitable for two-dimensional inverse dynamic analysis. Given the ground reaction force (filtered with a zero-lag fourth order Butterworth filter, cut-off frequency 30 Hz) and its point of application, the unknown net joint forces, and net joint moments ultimately responsible for the observed movement could be calculated by solving the equations of motion for each segment starting at the feet (e.g. Elftman 1939; Winter 1979; Aerts 1998). Power output at each joint was obtained by multiplying joint moment with joint angular velocity. Integration of joint power with respect to time yielded joint work.
For the inverse dynamic analysis, the subject was represented as a linkage of six rigid segments defined by the coordinates of the tip of the longest toe, ankle, knee, hip, shoulder, elbow and wrist (figure 1). The inertial properties of the segments (table 1) were estimated by scaling the segment parameters obtained from the cadaver dissected by Payne (2001, unpublished work) and are comparable to the segment parameters measured for a 33-kg chimpanzee by Crompton et al. (1996).
Table 1.
Inertial properties of the body segments for the bonobo, as used in the inverse dynamic analysis.
| segment | length (m) | mass (kg) | SCM (%)a | J (kg m2)b |
|---|---|---|---|---|
| feet | 0.17 | 1.41 | 53.8 | 0.0038 |
| shanks | 0.24 | 2.10 | 49.7 | 0.0062 |
| thighs | 0.27 | 4.89 | 51.3 | 0.0161 |
| trunk/head | 0.52 | 20.47 | 50.0 | 0.44 |
| upper arms | 0.26 | 2.52 | 43.7 | 0.0070 |
| forearms/hands | 0.23 | 2.61 | 45.0 | 0.0067 |
position of the segment centre of mass (SCM) relative to the proximal joint centre as a percentage of segment length.
Moment of inertia with respect to the SCM.
The pedobarometric and video data confirmed that the push-offs were symmetrical (asymmetries in arm movement were ignored), so that there was no need to consider left and right limb segments separately. In accordance with animal park policy, no markers were placed on the subject's body. Instead, anatomical landmarks were located through visual inspection, digitized manually (Didge Image Digitizing Software for Windows, courtesy of A. J. Cullum) and fitted with a polynomial (fourth order or higher). The reference grid provided by the ‘catwalk’ was used for calibration. The instant of toe-off was used for temporal synchronization of kinematics and forces. Joint angles were calculated as the difference between the segment angles of the proximal and the distal segment with respect to the right horizontal.
(d) Policy on animal testing
This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour website), the legal requirements of the country in which the work was carried out and all institutional guidelines.
(e) Human jumping
For comparison with the bonobos, four physically active male human subjects (age, 26±1 years; mass, 71.6±8.1 kg; height, 1.78±0.12 m) were asked to perform regular squat jumps from their preferred starting position (no counter movement, no arm swing). All subjects signed informed consent and reported to engage in various sport activities on a recreational level (i.e. volleyball, ice skating, climbing). Kinematics and ground reaction forces were recorded at 200 Hz (Northern Digital Inc. Optotrak 3020; Kistler 9281b). A representative jump by the best subject was processed analogously to the bonobo jumps. The subject's characteristics were: age, 27 years; mass, 61.5 kg; height, 1.68 m. Segment properties were estimated from segment length according to Winter (1979).
3. Results
Bonobo jump execution in terms of kinematics and ground reaction forces is illustrated in figure 1. The relevant aspects of the performance will be addressed below.
(a) Jump height
All bonobos achieved jump heights exceeding 0.7 m. The heights of the best squat jumps (without run-up) for each bonobo are listed in table 2, along with the performance of the best human subject and that of top level athletes (track and field sprinters and jumpers) as reported in the literature (Bosco et al. 1995; Rahmani et al. 2004). Where possible, jump heights calculated from the vertical ground reaction force are presented next to the jump heights calculated by the flight-time method.
Table 2.
