Abstract
The transmembrane ubiquitin ligase K5/MIR2 of Kaposi sarcoma herpesvirus (KSHV) mediates internalization and lysosomal degradation of glycoproteins involved in antigen presentation and co-stimulation. In endothelial cells (ECs), K5 additionally reduced expression of CD31/platelet–endothelial cell adhesion molecule (PECAM), an adhesion molecule regulating cell-cell interactions of ECs, platelets, monocytes, and T cells. K5 also reduced EC migration, a CD31-dependent process. Unlike other K5 substrates, both newly synthesized and pre-existing CD31 molecules were targeted by K5. K5 was transported to the cell surface and ubiquitinated pre-existing CD31, resulting in endocytosis and lysosomal degradation. In the endoplasmic reticulum, newly synthesized CD31 was degraded by proteasomes, which required binding of phosphofurin acidic cluster sorting protein-2 (PACS-2) to acidic residues in the carboxyterminal tail of K5. Thus, CD31, a novel target of K5, is efficiently removed from ECs by a dual degradation mechanism that is regulated by the subcellular sorting of the ubiquitin ligase. K5-mediated degradation of CD31 is likely to affect EC function in KS tumors.
Introduction
Kaposi sarcoma (KS), the most common AIDS-associated malignancy, is characterized by disorganized networks of abnormal microvasculature composed of spindle-shaped cells of endothelial cell (EC) origin.1 KS herpesvirus (KSHV) is consistently found in KS lesions, suggesting that infection with KSHV is a necessary, but not sufficient, prerequisite for the development of KS.2 KSHV belongs to the family of γ2-herpesviruses, or Rhadinoviruses, which includes tumorigenic viruses of primates and rodents.3 In addition to a generally conserved genomic organization and conservation of essential genes, this group of viruses also shares the characteristic of encoding genes pirated from the genomes of their hosts. Examples are KSHV-encoded homologs of cellular CD21, CD200, chemokines, IL-6, BCL-2, interferon regulatory factors, FLICE inhibitory protein (FLIP), cyclin D, and several DNA synthetic enzymes.2 These cellular homologs function predominantly in host-virus interactions (eg, regulating viral transformation of the host cell as well as modulation of the host's immune response to the virus).4
Sequence analysis of 2 related open reading frames (ORFs) in the KSHV genome, K3 and K5, indicated that these genes are also host derived.5 Studies from a number of laboratories indicated that K3 and K5 function as immunomodulators (reviewed in Früh et al6), hence their alias as modulators of immune recognition (MIR).7 K3 (MIR1) and K5 (MIR2) are transmembrane-spanning ubiquitinligases that mediate the ubiquitination of cytoplasmic lysines or cysteines of other transmembrane proteins.7,8 Both K3 and K5 target major histocompatibility complex class I (MHC I) molecules, thereby inhibiting presentation of viral antigen to cytotoxic T cells.9,10 Similarly, the murine gammaherpesvirus 68 (MHV68), which contains the single K3-related ORF MK3, inhibits antigen presentation to T cells, and deletion of MK3 affects the establishment of viral latency due to increased surveillance by CD8+ T cells.11-13 Despite their sequence similarity and similar genomic localization, the molecular mechanisms by which the KSHV or MHV68 K3-related ORFs target MHC I seem to be very different. Ubiquitination of MHC I by either KSHV-K3 or KSHV-K5 results in their endocytosis and destruction in lysosomes via the multivesicular body pathway.9,14-16 In contrast, MK3 becomes an integral part of the peptide-loading complex where it ubiquitinates not only MHC I, but also other members of this complex, including the peptide transporter TAP and the chaperone tapasin, all of which are subsequently destroyed by the proteasome (reviewed in Lybarger et al17). It is not known why 2 related viruses that express related immunomodulators and target similar substrates use such divergent intracellular routes of destruction. A possibility that is supported here is that the subcellular targeting of the ubiquitin ligase determines the selection of the substrate as well as the degradative pathway.
Essential for the ubiquitin ligase function of K3 and K5 is an N-terminal RING domain that diverges in sequence, but not in structure, from the canonical RING and RING-H2 domains.18 This so-called RING-CH domain is found in all eukaryotic genomes, including yeast.19 Homologs in the human genome, called membrane-associated RING-CH (MARCH) proteins, or c-MIR, seem to function similarly to their viral counterparts since overexpression of these homologs results in the internalization of ubiquitinated target proteins.20,21 While the KSHV-K3 protein seems to specifically target MHC I–like molecules, K5 also targets the costimulatory molecules B7.2 and ICAM-1.22-24
Understanding the mechanisms by which KSHV perturbs the characteristics of ECs is crucial for a better appreciation of KS etiology and the development of novel therapies. Such studies have been greatly facilitated by the development of in vitro models based on infecting immortalized or primary dermal microvascular endothelial cells (DMVECs).25-27 Adhesive interactions between ECs are essential for maintaining the integrity of the vascular lining. An important regulator of EC-EC adhesion is the platelet–endothelial cell adhesion molecule 1 (PECAM-1), or CD31, which is abundantly expressed on ECs.28 CD31 is also expressed on monocytes, neutrophils, platelets, and T-cell subpopulations. Homophilic interaction of CD31 molecules facilitates not only the formation of intercellular junctions between ECs, but also the adhesion of CD31-positive leukocytes to the vasculature and the ensuing transmigration, or diapedesis, of leukocytes across the vessel wall.29 CD31 is a signal-transducing transmembrane glycoprotein with an extensive cytoplasmic tail containing immunoregulatory tyrosine-based activation (ITAM) and potentially inhibiting (ITIM) motifs.30 Any regulation of CD31 expression by KSHV is therefore bound to have a profound effect on the vascular architecture. There are, however, conflicting data with respect to CD31 expression in KSHV-infected ECs, a finding that likely reflects subtle differences in virus-host dynamics in the different cell culture system used. For example, latent KSHV infection of DMVECs immortalized with the E6 and E7 proteins of human papilloma virus (HPV) (E-DMVECs) does not alter CD31 surface expression.31 In contrast, KSHV-infected DMVECs that are life-extended by telomerase transfection (TIME-DMVECs) show a profound down-regulation of CD31, suggesting that a different viral gene expression program predominates in infected TIME-DMVECs.32 We now demonstrate that CD31 is removed from the EC surface by the KSHV immediate early gene product K5, a finding that helps to resolve the discrepancies noted in different systems. K5 employs a vesicular targeting mechanism and both major cellular degradation pathways to completely eliminate CD31 from ECs. Given the pivotal role of CD31 in EC biology, our findings implicate K5 in the tumorigenesis of KS.
