Abstract
Astronauts lose 1–2% of their bone minerals per month during space flights. A systematic search for a countermeasure relies on a good understanding of the mechanism of bone formation at the molecular level. How collagen fibers, the dominant matrix protein in bones, are mineralized remains mysterious. Atomic force microscopy was carried out, in combination with immunostaining and Western blotting, on bovine tibia to identify unrecognized building blocks involved in bone formation and for an elucidation of the process of collagen calcification in bone formation. Before demineralization, tiles of hydroxyapatite crystals were found stacked along bundles of collagen fibers. These tiles were homogeneous in size and shape with dimensions 0.69 × 0.77 × 0.2 μm3. Demineralization dissolved these tiles and revealed small spheres with an apparent diameter around 145 nm. These spheres appeared to be lipid particles since organic solvents dissolved them. The parallel collagen bundles had widths mostly <2 μm. Composition analysis of compact bones indicated a high content of apolar lipids, including triglycerides and cholesterol esters. Apolar lipids are known to form lipid droplets or lipoproteins, and these spheres are unlikely to be matrix vesicles as reported for collagen calcification in epiphyseal cartilages. Results from this study suggest that the layer of round lipid particles on collagen fibers mediates the mineral deposition onto the fibers. The homogeneous size of these lipid particles and the presence of apolipoprotein in demineralized bone tissue suggest the possibility that these particles might be of lipoprotein origin. More studies are needed to verify the last claim and to exclude the possibility that they are secreted lipid droplets.
INTRODUCTION
Much is known about bone formation at the molecular level; however, the mechanism of collagen mineralization and the role of lipids in the process remain unclear. This hinders our effort in developing countermeasures against bone loss for astronauts. As a consequence of the body's adaptation to microgravity, astronauts lose ∼1–2% of their bone mass per month (whole body). Exercise can reduce this to ∼0.5% (whole body), but some of the major load-bearing bones still lose 1–2% per month. On a 6-month mission, astronauts may lose up to 20% of their bone mass at certain sites (1–3). This increases the risk of bone fractures, as astronauts readjust to gravity. Osteoporosis, either induced by disuse or hormonal change, is also a major medical issue for the general public, especially for patients with spinal core injuries. In the United States, 50% of women and 12% of men at age 50 or older are at various levels of abnormal bone loss and more than 20 million are suffering from osteoarthritis, the most common form of arthritis.
In addition to its protective role to the soft tissues, bone also serves as a reservoir for calcium minerals, balances the body's pH, Ca2+, and phosphate, and houses cells for hematopoiesis. Human bones are composed of an organic matrix, ∼30% of the mass, and inorganic hydroxyapatite (4). Collagen, mainly type I, accounts for 90% of the total protein mass. Bone formation, or osteogenesis, involves three main steps: 1), production of collagen; 2), mineralization of collagen to form bones; and 3), remodeling of the bone by resorption and reformation. Mineralization, the process of depositing calcium phosphate onto collagen fibers, is thought to be mediated by matrix lipid vesicles (5–11). In the absence of a mediator, calcium deposition on collagen in vitro does not occur at physiological pH, but at pH above 12 (12). Although a unified theory still seems far from the horizon after nearly half of a century's effort, reflecting the complexity of the problem and the difficulty of the subject to study, a popular hypothesis based largely on the work of cartilage states that these vesicles are the budding product of a cellular process of osteoblasts and chondrocytes (13,14). Within a confined space, Ca2+ channels, possibly annexins, could supersaturate the vesicles with Ca2+ ions (15). Although much remains in dispute regarding how such a saturation point is derived, the crystals formed inside the vesicles are thought to serve as the nucleation sites for the continued growth of the minerals after the fragmentation of the membrane. Depending on the bones used, these vesicles are reportedly 10–200 nm in diameter under an electron microscope. Round solid particles known as granules are also present (10).
Although the matrix vesicle model in cartilage mineralization is being established, much remains unknown regarding the collagen calcification in compact bones. Understanding this is important for NASA since astronauts' mineral loss is associated with an erosion of the compact bones. Lipid composition analysis indicates a high quantity of TG (70%) and CE in compact bones (6%) (16,17). Neither of the apolar lipids are structural components of plasma membranes nor intracellular membranes, from which matrix vesicles are supposedly derived. Thus, it is unlikely that matrix vesicles found in cartilage contribute significantly to the lipid composition in compact bones. It is also impossible to make vesicles with such a high concentration of apolar lipids in vitro. A lipid droplet is formed if triglycerides or cholesterol esters are involved. Two kinds of lipid particles known to be secreted by cells and to consist of a large quantity of TG and CE in our body are lipoproteins and lipid droplets. Then, the questions are: Where do these lipids come from? Do they mediate collagen calcification, and how?