Jump height as defined by the rise of the COM during the airborne phase for bonobos and humans. (Jump heights were calculated by the flight-time method and from the vertical ground reaction force, where available.)
| subject | jump height (flight-time; m) | jump height (force; m) |
|---|---|---|
| Djanoa | 0.73 | |
| Vifijo | 0.78 | |
| 0.78 | ||
| Kidogo | 0.77 | |
| 0.72 | ||
| 0.64 (3j-04) | 0.65 | |
| 0.67 (3j-15) | 0.67 | |
| 0.53 (3j-05) | 0.54 | |
| human | 0.32 | 0.34 |
| top level athletes | ||
| Bosco et al. (1995) | mean, 0.43; s.d., 0.05 | |
| Rahmani et al. (2004) | mean, 0.47; s.d., 0.04 |
(b) Work and power output
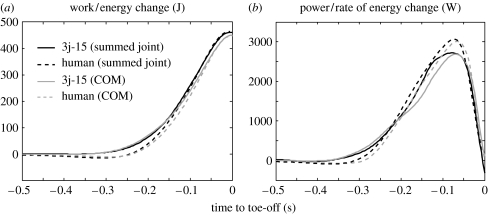
The total work and power output as a function of time (summed over all joints) as calculated from the inverse dynamic analysis for the highest bonobo jump analysed (3j-15) are shown in figure 2 together with the human data. Peak values of summed joint work and power for all jumps are given in tables 3 and 4. The change and rate of change in COM energy, derived directly from the measured ground reaction forces, are also shown in figure 2 (time-course) and tables 3 and 4 (peak values). The close match of the change and rate of change in COM energy with the sum of joint mechanical output as calculated by inverse dynamic analysis serves as a global indication for the validity of the inverse dynamic analysis because in vertical squat jumping, most of the mechanical output delivered by the muscles at the joints goes into (rate of) change of COM energy (Aerts 1998).
Figure 2.
Total joint work and joint power (summed over all joints) as calculated in the inverse dynamic analysis (black) for bonobo jump 3j-15 (solid) and a human squat jump (dashed). Change in COM energy and rate of change in COM energy as calculated from the ground reaction force (grey) for the same jumps.
Table 3.
Work (J) delivered during the push-off at individual joints (left and right taken together) and summed over all joints as well as the total change in COM energy (J) calculated from the ground reaction force (bracketed).
| ankles | knees | hips | arms (shoulders + elbows) | summed joint work (change in COM energy) | |
|---|---|---|---|---|---|
| bonobo | |||||
| 3j-04 | 42 | 30 | 293 | 50 | 414 (390) |
| 3j-05 | 46 | −25 | 270 | 84 | 377 (381) |
| 3j-15 | 62 | −9 | 341 | 66 | 460 (450) |
| human | 95 | 175 | 194 | 0 | 464 (447) |
Table 4.
Maximal power output (W) during the push-off at individual joints (left and right taken together) and summed over all joints as well as the maximal rate of change in COM energy (W) as calculated from the ground reaction force (bracketed).
| ankles | knees | hips | arms (shoulders + elbows) | summed joint power (rate of change in COM energy) | |
|---|---|---|---|---|---|
| bonobo | |||||
| 3j-04 | 890 | 313 | 2059 | 482 | 3054 (2667) |
| 3j-05 | 967 | 0 | 1975 | 702 | 2679 (2480) |
| 3j-15 | 1029 | 100 | 2080 | 457 | 2719 (2688) |
| human | 974 | 1515 | 1243 | 0 | 3064 (3008) |
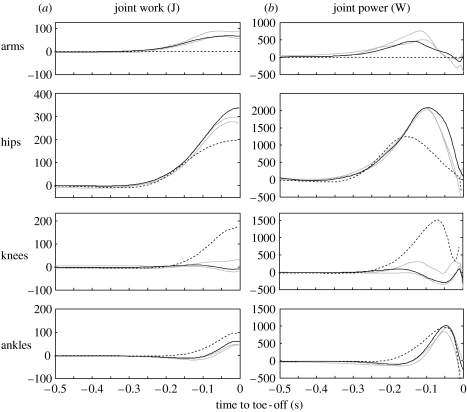
Figure 3 shows work and power output at the ankles, knees, hips and arms (shoulders and elbows combined) for the bonobo jumps and the human jump, and tables 3 and 4 present the peak values.
Figure 3.
Joint work and joint power output summed over left and right limbs for the bonobo jumps (solid) and for a human squat jump (dashed). The highest bonobo jump (3j-15) is in black; the other bonobo jumps (3j-04, 3j-05) are plotted in grey.