Materials and methods
Viruses and cell culture
KSHV-infected E-DMVECs were established and maintained as described previously.25 Primary DMVECs (Cambrex, Charles City, IA) were infected at 1:100 with KSHV in 1 mL OptiMEM (Invitrogen, Carlsbad, CA) and spun at 1000 rpm for 2 hours at 20°C, after which the inoculum was removed and replenished with fresh medium. Recombinant adenovirus (Ad) expressing WT-PACS-1, DN-PACS-1, WT-PACS-2, DN-PACS-2, DYNK44A, and DN-AP2 were described.33-35 CD31 plasmid was a kind gift from Dr J. A. Madri (Yale University School of Medicine).36 Ad expressing N-terminally (MK3, K3) or C-terminally (K5) FLAG-tagged inserts under a Tet-transactivator (tTA)–dependent promoter were generated using a plasmid-based recombinant system.37 tTA-expressing AdTet was obtained from Dr D. Johnson (Department of Microbiology and Immunology, Oregon Health and Science University).38
Antibodies and reagents
Anti–human CD31 monoclonal antibody JC/704 (DAKO, Carpinteria, CA) was used in cytometry and immunofluorescence assay (IFA). Anti-CD31 (clone H-300), used for immunoprecipitation experiments, and antiubiquitin (clone P4D1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–human human leukocyte antigen (HLA) W6/32, anti-FLAG antibody, either unconjugated or conjugated to FITC or phycoerythrin (PE), were purchased from Sigma (St Louis, MO). K5-specific monoclonal antibody 328C7 was obtained from Drs Koichi Yamanishi and Keiji Ueda (Osaka University Medical School, Japan).39 A monoclonal antibody to ORF59 was a kind gift from Bala Chandran (Department of Microbiology and Immunology, Rosalind Franklin University). Concanamycin A (Sigma), lactacystin, and MG132 (both from Boston Biochem, Cambridge, MA) were used at final concentrations of 50 nM, 50 μM, and 20 μM, respectively. Cells were treated with 10 μg/mL of brefeldin A (MP Fine Chemicals; MP Biochemicals, Solon, OH). Protein A or G beads were obtained from Santa Cruz Biotechnology. Phorbol 12-myristate 13-acetate (PMA) was obtained from Sigma and used at 20 ng/mL.
Cell-surface protein biotinylation and purification
Biotinylation with EZ-Link TM NHS-SS_Biotin was performed according to the manufacturer's protocol (Pierce, Rockford, IL). Briefly, cells were washed 3 times in ice-cold phosphate-buffered saline (PBS) and proteins at the cell surface were labeled with Sulfo-NHS-SS-Biotin for 30 minutes at 4°C. The cells were washed and either lysed immediately or incubated with medium for 24 hours at 37°C before lysing with a nonionic detergent. Labeled proteins were isolated with immobilized NeutrAvidin Gel (Pierce, Rockford, IL). The bound proteins were released by incubating the resin with SDS-PAGE sample buffer containing 50 mM DTT.
Flow cytometry and IFA
For flow cytometric analysis of surface proteins, E-DMVECs were removed from tissue culture dishes with 0.05% trypsin-EDTA (Invitrogen), washed with ice-cold PBS, and incubated with primary antibodies for 30 min at 4°C. After several washes in PBS, cells were incubated with PE-conjugated goat antimouse secondary antibody (DAKO) and washed again before analysis with a BD Biosciences FACScalibur flow cytometer (Palo Alto, CA). For intracellular staining, trypsinized and washed cells were fixed in 2% paraformaldehyde for 15 minutes at room temperature (RT) followed by 3 washes in a permeabilizing solution (1% saponin, 10% NaN3, 10% fetal calf serum [FCS], in PBS). Cells were incubated with antibodies as described for flow cytometric analysis but washed in permeabilizing solution. IFA was performed as previously described.20 Images were captured with a Zeiss Axioskop 2 microscope and an AxioCam (Carl Zeiss, Göttingen, Germany). Coverslips were mounted on slides and covered with Vectashield H-1200 plus DAPI. All pictures were taken in monochrome and contrast-enhanced and artificially colored by using Openlab 4.0.3 software (Improvision, Lexington, MA). For Figures 1C, 2B, and 7A and C, a 40×/0.75 NA oil objective was used; for Figure 3A, a 10×/0.30NA objective was used. Appropriate specificity controls were included for all experiments.
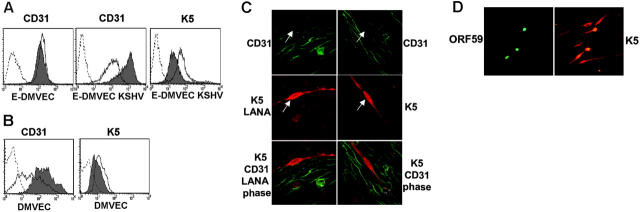
Figure 1.
Reduced CD31 expression on KSHV infected E-DMVECs correlates with spontaneous or PMA-induced expression of K5. (A) Flow cytometry of CD31 and K5 expression in uninfected (E-DMVEC) or infected cells (E-DMVEC KSHV) treated with PMA (dark line, no shading) or untreated (gray shading) for 48 hours. Cell-surface–expressed CD31 was detected on intact cells using monoclonal antibody JC/704; the dotted line indicates fluorescently labeled secondary antibody only. To reveal K5 expression, KSHV-infected cells or uninfected E-DMVECs (dotted line) were permeabilized and stained with K5-specific antibody 328C7 as well as fluorescently labeled secondary antibody. (B) Flow cytometry of CD31 or K5 expression of uninfected primary DMVECs ( ) or primary DMVECs 48 hours after infection with KSHV (dark line, no shading). (C) Spontaneous K5 expression in latently infected E-DMVECs in the absence of PMA. Left column shows KSHV-infected E-DMVECs stained for CD31 (green), K5 (red), and LANA-1 (red). LANA-1 staining is evident by a typical punctate nuclear pattern. Right column shows staining as in the left column, but without antibodies to LANA-1. The arrow indicates a K5-expressing cell. Note the lack of CD31 expression on K5-expressing cells, whereas LANA-expressing, but K5-negative, cells still express CD31. (D) Simultaneous evaluation of K5 and ORF59 in KSHV-infected DMVECs after PMA induction (48 hours). Monolayers were stained for expression of K5 and ORF59 using specific antibodies and isotype-specific secondary antibodies (Alexa 488 IgG1 for ORF59 and Alexa 594 IgG2b for K5). All cells expressing the IE protein K5 expressed the E protein Orf59, but 4 to 5 times as many cells expressed K5 only. Similar ratios, but lower absolute numbers, were observed in the absence of PMA (not shown).
) or primary DMVECs 48 hours after infection with KSHV (dark line, no shading). (C) Spontaneous K5 expression in latently infected E-DMVECs in the absence of PMA. Left column shows KSHV-infected E-DMVECs stained for CD31 (green), K5 (red), and LANA-1 (red). LANA-1 staining is evident by a typical punctate nuclear pattern. Right column shows staining as in the left column, but without antibodies to LANA-1. The arrow indicates a K5-expressing cell. Note the lack of CD31 expression on K5-expressing cells, whereas LANA-expressing, but K5-negative, cells still express CD31. (D) Simultaneous evaluation of K5 and ORF59 in KSHV-infected DMVECs after PMA induction (48 hours). Monolayers were stained for expression of K5 and ORF59 using specific antibodies and isotype-specific secondary antibodies (Alexa 488 IgG1 for ORF59 and Alexa 594 IgG2b for K5). All cells expressing the IE protein K5 expressed the E protein Orf59, but 4 to 5 times as many cells expressed K5 only. Similar ratios, but lower absolute numbers, were observed in the absence of PMA (not shown).