In arterial subendothelial matrices, unilamellar and multilamellar vesicles and lipid droplets with diameters between 35 and 350 nm have long been identified, experimentally proved, and generally accepted to be of lipoprotein origin (18–23). The deposition of these granules onto collagen is thought to protect collagen from metalloproteinase digestion and to induce calcification in the atherosclerotic plaques (18–23). Whether analyzed in situ or isolated and analyzed in vitro, these vesicles and granules can be labeled by anti-apoB and anti-apo(a) antibodies. Collagen, at high concentrations, promotes the generation of these vesicles from lipoproteins. The same molecules involved in bone formation are also found in calcified atherosclerotic plaque, namely collagen, calcium, and lipids. An analogy between bone formation and atherosclerotic plaque formation has also been proposed recently by Demer and colleagues (24,25). Bone-like structures have been observed in the arterial wall by a number of groups.
Bone marrow is known to consume about half of the chylomicrons (26). Triglycerides are the major lipids in bone marrow. Essential fatty acids in bone marrow play an important role in bone metabolism, indicating that at least some of the lipids must be taken up from circulation either in the form of free fatty acids or lipoproteins (27). Consistent with this, animal studies show an influence of dietary fat on bone marrow lipid composition, and a decrease in the essential fatty acid content of the bone marrow occurs during starvation (28). Despite this, lipoprotein metabolism in bones remains largely unknown and some claim bone is a forgotten organ in lipidology (29). All of these issues call for a thorough examination of the role of lipoproteins in bone formation.
AFM is a powerful tool in the study of the structure and dynamics of assembly of macromolecules (30–33). Recently, AFM was used to investigate bone structure and mechanical properties (34). When the exterior surface of trabecular bone was analyzed, AFM revealed a densely woven structure of collagen fibrils, banded with a 67 nm periodicity, and densely packed mineral plates. The mineral plates on the collagen fibrils overlap and exhibit diameters ranging from 30 to 200 nm. On the collagen fibrils, small nodular features, spaced 20–30 nm, run perpendicular to the fibrils (35). In some cases, these nodules are also found on filaments extending between collagen fibrils.
Work presented in this article aims at an understanding of the molecular pathway of compact bone formation with a focus on the following: 1), the presence of the lipid particles in compact bone; 2), the structure and structural relationship among mineral crystals, lipid particles, and collagen fibers; 3), the mechanism of collagen mineralization in compact bone; and 4), whether the lipid particles in compact bone are derived from lipoproteins or lipid droplets.
MATERIALS AND METHODS
Bovine tibia preparation
Compact bovine tibia from a local grocery store was cleaned by removing the yellow marrow, the periosteum, the endosteum, and tissue debris, and then rinsed with water and air dried. The bone was sawed into cubes ∼0.5 cm wide. The cuts for the AFM image were radial and those used in dye and immunohistology were transverse, meaning the former is parallel to the osteon whereas the latter is perpendicular.
Demineralization
The cubic bone was demineralized by an incubation in 0.2 N HCl at room temperature until its weight stabilized (∼3–5 weeks).
Microtomed tissue preparation
Demineralized bone tissue was incubated in 20% sucrose for 2 days and was sliced with a cryotome to a thickness around 8 μm.
AFM
AFM imaging was carried out in air (Pico+ from Molecular Imaging, Tempe, AZ) with a long narrow-legged cantilever tip. The gains were maximized for topography mode and minimized for deflection mode. The loading force was minimized by lowering the value of the set point.
Lipid stain
Microtomed tissue slices placed on a glass substrate were first incubated with a few drops of PBS buffer for 1 min. After removal of the buffer, a couple drops of oil red O or Sudan black were applied to the tissue and the substrate was then placed on a heating block and incubated at 70°C for 10 min. The stain was rinsed off with tap water and the stained tissue was examined with an optical microscope (Olympus IX70). Images of interest were captured with MagaFire-SP (Meyer Instruments, Houston, TX).
Immunolight microscopy
Demineralized tibia bone tissue sliced with a cryotome was placed on a glass substrate. After a 5 min incubation in blocking solution (1% bovine serum albumin, 10 mM sodium phosphate, 150 mM NaCl), the tissue was labeled with anti-human LDL antibody overnight. The labeled tissue was then blocked with bovine serum albumin solution three times, each for 10 min, and was then treated with the secondary antibody conjugated with peroxidase. After the substrate was rinsed with PBS buffer, it was developed with 3,3-diaminabenzidine solution in the presence of nickel ions for 2 min, rinsed with water, and sealed with a coverslip and nail polish.