(c) Jump execution
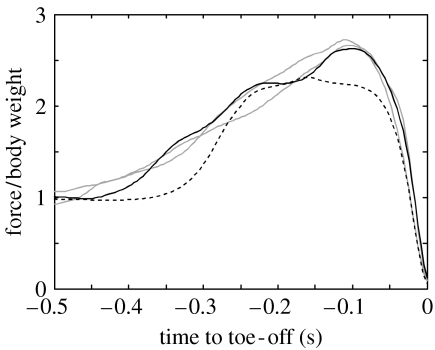
In figure 3 it can be seen that bonobo jumps, like the human jump, are characterized by a proximo-distal sequence of joint extension. The bonobo starts from an extremely low starting position, too low to allow for a counter movement, and raises its COM about 0.6 m prior to toe-off. For the human jump, the rise of the COM before toe-off was 0.4 m. Peak joint angular velocities in the bonobo jumps were 19.8±3.2, 17.4±1.1 and 14.0±2.7 rad s−1 for ankles, knees and hips, respectively. Figure 1 shows original video data for jumps 3j-04 and 3j-05 as well as stick figures for jump 3j-15. Ground reaction force is depicted as a vector in the stick figures for jump 3j-15. In figure 4, vertical ground reaction forces for all available bonobo jumps are shown as a time-series, normalized to body weight for comparison with the human data. For the bonobo jumps, vertical ground reaction force peaks at 2.6 times body weight, for the human jump the peak is at 2.3 times body weight.
Figure 4.
Vertical ground reaction forces normalized to body weight, for bonobo jumps (solid) and human squat jump (dashed). The highest bonobo jump (3j-15) is in black; the other bonobo jumps (3j-04, 3j-05) are plotted in grey.
4. Discussion
This study shows that untrained bonobos of various sex and age easily outperform even highly trained human athletes. It is not even known for sure if the bonobos' performance was maximal.
To explain the difference in performance between bonobo and human, the first step is to compare the mechanical output delivered during the push-off to the available jumping muscle mass. Subsequently, the amount of specific work (work delivered per kilogram muscle) can be calculated for both species to reveal differences in the intrinsic work-producing capabilities of the muscle tissue. In the same way, the maximal specific power (peak power output per kilogram muscle) can be calculated. Note that unlike specific work, specific power calculated in this way can be influenced by elastic energy storage. With the species currently under study (bonobo, human), however, amplification of power output through elastic energy storage is expected to play a minor role, so that most power is likely to be delivered directly by the muscle fibres. There are no indications for power-amplifying mechanisms as found in specialized jumpers; this coincides with the fact that elastic tendinous or aponeurotic structures are not very conspicuous (e.g. the bonobo barely has an Achilles' tendon; Payne 2001).
Surprisingly, the mechanical output delivered during the push-off, estimated by the total change and the peak rate of change in mechanical energy of the COM (as calculated from the ground reaction forces), was similar in bonobo and human despite their difference in size: about 450 J and close to 3000 W, respectively (tables 3 and 4). Values for human squat jumping found in this study were similar to those reported in other studies for similar subjects in terms of body mass, height and age (Cavagna et al. 1971; Hubley & Wells 1983).
Given that the bonobo is substantially smaller than the human (34 versus 61.5 kg), it is expected that, in an absolute sense, the bonobo has less jumping muscle mass than the human. This is confirmed by anatomical studies (Thorpe et al. 1999; Payne et al. in press a). A list of the main hindlimb extensors and their estimated mass for the bonobo and the human subject are given in table 5. Muscle masses for the bonobo were taken in a dissection of a fresh 30-year-old male bonobo who died of heart failure and was fully active up until a week before death (Payne et al. in press a) and scaled to the body mass of the current subject. As far as we know, this is the only publication that reports masses of individual bonobo muscle groups. Muscle masses for the human subject were obtained from Thorpe et al. (1999), presenting MRI data for healthy human subjects. The total estimated mass of the hindlimb extensors is 9.54 kg for the human and only 3.79 kg for the bonobo.
Table 5.