Figure 2.
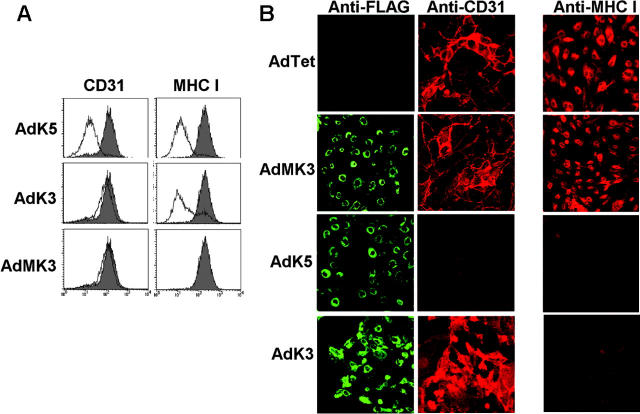
K5 inhibits CD31 expression. (A) E-DMVECs infected with AdK5, AdK3, AdMK3, or AdTet control were analyzed for CD31 or MHC I expression by flow cytometry. The shaded graph represents AdTet-infected cells, whereas the unshaded graph represents cells transduced with the indicated adenovirus constructs. (B) At 24 hours after transduction with indicated viruses, E-DMVECs were stained with fluorescently labeled FLAG antibody to detect MK3, K5, or K3 (left panel; green) and costained for CD31 (middle panel, red) or MHC I (right panel; red).
Figure 7.
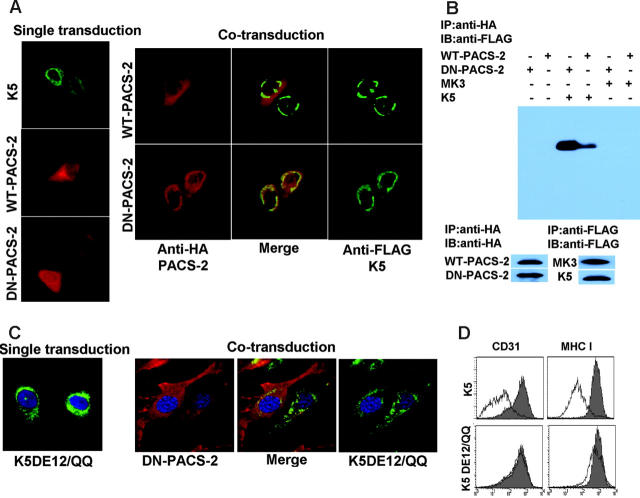
PACS-2 interacts with acidic clusters in the carboxyterminus of K5. E-DMVECs were transduced with the indicated recombinant Ad constructs either alone (left panel) or in combination (right panel). After 24 hours of infection, cells were permeabilized and K5 was detected using anti-FLAG (green), whereas PACS-2 was visualized using anti-HA (red). Note that DN-PACS-2 localization changes from cytoplasmic to perinuclear in cells infected with AdK5. Merging the images (center) indicates colocalization of DN-PACS-2, but not WT-PACS-2, with K5. (B) CD31 was immunoprecipitated using anti-HA from E-DMVECs transduced with the indicated adenovirus constructs. Coprecipitated K5 was detected using anti-FLAG by immunoblotting the PACS-2 preciptiates. In contrast, MK3 was absent. Control blots using anti-FLAG and anti-HA antibodies confirm expression of the respective proteins. (C) DN-PACS-2 (red) does not colocalize with K5DE12/QQ (green). (D) CD31 and MHC I expression at the cell surface was monitored by flow cytometry at 24 hours after transduction of E-DMVECs with AdK5 or AdK5DE12 (unshaded). AdTet-infected cells were used as positive control (shaded).
Figure 3.
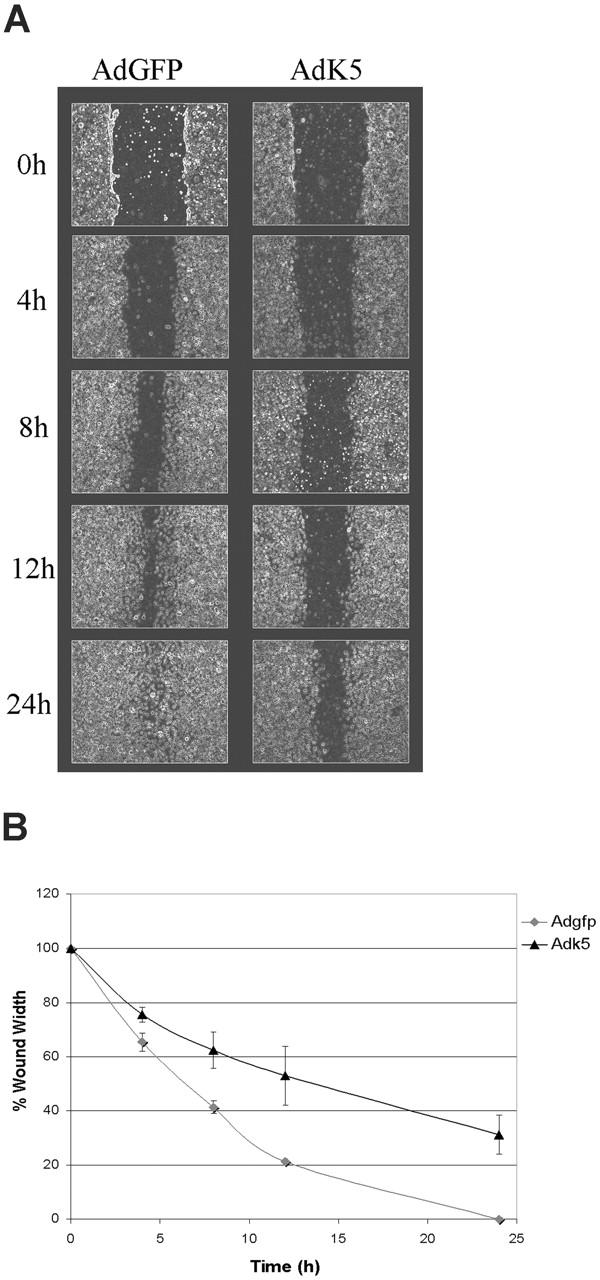
K5 inhibits migration of endothelial cells. HUVECs were transduced with AdEGFP or AdK5 as described in “Materials and methods.” The monolayers were scratched using a pipet tip and the resealing of the wounds was monitored over the indicated time points by phase microscopy. (A) Photographs were taken at regular intervals of the identical region of a representative part of each wound. (B) The width of each wound was measured and plotted as the percentage of the initial wound width versus time. The average of 3 independent experiments is shown.
Infection, metabolic labeling, immunoprecipitation, and immunoblotting
E-DMVECs grown in 100-mm tissue culture dishes were infected with Ad constructs at a multiplicity of infection (MOI) of 250 plaque-forming units (PFUs) per cell. For tTA-dependent constructs, AdTet was included at a 1:5 ratio. At 20 hours after infection, cells were labeled and immunoprecipitated as previously described.40 For immunoblotting, the WesternBreeze chemiluminescence detection system (Invitrogen) was used following semidry transfer to polyvinylidene difluoride membranes (Millipore, Billerica, MA).