Western blot
To examine whether lipoprotein was present in bone matrix, demineralized bone tissues (1 g) were milled in liquid nitrogen and dissolved in 0.5 M acetic acid. Insoluble materials were removed by centrifugation (10,000 × g for 15 min). Lipids were removed by a chloroform/trichloroacetic acid extraction (sample, CHCl3:50%trichloroacetic acid, 2:2:1, v/v). The insoluble portion, collected from the water-chloroform interface upon centrifugation with a microfuge, was subjected to Western blot analysis for apolipoprotein B. The insoluble portion was analyzed on a 4–12% Tris-Glycine NuPAGE gel (Invitrogen, Carlsbad, CA). The proteins from the gel were transferred to polyvinylidene nylon membrane and anti-human LDL antibody was used as the primary binding (dilution factor 1:100 in blocking buffer, incubation time, 1 h at room temperature with agitation). After the membrane was rinsed four times with TBS (50 mM Tris, 150 mM NaCl, pH 7.6), 10–15 min each time, it was incubated with secondary antibody, anti-goat IgG conjugated with horseradish peroxidase in blocking buffer, 1:1500, for 30 min at room temperature with constant agitation. After another four washes with TBS, the membrane was developed with ABC-AP kit (Vector Labs, Burlingame, CA).
RESULTS
Bovine tibia compact bone was analyzed for its structure before and after demineralization for an identification of materials that mediate the mineral deposition on the matrix protein of collagen. AFM was used in combination with Western blot and immunohistochemical techniques. The data indicate that calcium phosphate crystals are not in direct contact with collagen bundles. Beneath the mineral crystals, a layer of round lipid particles are deposited on the surface of the collagen bundles. These lipid particles, thought to be solid, not hollow as in vesicles, may have mediated calcium phosphate deposition onto collagen. We also investigated the source and nature of these lipid particles.
Calcium minerals stack along the collagen fiber bundles
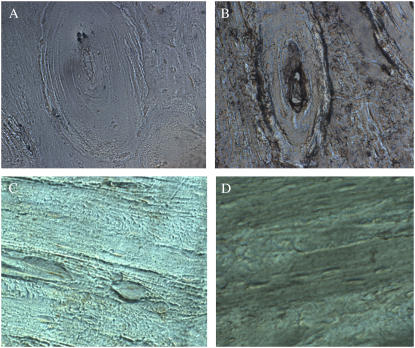
AFM images of bovine tibia compact bone reveal that the hydroxyapatite crystals appear as squared sheets or tiles. These tiles stacked on top of each other and along the axis of the collagen bundles. The plane of the crystals is tilted slightly from the perpendicular orientation toward the collagen bundles (Fig. 1). This allows for the partial image of the top plane rather than the side of the sheets. The sheets, homogeneous in size and shape, are ∼0.69 μm × 0.77 μm × 0.2 μm (n = 30), and are compatible in the lateral dimension to the diameter of the collagen bundles. These bundles remain visible, especially at low magnifications, in the presence of the minerals, suggesting monolayer crystal deposition.
FIGURE 1.
AFM image of bovine tibia compact bone. A rectangular piece of bovine tibia compact bone was placed on AFM sample stage. Images are collected in air and representatives at three different resolutions are presented.
Lipid particles cover the entire surface of collagen bundles
Round particles were found decorating the collagen bundles of the demineralized compact bone (Fig. 2). These particles, with a diameter around 145 nm ± 15 nm, are ∼1/5 of the lateral size of the mineral crystals shown in Fig. 1. The coverage of the bundles by these round particles is complete and appears to be one or a few layers thick since the outline of the bundles can still be recognized at low resolution images. The bundles are densely packed (shoulder to shoulder) in parallel and have different diameters, mostly 2 μm or less.
FIGURE 2.
AFM image of demineralized bones. Compact bone from bovine tibia (cut to the size of ∼3 mm × 0.5 cm × 0.5 cm) was demineralized with mild acid (0.2N HCl) until no more reduction in weight (∼2–3 weeks). A piece of the demineralized bone sliced with a cryotome to a thickness of 8 μm was air dried on a glass coverslip and imaged with AFM. Four images at different resolutions are presented (A–D).
The lipid particles can be removed by incubating the demineralized bone in chloroform-methanol solution
An incubation of the demineralized bones, such as those used in Fig. 2, in chloroform solution (CHCl3:MeOH:0.2NHCl, 200:100:1, v/v) overnight appears to eliminate the round particles. The AFM images reveal few round particles after the organic solvent incubation (Fig. 3). The desolation in chloroform solution indicates that these small round particles are made of hydrophobic molecules. Based on their appearance, a significant presence of lipids in bone matrix (16,17), and their solubility in organic solvents, we assume these round particles are made of lipids and name them lipid particles. These lipid particles may mediate calcification of collagen fibers. The space between collagen bundles appears to have something that was not removed by chloroform-methanol solution.
FIGURE 3.