Estimated hindlimb extensor mass (kg), summed over left and right hindlimbs, in a 34-kg bonobo, a 61.5-kg human and a 37-kg chimpanzee.
| muscle group | function (sagittal plane) | 34-kg bonobo | 61.5-kg human | 37-kg chimpanzee (Thorpe et al. 1999) |
|---|---|---|---|---|
| gluteals (m. gluteus maximus, m. gluteus medius, m. gluteus minimus, m. scansorius) | hip extension | 0.95 | 3.08 | 1.28 |
| hamstrings (m. biceps femoris, m. semitendinosus, m. semimembranosus) | hip extension, knee flexion | 0.50 | 1.69 | 0.60 |
| quadriceps (m. rectus femoris, mm. vasti) | knee extension, hip flexion (m. rectus femoris) | 0.97 | 3.23 | 1.10 |
| triceps surae (m. gastrocnemius, m. soleus, m. plantaris) | ankle extension, knee flexion | 0.54 | 1.54 | 0.57 |
| adductors (m. adductor magnus, m. adductor longus, m. adductor brevis) | hip extension (only in apes) | 0.83 | 1.06 | |
| total | 3.79 | 9.54 | 4.61 |
To account for the similar work and power output (450 J and 3000 W, respectively), the human hindlimb muscles must deliver 47 J kg−1 and 314 W kg−1, respectively. For the bonobo hindlimb muscles, this is 119 J kg−1 and 792 W kg−1, respectively. Either the bonobo can generate more than twice the work and power per unit of muscle mass compared with the human or the bonobo involves a considerable amount of muscle mass other than hindlimb extensors. At first sight, the second option seems reasonable; after all, the bonobo might have small hindlimbs, but its forelimbs are very well developed compared to humans (Payne 2001). For humans, it has been shown that the arm swing can increase jump height by 21% (Harman et al. 1990). However, the inverse dynamic analysis revealed that the mechanical output at the hips, rather than at the arms, is the key to bonobo jumping (figure 3). In fact, it is interesting to note that the mechanical output at the knee was near zero in bonobo jumping. The net moment at the knee remained close to zero, because the length of the hindlimb segments is such that the ground reaction force vector passed close to the knee during the entire push-off (figure 1) and, hence, work output at the knee is negligible. The bonobo had to coordinate its push-off in this way because a large knee extension moment would result in an undesirable backward acceleration (Bobbert & Van Zandwijk 1999). It is highly unlikely, however, that the bonobo did not use its knee extensors. The quadriceps is the bonobo's largest hindlimb muscle and well suited to produce work and power. Presumably, the mechanical output of the quadriceps was transferred to the hip by the biarticular hamstrings, which, when coordinated appropriately, are the only muscles that can counteract the quadriceps knee extension moment without dissipating energy in a lengthening contraction. Interestingly, in human jumping, there is no evidence for transfer of mechanical work and power from the knee to the hip (Pandy & Zajac 1989).
To eliminate any effect of the arm swing on the calculation of the muscle-mass-specific mechanical output, let us focus on the mechanical output at the hips and the muscle mass available to actuate the hip joints. At the hip joint level, the bonobo produced ca 300 J and a peak power output of ca 2000 W. The estimated mass of the muscles that actuate the hip directly or indirectly via biarticular muscles (gluteals, hamstrings, adductors and quadriceps, see table 5) is 3.25 kg. This yields a specific work of 92 W kg−1 and a specific power of 615 W kg−1, which is still twice as much as human specific work and power output.
Before putting these results in context with other literature on muscle mechanics and jumping animals, some possible explanations for the above findings are discussed:
Mechanical output at the hip joint is overestimated in the inverse dynamic analysis which uses a rigid trunk model while, in reality, the trunk was extending;
the bonobo's hip and knee extensor muscle mass is extremely underestimated for the individual in this study;
bonobo and human have similar muscle properties but humans can only recruit a fraction of their muscle mass voluntarily;
the properties of bonobo muscle are different from human muscle properties so that the same mechanical output can be achieved with a smaller muscle volume (i.e. higher force per cross-sectional area or higher maximal shortening velocity in fibre lengths per second).