Wound-healing assay
Human umbilical vein endothelial cells immortalized with E6 and E7 from human papilloma virus (E-HUVECs) were plated in 12-well dishes and grown to confluency. The cells were coinfected with AdK5 and AdTet, or AdEGFP and AdTet, each at an MOI of 100 in serum-free medium (Human Endo-SFM; Invitrogen) in the presence of 4 μg/mL polybrene for 2 hours at 37°C. The inoculum was replaced with complete medium (Endo-SFM plus 10% human serum and 37 μg/mL endothelial cell growth supplement [ECGS]), and the cells were incubated for another 20 hours. A scratch wound was induced in the monolayer with a 1-mL pipet tip, and fresh media were replaced. Pictures of the same field were taken at regular intervals up to 24 hours to monitor cell migration into the wound. Quantitation of the wound width was measured using Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij).
Results
CD31 down-regulation by K5
We previously reported a robust expression of CD31 on E-DMVECs long-term–infected by KSHV, a state where latency predominates.31 In contrast, reduced CD31 surface expression was observed in another study using newly infected telomerized DMVECs.32 Similarly, when lytic gene expression in latently KSHV-infected E-DMVECs was induced by the phorbol-ester PMA,25 KSHV-infected E-DMVECs showed a pronounced reduction in CD31 expression, whereas CD31 expression of uninfected E-DMVECs was not altered by PMA (Figure 1A). This result suggested that a lytic viral gene product was responsible for CD31 down-regulation. In contrast to E-DMVECs that are typically infected for at least 3 weeks to establish a latent infection, CD31 expression was reduced in primary DMVECs at 48 hours after KSHV infection, even in the absence of PMA (Figure 1B). This observation is similar to the earlier observation in telomerized DMVECs and is consistent with the reported lytic gene expression at early times after infection.41 The immediate early gene product K5 was the most likely candidate for CD31 down-regulation because reduction of CD31 expression correlated with that of MHC I and ICAM-1,32 known targets of K5, and because K5 mRNA is expressed for days during initial infection, whereas most other lytic genes are shut off.41 Moreover, similar to other K5 targets, CD31 expression was reduced by a posttranscriptional mechanism, since CD31 mRNA levels did not change upon PMA induction (data not shown). Although K5 expression did not correlate with reduced CD31 levels in the previous study,32 we observed that K5 was detectable in approximately the same percentage of cells that showed reduced CD31 surface levels in PMA-induced E-DMVECs (Figure 1A). Spontaneous K5 expression in newly infected primary DMVECs was also detectable, albeit at a lower level compared to PMA-induced E-DMVECs (Figure 1B). Thus, we conclude that early after infection, K5 may be expressed at levels sufficient to influence CD31 expression but not accurately quantifiable by flow cytometry. To further examine whether K5 expression correlated with reduced CD31 expression, we took advantage of the fact that a small percentage of KSHV-infected E-DMVECs spontaneously express lytic genes.25 We found that K5 was expressed in some of the uninduced E-DMVECs, and CD31 was consistently absent from cells that expressed K5 (Figure 1C). In contrast, CD31 was well expressed on adjacent cells expressing the latency-associated protein LANA-1, but not K5 (Figure 1C). The correlation between K5 expression and reduced CD31 levels strongly suggested that K5 is responsible for the decrease in CD31 expression.
Interestingly, at any one time, expression of K5 was consistently observed in a much higher percentage of infected E-DMVECs than that of the early gene product ORF59, a processivity factor for the viral DNA polymerase (Figure 1D). Counting K5- and ORF59-positive cells in 3 independent experiments revealed that K5 was expressed in approximately 5 times more cells than ORF59 in uninduced E-DMVECs and in approximately 4 times more cells upon PMA induction (ratio of 4.8 ± 0.3 and 3.96 ± 0.7, respectively, for 3 independent experiments). That the percentage of E-DMVECs spontaneously expressing K5 is below 5% explains why the concomitant reduction of CD31 surface expression is not observed by flow cytometry (Figure 1A).
To determine whether K5-expression was sufficient to reduce surface expression of CD31, K5 was expressed in uninfected E-DMVECs by adenovirus (Ad) transduction, and CD31 surface expression was monitored by flow cytometry. In parallel, we also transduced cells with KSHV-K3 and MK3. As expected, both K3 and K5 reduced MHC I expression, whereas MK3 or AdTet alone had no effect (Figure 2A). Staining the same cells for CD31 expression revealed a very dramatic reduction of CD31 surface expression by K5, but not by K3 or MK3 (Figure 2A). Taken together, these data suggest that K5 is uniquely responsible for the down-regulation of CD31 in KSHV-infected cells. The dramatic reduction of CD31 expression by K5, but not MK3, was confirmed by IFA. As shown in Figure 2B, cells expressing high levels of K5 were completely negative for CD31. In contrast, MHC I staining was reduced and confined to intracellular compartments upon expression of K5 or K3 (Figure 2B). This was consistent with previous observations that MHC I assembly and maturation in the ER is not affected by K5.9,10 Robust expression of CD31 and MHC I was maintained on cells infected with AdMK3, K3, or the AdTet control.
K5 inhibits EC migration
CD31 regulates neoangiogenesis and leukocyte transmigration through the endothelial monolayer.28,29 In vitro, CD31 modulates migration of ECs in response to directional signals such as those occurring during mechanical wounding of the monolayer.42,43 To examine whether K5 affect EC migration we performed a wound scratch assay in E-HUVECs transduced with AdGFP or AdK5. Upon wounding, the closure of the scratch by EC migration was recorded over a period of 24 hours. AdGFP-transduced HUVECs closed the wound almost completely as early as 13 hours after wounding (Figure 3A). In contrast, migration of AdK5-transduced HUVECs was significantly delayed in several independent experiments (Figure 3B). These observations are consistent with the previously reported delay of CD31-deleted endothelial cells to respond to wounding.42,43 Thus, it is likely that CD31-down-regulation by K5 is the primary reason for this inhibition of EC migration, although a role of other K5 targets cannot be ruled out at this point. These data suggest a broader role for K5 in perturbation of EC function.
Proteasomal degradation of newly synthesized CD31
K5 ubiquitinates MHC I that has already traversed the endoplasmic reticulum (ER) and Golgi.16 Once ubiquitinated, MHC I is targeted for lysosomal degradation via the multivesicular body pathway.9,10 However, the complete absence of CD31 in K5-transduced E-DMVECs (Figure 2B) suggested that CD31 might be degraded prior to ER exit. To examine the fate of newly synthesized CD31, we pulse/chase-labeled AdK5-transduced or AdTet-transduced E-DMVECs. CD31 was immunoprecipitated with a specific antibody and half of each sample was treated with endoglycosidase H (EndoH) to monitor the maturation of N-linked glycans upon transit to the medial Golgi. CD31 is highly glycosylated and matures relatively slowly, as evident from the 3 hours required for CD31 to acquire complete EndoH resistance in AdTet-transduced DMVECs (Figure 4A). In AdK5-transduced DMVECs, however, newly synthesized CD31 was degraded prior to acquiring EndoH resistance. This degradation occurred relatively rapidly, since the amounts of CD31 recovered were already reduced at the end of the 45-minute pulse.