These possibilities are discussed point by point:
it is possible that the work and power output calculated at the hips is overestimated due to trunk extension: a rigid trunk was used in the inverse dynamic analysis, while in reality the trunk was extending during push-off. The position of the shoulder was used to mark the endpoint of the trunk segment, and the movement of the shoulder with respect to the hip that was due to trunk extension may have led to an overestimation of hip joint extension angle by a few degrees. In this way, work and power delivered by the trunk extensor muscles may have been attributed to the hip. Hip joint moment, however, is not influenced by the rigid trunk assumption. Also, take-off velocity of the COM when calculated from the ground reaction force (exerted by a real bonobo with extending trunk) differed by no more than 0.09 m s−1 from the take-off velocity of the COM in the rigid-segment model, which indicates that rigidity of the segments is an adequate assumption. Even though the mass of the trunk extensor muscles in bonobo is not known, it is unlikely that trunk extension unintentionally accounts for the high mechanical output at the hips;
the bonobo in this study (body mass 34 kg) was much smaller than the human subject (61.5 kg). Geometric scaling alone predicts that the hindlimb muscle mass in the bonobo is about half of the human hindlimb muscle mass. Additionally, the hindlimbs of bonobos (and chimpanzees) weigh less in relation to their total body mass than do human legs (Crompton et al. 1996). Hence, it is in line with expectation that the bonobo has substantially less hindlimb muscle mass than the human subject. Unscaled data for a 37-kg a male chimpanzee, as presented in table 5, also agree well with the estimated bonobo muscle mass;
while the fraction of muscle mass that can be voluntarily recruited during squat jumping is not known, it has been reported that humans can voluntarily activate more than 90% of their quadriceps muscle fibres in isometric and slow shortening contractions (Beltman et al. 2004). At present, there is no reason to believe that the activation level is substantially lower in other muscles or during jumping;
since possibilities (i–iii) are unlikely to account for the difference between the specific work and power delivered by bonobo and human muscle, possibility (iv) is the last available option: bonobo muscle properties substantially differ from human muscle properties.
Without further experiments, there are arguments in favour and against proposition (iv).
The specific work output of 92 J kg−1 for the bonobo is much higher than one would expect form previous studies on jumping animals (Peplowski & Marsh 1997; Aerts 1998; Henry et al. 2005). The bonobo's specific power of 615 W kg−1, presumably achieved by direct muscle action, is also very high, although higher specific power outputs have recently been measured in vitro for lizards (Curtin et al. 2005). On the other hand, the bonobo's jumping performance has been established and it cannot be attributed to forelimb muscle mass because the problem manifests itself at the level of the hips, where most of the mechanical output is generated. Unfortunately, it is impossible to calculate the muscle force or muscle fibre shortening velocity of individual muscles from the available data, but there is a good basis for speculation. Chimpanzee and bonobo morphology is characterized by relatively long muscle fibres and relatively short muscle moment arms (except in the adductors), presumably an adaptation to exert force over a wide range of motion as evidenced by their prehensile limbs (Thorpe et al. 1999; Payne et al. in press b). Combining the short muscle moment arms with the peak joint angular velocities that occur during bonobo jumping, which are similar to those of human jumping, it is speculated that muscle fibre shortening velocities are not impressively high. To explain the two-fold difference in specific power output with respect to human muscle tissue, the force delivered at a certain shortening velocity must have been very high. Higher muscle force at a given fibre shortening velocity can be explained by a two-fold difference in the maximal force per cross-sectional area (specific force) or a two-fold difference in the maximal shortening velocity (vmax, in fibre optimum lengths per second), or a combination both. Across species, vmax seems to be more variable than the specific force (Medler 2002), with a tendency for higher vmax in smaller animals. The observations by Bauman (1923, 1926) and Edwards (1965), however, where chimps outperformed humans in isometric tasks (leg press, one-armed pull), clearly favours a difference in specific force.
To summarize, this study offers strong evidence that in an explosive task, bonobo muscle performs superiorly to human muscle, most likely due to a higher specific force. Whether the difference is due to higher density of contractile material or due to differences in the contractile machinery per se (i.e. myosin heavy chain isoform) remains to be investigated.
Acknowledgments
We gratefully acknowledge Katleen Huyghe and the caretakers at the animal park ‘Planckendael’ for their assistance and cooperation during the measurements. This study was supported by the Fund for Scientific Research-Flanders and by the Flemish government through the Centre for Research and Conservation (KMDA, Antwerp).