Figure 4.
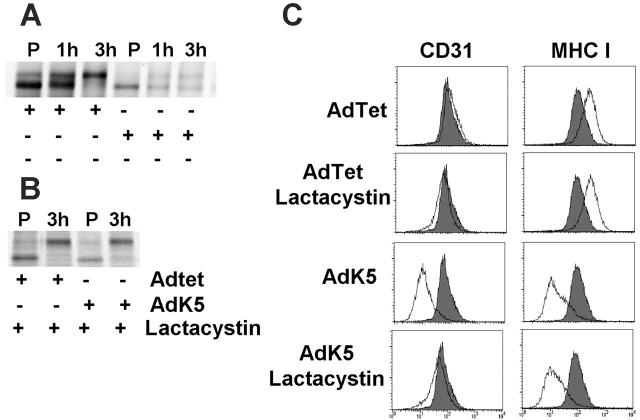
K5 mediates proteasomal destruction of newly synthesized, ER-resident CD31. (A) AdTet or AdK5-transduced E-DMVECs were metabolically labeled for 45 minutes (P) at 24 hours after infection, and the label was chased for the indicated times. Immunoprecipitates were EndoH treated. (B) Cells were infected and labeled as in panel A except that lactacystin was added prior to labeling and immunoprecipitation and EndoH treatment. (C) Surface expression of MHC I and CD31 was simultaneously monitored by flow cytometry in E-DMVECs transduced by AdTet or AdK5 (unshaded) with or without lactacystin treatment. CD31 or MHC I expression on uninfected E-DMVECs is shaded.
Misfolded ER proteins are degraded in the cytosol by the proteasome.44 ER-associated degradation (ERAD) by the proteasome is also observed for ER-resident proteins targeted for degradation by viral proteins.45 Indeed, treatment of AdK5-transduced cells with the proteasomal inhibitor lactacystin prevented the degradation of newly synthesized CD31 (Figure 4A). Different from proteins of cytomegalovirus that bind to and reverse-translocate MHC I to the cytosol,45 we did not observe a deglycosylated cytosolic intermediate of CD31. Instead, CD31 seemed to exit the ER upon inhibition of proteasomal degradation. This was further confirmed by analyzing cell-surface expression of CD31, as treatment of AdK5-transfected E-DMVECs with lactacystin prevented the K5-mediated down-regulation of CD31 (Figure 4B). In the same cells, MHC I was still down-regulated by K5, thus ruling out a general effect of inhibitor treatment on K5 modulation of target molecules. These data suggested that K5 targets CD31 for proteasomal degradation, which is in contrast to other known K5 targets, which are all targeted to the endosome/lysosome pathway.
Down-regulation of pre-existing CD31 by cell-surface–expressed K5
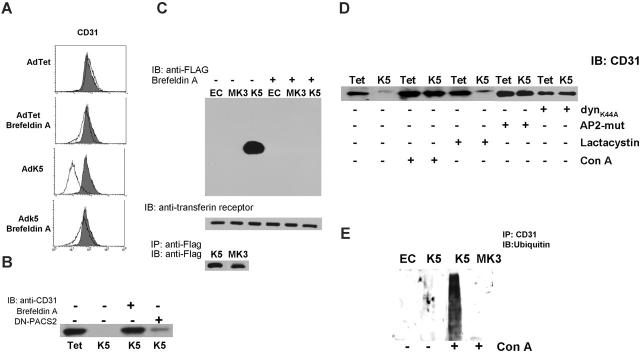
CD31 was no longer down-regulated by K5 when cells were treated with brefeldin A (BFA), a fungal metabolite that prevents retrograde vesicular transport by inhibiting coatomer assembly (Figure 5A). This result suggested that vesicular transport of either K5 or CD31 might be required prior to proteasomal degradation. However, proteasomal degradation of ER-associated proteins is generally unaffected by BFA.45 Therefore, we considered the alternative hypothesis that CD31 down-regulation involved a second molecular mechanism that can be inhibited by BFA. Conceivably, intracellular transport of K5 could be required to down-regulate pre-existing CD31 at the cell surface. To examine whether preexisting CD31 is removed by K5, we biotinylated cell-surface proteins immediately after infection with AdK5 or control virus. Upon removal of excess biotin and continued incubation for 16 hours, cells were lysed and biotinylated CD31 was detected by immunoblotting with anti-CD31 antibody (Figure 5B). In contrast to control cells, biotinylated CD31 was completely absent from lysates of E-DMVECs expressing K5, consistent with K5 efficiently removing CD31 from the cell surface. Moreover, treatment of cells with BFA inhibited this removal (Figure 5B). To examine whether BFA prevented intracellular transport of K5, we biotinylated the cell surface of AdK5-transduced E-DMVECs. MK3 was included as control since MK3 is an ER-resident protein17 (Figure 2B). K5 and MK3 were expressed at equal levels, as shown by immunoblot of total lysates (Figure 5C). However, only K5 was detected in avidin precipitates, consistent with cell-surface expression of K5 but not MK3. Intracellular transport and surface expression of K5 was completely inhibited by BFA.
Figure 5.
K5 transport to the cell surface is required for removal of pre-existing CD31 from the plasma membrane. (A) CD31 surface expression was monitored by flow cytometry of AdTet- or AdK5-transduced E-DMVECs at 20 hours after infection. Where indicated, cells were treated with BFA (10 μg/mL) shortly after adenovirus infection. Uninfected E-DMVECs were stained for CD31 as control (shaded). (B) E-DMVECs were infected with AdTet, AdK5, or AdDN-PACS-2, and proteins at the cell surface were biotinylated immediately after infection and prior to K5 expression. Cells were washed 3 times in cold PBS and incubated with medium in the presence or absence of BFA. Cells were lysed after 20 hours and biotinylated proteins were captured using NeutrAvidin followed by SDS-PAGE separation, transfer to nylon membrane, and immunoblot with anti-CD31. (C) Untreated E-DMVECs (EC) or E-DMVECs transduced for 24 hours with AdK5 or AdMK3 were BFA treated or untreated and biotinylated as in panel B. Cell lysates were split in 3 fractions. Top panel shows biotinylated proteins precipitated with NeutrAvidin and immunoblotted with anti-FLAG to detect K5 or MK3. Middle panel shows precipitated, biotinylated proteins immunoblotted with antitransferrin receptor as control. Bottom panel shows total amounts of K5 and MK3 detected in cell lysates using anti-FLAG for both immunoprecipitation and immunoblotting. (D) Cells were transduced with AdTet, AdK5, AdDynK44A, or AdAP-2 as shown, and cell-surface proteins were biotinylated. Where indicated, concanamycin A or lactacystin were added to the medium during biotinylation. Biotinylated CD31 was detected by immunoblot as in panel C. (E) E-DMVECs were transduced with AdK5 or AdMK3 or untreated (EC) for 20 hours. Where indicated, the lysosomal inhibitor concanamycin A was added for 3 hours prior to immunoprecipitation of CD31 followed by immunoblotting with antiubiquitin.
As expected, destruction of pre-existing CD31 was not inhibited by proteasomal inhibitors (Figure 5D). In contrast, inhibitors of endosomal acidification, such as concanamycin A (conA), restored expression of biotinylated CD31 despite the presence of K5. Furthermore, cotransduction with adenoviruses expressing dominant negative mutants of the clathrin adaptor AP-2 (AdAP-2) or dynamin (AdDynK44A) prevented K5-mediated degradation of biotinylated CD31 (Figure 5D). We conclude that the degradation of pre-existing CD31 by K5 involves clathrin-dependent endocytosis, suggesting that CD31 is ultimately degraded in lysosomes. Thus, cell-surface–expressed pre-existing CD31 is targeted by K5 to a different degradation compartment than newly synthesized CD31.
To further determine whether CD31 was ubiquitinated by K5, CD31 was immunoprecipitated from E-DMVECs in the presence or absence of K5 and immunoblotted with antiubiquitin antibody. A faint smear of ubiquitinated CD31 was observed in the presence of K5 (Figure 5E). These ubiquitinated intermediates could be stabilized upon treatment with conA consistent with a rapid internalization and degradation of ubiquitinated CD31.
Involvement of PACS-2 in the ER degradation of CD31
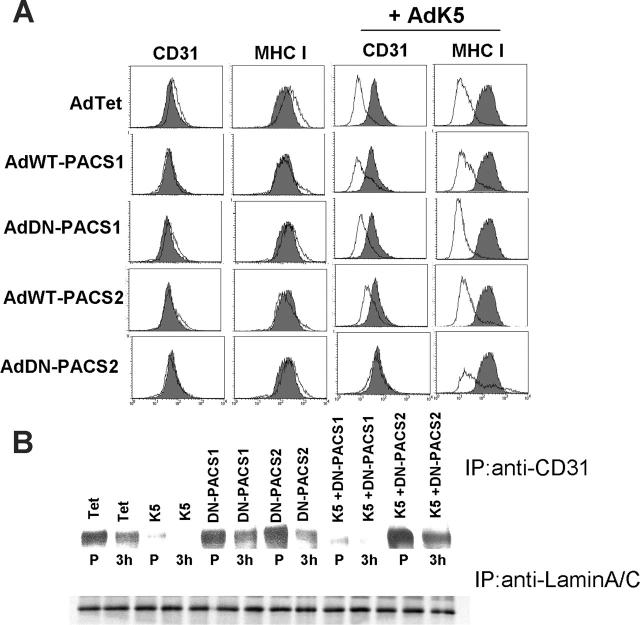
During our exploration of adaptor proteins that are potentially involved in the subcellular targeting of CD31 by K5, we observed that a dominant-negative (DN) version of the recently discovered phosphofurin acidic cluster sorting protein-2 (PACS-2)34,46 restored CD31 surface expression in the presence of K5 (Figure 6A). A slight but reproducible restoration of CD31 surface levels was also observed upon overexpression of wild-type PACS-2 (Figure 6A). Interestingly, neither wild-type (WT) nor DN-PACS-2 restored MHC I expression in the presence of K5, suggesting that DN-PACS-2 interfered with a K5-dependent process that differs between MHC I and CD31. Similar to its more extensively studied homolog PACS-1, PACS-2 is a cytosolic protein that binds to acidic cluster motifs in the cytoplasmic domains of cargo proteins. However, PACS-1 binds to the adaptor proteins AP-1 and AP-3 and localizes membrane cargo to the trans-Golgi network (TGN),33 whereas PACS-2 binds to COP-I coatomer to localize membrane cargo the ER.34,46 DN-PACS-1 and DN-PACS-2 bind cargo but not adpators or COPI, respectively.33,46 In contrast to PACS-2, neither WT- nor DN-PACS-1 interfered with MHC I or CD31 down-regulation by K5 (Figure 6A), suggesting that PACS-1 is not involved in K5 function. Interestingly, DN-PACS-2 did not interfere with the degradation of biotinylated CD31 (Figure 5B), suggesting that it does not affect the intracellular transport of K5 to the cell surface or the subsequent degradation of pre-existing CD31. In contrast, degradation of newly synthesized CD31 was inhibited in the presence of DN-PACS-2 (Figure 6B). DN-PACS-2 restored CD31 surface levels (Figure 6A) similar to the restoration of CD31 surface expression upon lactacystin treatment (Figure 4B). Since removal of pre-existing CD31 by K5 is not inhibited in either case (Figure 5B,D), it seems likely that the supply of newly synthesized CD31 overcomes the capacity of K5 to remove CD31 from the cell surface. That ER degradation is the predominant pathway of CD31 removal is also supported by our observation that inhibiting the clathrin pathway did not restore CD31 surface levels in K5-transduced cells (not shown).
Figure 6.
DN-PACS-2 prevents degradation of newly synthesized CD31 by K5. (A) E-DMVECs were transduced with the indicated recombinant adenoviruses for 24 hours, and cell-surface expression of MHC I or CD31 was analyzed by flow cytometry (unshaded). Untreated cells stained for CD31 or MHC I are shown for comparison (shaded). Note that both CD31 and MHC I are down-regulated by K5, but only CD31 surface expression is restored upon cotransduction of DN-PACS-2. (B) Top panel shows immunoprecipitation of CD31 from E-DMVEC transduced with the indicated adenoviruses and metabolically labeled for 45 minutes (P) followed by a 3-hour chase. Bottom panel shows one-fourth of each sample immunoprecipitated using lamin A/C antibody as a loading control.
Since DN-PACS-2 did not affect the intracellular transport and surface expression of CD31, we hypothesized that DN-PACS-2 interfered with the trafficking of K5. Consistent with previous reports,47 K5 predominantly showed a perinuclear, ER-like staining pattern in IFA (Figure 7A), suggesting that most K5 remains intracellular. This perinuclear localization did not seem to change significantly upon coexpression of DN-PACS-2. However, the subcellular distribution of DN-PACS-2, but not WT-PACS-2, changed from a diffuse cytoplasmic staining to a staining that overlapped with K5 (Figure 7A), suggesting that PACS-2 physically interacted with K5. This interaction is likely more stable for the DN version than the WT since DN-PACS-2 lacks the ability to dissociate upon completion of the trafficking process.
To confirm a physical interaction of PACS-2 and K5, we immunoprecipitated HA-tagged PACS2 and immunoblotted the precipitate using antibodies to the FLAG-tag of K5. As shown in Figure 7B, DN-PACS-2 coprecipitated with K5, but not with MK3 used as a control. K5 also coprecipitated with WT-PACS-2, albeit with less efficiency, consistent with their differential localization seen by IFA.