References
- Aerts P. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Phil. Trans. R. Soc. B. 1998;353:1607–1620. doi:10.1098/rstb.1998.0313 [Google Scholar]
- Bauman J.E. The strength of the chimpanzee and orang. The Sci. Monthly. 1923;16:432–439. [Google Scholar]
- Bauman J.E. Observations on the strength of the chimpanzee and its implications. J. Mammal. 1926;7:1–9. [Google Scholar]
- Beltman J.G.M, Sargeant A.J, van Mechelen W, de Haan A. Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. J. Appl. Physiol. 2004;97:619–626. doi: 10.1152/japplphysiol.01202.2003. doi:10.1152/japplphysiol.01202.2003 [DOI] [PubMed] [Google Scholar]
- Bobbert M.F, Van Zandwijk J.P. Dynamics of force and muscle stimulation in human vertical jumping. Med. Sci. Sports Exerc. 1999;31:303–310. doi: 10.1097/00005768-199902000-00015. doi:10.1097/00005768-199902000-00015 [DOI] [PubMed] [Google Scholar]
- Bosco C, Luhtanen P, Komi P.V. A simple method for measurement of mechanical power in jumping. Eur. J. Appl. Physiol. Occup. Physiol. 1983;50:273–282. doi: 10.1007/BF00422166. doi:10.1007/BF00422166 [DOI] [PubMed] [Google Scholar]
- Bosco C, et al. A dynamomenter for evaluation of dynamic muscle work. Eur. J. Appl. Physiol. Occup. Physiol. 1995;70:379–386. doi: 10.1007/BF00618487. doi:10.1007/BF00618487 [DOI] [PubMed] [Google Scholar]
- Cavagna G.A, Komarek L, Cittero G, Margaria R. Power output of the previously stretched muscle. In: Vredenbregt J, Wartenweiler J, editors. Biomechanics II. S. Karger; Basel, Switzerland: 1971. pp. 159–167. [Google Scholar]
- Crompton R.H, Li Y, Günther M.M, Alexander R.M. Segment inertial properties of primates: new techniques for laboratory and field studies of locomotion. Am. J. Phys. Anthropol. 1996;99:547–570. doi: 10.1002/(SICI)1096-8644(199604)99:4<547::AID-AJPA3>3.0.CO;2-R. doi:10.1002/(SICI)1096-8644(199604)99:4<547::AID-AJPA3>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Curtin N.A, Woledge R.C, Aerts P. Muscle directly meets the vast power demands in agile lizards. Proc. R. Soc. B. 2005;272:581–584. doi: 10.1098/rspb.2004.2982. doi:10.1098/rspb.2004.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aout K, Aerts P, De Clercq D, Schoonaert K, Vereecke E, Van Elsacker L. Studying bonobo (Pan paniscus) locomotion using an integrated setup in a zoo environment: preliminary results. Primatologie. 2001;4:191–206. [Google Scholar]
- Edwards W.E. Factors in relative strength of ape and man. Am. J. Phys. Anthropol. 1963;21:413. [Google Scholar]
- Edwards W.E. Holloman Air Force Base, N.M.: 6571st Aeromedical Research Laboratory; Holloman, New Mexico: 1965. Study of monkey, ape and human morphology and physiology relating to strength and endurance. Phase IX: The strength testing of five chimpanzee and seven human subjects. [Google Scholar]
- Elftman H. Forces and energy changes in the leg during walking. Am. J. Physiol. 1939;125:339–356. [Google Scholar]
- Finch G. The bodily strength of chimpanzees. J. Mammal. 1943;24:224–228. [Google Scholar]
- Gardner R.A, Gardner B.T. Teaching sign language to a chimpanzee. Science. 1969;165:664–672. doi: 10.1126/science.165.3894.664. [DOI] [PubMed] [Google Scholar]
- Harman E.A, Rosenstein M.T, Frykman P.N, Rosenstein R.M. The effects of arms and countermovement on vertical jumping. Med. Sci. Sports Exerc. 1990;22:825–833. doi: 10.1249/00005768-199012000-00015. [DOI] [PubMed] [Google Scholar]
- Henry H.T, Ellerby D.J, Marsh R.L. Performance of guinea fowl numida meleagris during jumping requires storage and release of elastic energy. J. Exp. Biol. 2005;208:3293–3302. doi: 10.1242/jeb.01764. doi:10.1242/jeb.01764 [DOI] [PubMed] [Google Scholar]