Previously it was pointed out that K3 and K5 each contain 2 acidic clusters in their carboxy-terminal, cytoplasmic domains.15 To determine whether these motifs are involved in the binding of PACS-2 to K5, we expressed a mutant of K5 lacking both acidic clusters in adenovirus (AdK5DE12/QQ). This mutant no longer colocalized with PACS-2, suggesting that PACS-2 interacts with at least one of the 2 acidic clusters (Figure 7C). Furthermore, K5DE12/QQ was unable to down-regulate CD31 (Figure 7D) consistent with an essential role of the interaction of K5 with PACS-2 for CD31 down-regulation. However, K5DE12/QQ also failed to down-regulate MHC I (Figure 7D), a process that does not involve PACS-2, suggesting that this mutant has additional defects unrelated to PACS-2 interaction.
In summary, we conclude that K5 is sorted to specific subcellular compartments by cellular adaptor proteins for the efficient removal of both newly synthesized and pre-existing CD31 from ECs. Newly synthesized CD31 is degraded by the proteasome, whereas pre-existing CD31 is endocytosed and degraded in the endosomal/lysosomal system.
Discussion
Our data demonstrate that CD31 is a novel target for KSHV-K5. Different from previously described targets, CD31 is efficiently removed from ECs by a dual degradation mechanism targeting both newly synthesized and pre-existing molecules. Also different from all other targets of K5 described so far, CD31 is not expressed on cells of the B-cell lineage. KSHV-mediated transformation of B cells causes the lymphoproliferative diseases multicentric Castleman disease and primary effusion lymphoma.48 In contrast, neoplastic cells in KS are of EC origin. Thus, down-regulation of CD31 by K5 is expected to specifically play a role in KS. Given the important role of CD31 in many aspects of EC biology, it seems likely that K5 not only affects the interaction of cells of the immune system with KSHV-infected cells as previously thought, but might also dysregulate EC function during KS development. Consistent with this hypothesis, we observed a slowing of EC migration upon K5 expression. The finding that CD31 expression is unchanged in latently infected E-DMVECs, but significantly reduced during primary infection or upon induction of the lytic cycle, would be consistent with CD31 removal coinciding with the initial establishment of infection or during periods of reactivation of the latent genome. Although CD31 expression is abundantly found in KS tumors, including the characteristic spindle cells, which often harbor latent viral genome,49,50 K5 is also widely expressed in tumor tissue.51 From our data, we predict that those lesional cells expressing K5 would be CD31 negative, whereas most latently infected cells are K5 negative and CD31 positive.
Interestingly, expression of K5 is maintained for days in primary DMVECs, whereas most other lytic genes are shut off,41 and K5 is expressed in a higher percentage of cells than other lytic genes in E-DMVECs (Figure 1D). Thus, K5 expression might also occur in cells that do not support progression into a complete replication cycle or during the establishment latency. Such transient K5 expression might contribute to tumorigenesis by dysregulating normal EC functions. For example, it is possible that the loss of CD31 promotes detachment of KSHV-infected ECs from the local tumor environment, with entry to the circulation for seeding and development of new tumor masses. Indeed, others have hypothesized that circulating spindle cells may explain the multifocal nature of KS.52 Circulating KSHV-infected donor ECs may also be the seeding vehicle for posttransplantation KS.53
CD31 expands the number target molecules of K5 to a total of 6 (HLA-A, HLA-B, B7.2, ICAM-1, and CD1). There are few common features among these targets other than their identity as type I transmembrane-spanning glycoproteins of the Ig superfamily that contain lysines or cysteines in the cytoplasmic tail that are targeted for ubiquitination. However, many other glycoproteins that fulfill these criteria are not targeted by K5 (eg, HLA-C,10 MHC class II,23 or MIC-A [M. M., unpublished observations, August 2005]). Thus, a presently unsolved question is that of substrate specificity. Given the high divergence in primary structure of K5 targets, it seems unlikely that substrates share a common K5 interacting motif. Instead, it could be that targets are selected based on their subcellular transport routes intersecting with the intracellular location of K5. In support of this concept is our observation that removal of CD31 from the cell surface requires transport of K5 to the cell surface, whereas targeting of newly synthesized CD31 for proteasomal destruction seems to involve PACS-2 binding and thus COP-I–mediated retrograde transport.
An additional implication of our findings is that subcellular localization of the ubiquitination reaction seems to be the primary factor determining whether an ubiquitinated membrane protein is targeted for lysosomal or proteasomal destruction. Current models of ubiquitin-mediated targeting to lysosomal vesicles versus targeting to the multisubunit proteasomal particle imply different types of ubiquitination. Proteasomal recognition of ubiquitinated substrates is greatly enhanced by polyubiquitination with a threshold of at least 4 ubiquitins.54 In contrast, monubiquitination is generally assumed to be sufficient for internalization and lysosomal targeting.55 Thus, 1 possibility is that K5 mediates monoubiquitination at the cell surface and polyubiquitination in the ER. Alternatively, K5 could mediate the same type of ubiquitination in both locations, with local differences in the ubiquitin-interacting proteins directing the subsequent steps of targeting the transmembrane protein to the lysosome versus proteasome. The latter hypothesis seems more likely given the structurally unique RING-CH domain, which restricts the interaction with ubiquitin-conjugating enzymes that are ultimately responsible for substrate ubiquitination.18
The pivotal role of the subcellular targeting of K5 in selecting the degradation pathway, and possibly substrate selection, suggests that the cellular proteins that connect K5 and other members of this protein family to the cellular vesicular sorting machinery are important for their function. PACS-2 is the first cellular adaptor protein shown to interact with 1 of the viral K3-like proteins. However, a recent report demonstrated that MARCH-II, one of the cellular homologs of K3 and K5, interacts with syntaxin-6, a SNARE (soluble n-ethylmaleimide-sensitive factor attachment protein) located to the TGN and endosomes.56 Similar to the redistribution of DN-PACS-2 upon coexpression of K5, syntaxin-6 binds to and colocalizes with MARCH-II. While this was interpreted as MARCH-II regulating syntaxin-6 localization and thus vesicular traffic, it is also possible that the interaction with syntaxin-6 regulates the subcellular sorting of MARCH-II to the TGN and endosomes. Similar to the interaction of K5 with PACS-2, it was further shown that the carboxyterminal tail of MARCH-II contains the binding site for syntaxin-6, although the precise sequence has not been mapped. Thus, it seems that the carboxyterminal region of this viral and cellular protein family interacts with cellular proteins restricted to specific subcellular membranous compartments. This way, the transmembrane ubiquitin ligases seem to be targeted to specific subcellular localizations where they ubiquitinate substrate. The fate of the substrate is then determined by the subcellular localization of this reaction (ie, when ubiquitination reactions occur in the ER, the substrate will be targeted for proteasomal degradation), whereas ubiquitination serves as a sorting signal to the lysosome via the multivesicular body pathway if it occurs in a post-ER compartment.
The exploitation of the subcellular sorting machinery by K5 enables the rapid and efficient removal of CD31 via a dual degradation mechanism. Thus, removal of CD31 is likely one of the prime functions of K5. Similar to other immune modulators of KSHV, K5 could thus play a dual role both in immune evasion and tumorigenesis.4
Acknowledgments
We thank J. A. Madri for CD31 cDNA, D. Johnson for Ad constructs, B. Chandran for ORF59 antibodies, and Drs Koichi Yamanishi and Keiji Ueda for K5 antibodies.
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-11-4404.
Supported by National Institutes of Health (NIH) grants R01CA99906 (A.V.M.), R01CA/AI94011 (K.F.), and DK37274 and AI49793 (G.T.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2: 373-382. [DOI] [PubMed] [Google Scholar]
- 2.Moore PS, Chang Y. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356: 499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensser A, Fleckenstein B. T-cell transformation and oncogenesis by gamma2-herpesviruses. Adv Cancer Res. 2005;93: 91-128. [DOI] [PubMed] [Google Scholar]
- 4.Moore PS, Chang Y. Kaposi's sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu Rev Microbiol. 2003;57: 609-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholas J, Ruvolo V, Zong J, et al. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol. 1997;71: 1963-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Früh K, Bartee E, Gouveia K, Mansouri M. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Research. 2002;88: 55-69. [DOI] [PubMed] [Google Scholar]
- 7.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155: 1265-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309: 127-130. [DOI] [PubMed] [Google Scholar]
- 9.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97: 8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74: 5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc Natl Acad Sci U S A. 2000;97: 8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boname JM, Stevenson PG. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity. 2001;15: 627-636. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson PG, May JS, Smith XG, et al. K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat Immunol. 2002;3: 733-740. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo ME, Jung JU, Ploegh HL. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class I major histocompatibility complexes to late endocytic compartments. J Virol. 2002;76: 5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Means RE, Ishido S, Alvarez X, Jung JU. Multiple endocytic trafficking pathways of MHC class I molecules induced by a Herpesvirus protein. EMBO J. 2002;21: 1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewitt EW, Duncan L, Mufti D, Baker J, Stevenson PG, Lehner PJ. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 2002;21: 2418-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lybarger L, Wang X, Harris M, Hansen TH. Viral immune evasion molecules attack the ER peptide-loading complex and exploit ER-associated degradation pathways. Curr Opin Immunol. 2005;17: 71-78. [DOI] [PubMed] [Google Scholar]
- 18.Dodd RB, Allen MD, Brown SE, et al. Solution structure of the Kaposi's sarcoma-associated herpesvirus K3 N-terminal domain reveals a novel E2-binding C4HC3-type RING domain. J Biol Chem. 2004;279: 53840-53847. [DOI] [PubMed] [Google Scholar]
- 19.Swanson R, Locher M, Hochstrasser M. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 2001;15: 2660-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol. 2004;78: 1109-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto E, Ishido S, Sato Y, et al. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem. 2003;278: 14657-14668. [DOI] [PubMed] [Google Scholar]
- 22.Ishido S, Choi JK, Lee BS, et al. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13: 365-374. [DOI] [PubMed] [Google Scholar]
- 23.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7–2 and modulates T cell costimulation. J Clin Invest. 2001;107: 1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115: 1369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moses AV, Fish KN, Ruhl R, et al. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73: 6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciufo DM, Cannon JS, Poole LJ, et al. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J Virol. 2001;75: 5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagunoff M, Bechtel J, Venetsanakos E, et al. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J Virol. 2002;76: 2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci. 1994;714: 165-174. [DOI] [PubMed] [Google Scholar]
- 29.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66: 698-704. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540: 7-14. [DOI] [PubMed] [Google Scholar]
- 31.Raggo C, Ruhl R, McAllister S, et al. Novel cellular genes essential for transformation of endothelial cells by Kaposi's sarcoma-associated herpesvirus. Cancer Res. 2005;65: 5084-5095. [DOI] [PubMed] [Google Scholar]
- 32.Tomescu C, Law WK, Kedes DH. Surface down-regulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus. J Virol. 2003;77: 9669-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crump CM, Xiang Y, Thomas L, et al. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 2001;20: 2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmen T, Aslan JE, Blagoveshchenskaya AD, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24: 717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111: 853-866. [DOI] [PubMed] [Google Scholar]
- 36.Lu TT, Yan LG, Madri JA. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proc Natl Acad Sci U S A. 1996;93: 11808-11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pande NT, Powers C, Ahn K, Fruh K. Rhesus cytomegalovirus contains functional homologues of US2, US3, US6, and US11. J Virol. 2005;79: 5786-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegde NR, Tomazin RA, Wisner TW, et al. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J Virol. 2002;76: 10929-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haque M, Chen J, Ueda K, et al. Identification and analysis of the K5 gene of Kaposi's sarcoma-associated herpesvirus. J Virol. 2000;74: 2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansouri M, Bartee E, Gouveia K, et al. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J Virol. 2003;77: 1427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78: 3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003;17: 1458-1469. [DOI] [PubMed] [Google Scholar]
- 43.Rothermel TA, Engelhardt B, Sheibani N. Polyoma virus middle-T-transformed PECAM-1 deficient mouse brain endothelial cells proliferate rapidly in culture and form hemangiomas in mice. J Cell Physiol. 2005;202: 230-239. [DOI] [PubMed] [Google Scholar]
- 44.Plemper RK, Wolf DH. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24: 266-270. [DOI] [PubMed] [Google Scholar]
- 45.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84: 769-779. [DOI] [PubMed] [Google Scholar]
- 46.Kottgen M, Benzing T, Simmen T, et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24: 705-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulson E, Tran C, Collins C, Früh K. KSHV-K5 inhibits phosphorylation of the major histocompatibility complax class I tail. Virology. 2001;288: 369-378. [DOI] [PubMed] [Google Scholar]
- 48.Cesarman E, Knowles DM. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin Diagn Pathol. 1997;14: 54-66. [PubMed] [Google Scholar]
- 49.Nickoloff BJ. PECAM-1 (CD31) is expressed on proliferating endothelial cells, stromal spindle-shaped cells, and dermal dendrocytes in Kaposi's sarcoma. Arch Dermatol. 1993;129: 250-251. [PubMed] [Google Scholar]
- 50.Uccini S, Ruco LP, Monardo F, et al. Co-expression of endothelial cell and macrophage antigens in Kaposi's sarcoma cells. J Pathol. 1994;173: 23-31. [DOI] [PubMed] [Google Scholar]
- 51.Haque M, Ueda K, Nakano K, et al. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J Gen Virol. 2001;82: 1175-1180. [DOI] [PubMed] [Google Scholar]
- 52.Browning PJ, Sechler JM, Kaplan M, et al. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood. 1994;84: 2711-2720. [PubMed] [Google Scholar]
- 53.Barozzi P, Luppi M, Facchetti F, et al. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat Med. 2003;9: 554-561. [DOI] [PubMed] [Google Scholar]
- 54.Attaix D, Ventadour S, Taillandier D, Combaret L. The ubiquitin-proteasome pathway: limitations and opportunities. J Support Oncol. 2005;3: 221-222. [PubMed] [Google Scholar]
- 55.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2: 195-201. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura N, Fukuda H, Kato A, Hirose S. MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking. Mol Biol Cell. 2005;16: 1696-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]