- Hubley C.L, Wells R.P. A work–energy approach to determine individual joint contributions to vertical jump performance. Eur. J. Appl. Physiol. Occup. Physiol. 1983;50:247–254. doi: 10.1007/BF00422163. doi:10.1007/BF00422163 [DOI] [PubMed] [Google Scholar]
- Medler S. Comparative trends in shortening velocity and force production in skeletal muscles. Am. J. Physiol. 2002;283:R368–R378. doi: 10.1152/ajpregu.00689.2001. [DOI] [PubMed] [Google Scholar]
- Nagano A, Umberger B.R, Marzke M.W, Gerritsen K.G.M. Neuromuscular computer modeling and simulation of upright, straight-legged, bipedal locomotion of Australopithecus afarensis (A.L 288-1) Am. J. Phys. Anthropol. 2005;126:2–13. doi: 10.1002/ajpa.10408. doi:10.1002/ajpa.10408 [DOI] [PubMed] [Google Scholar]
- Pandy M.G, Zajac F.E. Dependence of jumping performance on muscle strength, muscle-fiber speed, and tendon compliance. In: Stein J.L, Ashton-Miller J.A, Pandy M.G, editors. Issues in the modeling and control of biomechanical systems, 1989 ASME winter annual meeting in San Francisco. The American Society of Mechanical Engineers; New York, NY: 1989. [Google Scholar]
- Payne, R. C. 2001 Musculoskeletal adaptations for climbing in hominoids and their role as adaptions for the acquisition of bipedalism. PhD thesis. Department of Human Anatomy and Cell Biology, The University of Liverpool.
- Payne, R. C., Crompton, R. H., Isler, K., Savage, R., Vereecke, E. E., Günther, M. M., Thorpe, S. K. S. & D'Août, K. In press a Morphological analysis of the hindlimb in apes and humans. Part I: comparative anatomy. J. Anat [DOI] [PMC free article] [PubMed]
- Payne, R. C., Crompton, R. H., Isler, K., Savage, R., Vereecke, E. E., Günther, M. M., Thorpe, S. K. S. & D'Août, K. In press b Morphological analysis of the hindlimb in apes and humans. Part II: moment arms. J. Anat [DOI] [PMC free article] [PubMed]
- Peplowski M.M, Marsh R.L. Work and power output in the hindlimb muscle of cuban tree frogs osteopilus septentrionalis during jumping. J. Exp. Biol. 1997;200:2861–2870. doi: 10.1242/jeb.200.22.2861. [DOI] [PubMed] [Google Scholar]
- Rahmani A, Locatelli E, Lacour J.-R. Differences in morphology and force/velocity relationship between Senegalese and Italian sprinters. Eur. J. Appl. Physiol. Occup. Physiol. 2004;91:399–405. doi: 10.1007/s00421-003-0989-x. doi:10.1007/s00421-003-0989-x [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh S.E, Shanker S.G, Taylor T.J. Apes, language and the human mind. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Sellers W.I, Cain G.M, Wang W, Crompton R.H. Stride lengths, speed and energy cost in walking of Australopithecus afarensis: using evolutionary robotics to predict the locomotion of early human ancestors. J. R. Soc. Interface. 2005;2:431–441. doi: 10.1098/rsif.2005.0060. doi:10.1098/rsif.2005.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S.K.S, Crompton R.H, Günther M.M, Ker R.F, Alexander R.M. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (pan troglodytes) Am. J. Phys. Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. doi:10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Winter D.A. Biomechanics of human movement. Wiley; New York, NY: 1979. [Google Scholar]
- Yerkes R.M. Chimpanzees: a laboratory colony. Yale University Press; New Haven, CT: 1943. [Google Scholar]
- Zihlman A.L. Body build and tissue composition in Pan paniscus and Pan troglodytes, with comparison to other hominoids. In: Susman R.L, editor. The pygmy chimpanzee. Plenum Press; New York, NY: 1984. pp. 179–200. [Google Scholar]