Chloroform-methanol solution appears capable of dissolving these lipid particles. Microtoned bone tissues were incubated in chloroform solution (CHCl3:MeOH:0.2NHCl, 200:100:1, v/v) overnight. Images at three different resolutions are presented (A–C) and only a few round particles are left on B and C as compared to their complete coverage on the collagen bundles shown in Fig. 2.
The possibility that these round particles might be of lipoprotein origin
Data from a Western blot of proteins extracted from demineralized bones indicated the presence of lipoproteins in bone. A band corresponding to a molecular mass around 500 kDa was recognized by the anti-human LDL antibody (Fig. 4). Apolipoprotein B, present in LDL, IDL, and VLDL, was expected to have a molecular mass around 500 kDa.
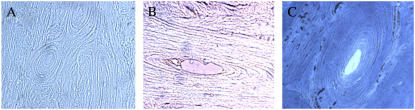
Results from immunohistochemical experiments suggest the lipoproteins may participate in bone formation. The identification of dark brown stains on the osteons along the rings in panel B of Fig. 5 suggested the tissue was labeled by the anti-human LDL polyclonal antibody. The Haversian canal showed strong labeling, indicating lipoproteins were present (36).
Lipids extracted from bones resemble those in lipoproteins and are very different from those found in plasma membranes. Cellular membranes (plasma or intracellular) are composed of phospholipids and cholesterols, but lack any significant amount of triacylglycerol or cholesterol esters (37). By contrast, the lipid particles extracted from bones show a high percentage of triacylglycerols (TG, 4.1%) and cholesterol esters (CE, 13.5%). On the other hand, lipoproteins of various types have significant amounts of TG and CE. Lipids extracted from bones have components that better resemble those isolated from LDL than those from cellular membranes (Table 1). Membrane vesicles cannot be formed in the presence of triacylglycerols or cholesterol esters.
Histochemical experiments suggest the presence of lipid particles in demineralized compact bones. Demineralized bones showed positive stains with oil red O and Sudan black, which suggested the presence of lipid droplets. Most of the dyes appeared to stain along the rings of the osteons both for oil red O (Fig. 6, panel B) and Sudan black (Fig. 6, panel C). Oil red O is often used in cardiovascular research to stain lipid droplets. These data further support the hypothesis that these particles might have a hydrophobic core like those found in lipid droplets or lipoproteins, not a water-filled core that would be expected for a vesicle.
LDL aggregation forms nucleation units and vesicles. Many articles have been published regarding lipoprotein aggregation, an apparently important area of medical research (38,18–23). Our preliminary studies showed the de novo pathway of LDL aggregation and the formation of membrane vesicles, sheets, granule particles or nucleation units, and other intermediates (41). Such lipid granules and vesicles have been reported in bone matrix and suggested to mediate collagen mineralization. Many chemicals and biochemicals are known to be capable of inducing lipoprotein aggregation. We have tested H2O2, Cu2+, Ca2+, and acidic pH and found that LDL aggregates in a similar pathway under these conditions.
FIGURE 4.
Western blot data suggest the presence of lipoproteins in compact bones. Demineralized bones were boiled in PBS buffer for 20 min and the insoluble particles were removed by centrifugation. Proteins were extracted with chloroform and precipitated with trichloroacetic acid (final concentration 10%). After a centrifugation with a microfuge for 10 min, the precipitates at the interphase were collected and analyzed on a 3–8% SDS-PAGE and a Western blot for reactivity with anti-human LDL antibody. The experiment was repeated three times and the same results were obtained.
FIGURE 5.
Immunohistochemical studies suggest the presence of lipoproteins in demineralized bovine tibia compact bones. (A and C) Control, secondary antibody only. Other controls include the absence of both primary and secondary antibodies and the absence of only secondary antibodies. (B and D) Microtomed tissues from demineralized bovine tibia compact bone were incubated with anti-human LDL polyclonal antibody (1:20 dilution in PBS buffer) for 2 days at 4°C. Panels A and B are transverse section whereas C and D are radial sections. After rinsed with PBS buffer three times, the tissue was incubated with the secondary antibody conjugated with peroxidase for 1 h at room temperature, four times with PBS buffer, and then subjected to substrate development along with all controls (magnification, 400×). All controls showed some background labeling as in panel A, but much less labeling than that shown in panel B (magnification, 400×).
TABLE 1.
Lipid composition of the matrix vesicles extracted from bones compared to those extracted from other sources
| Total lipids (%) | CE | C | TG | PL | FA |
|---|---|---|---|---|---|
| Compact bone* | 15.8 | 5.4 | 70.4 | 0.02 | 8.2 |
| Matrix vesicles† | 2.6–13.5 | 11.5–15.8 | 4.1–11.5 | 63.6 | 3.0–4.5 |
| Cellular membranes‡ | 0 | 3–30 | 0 | 70–97 | nd |
| Atheroma lipid in PL-FC phase§ | 7.8 | 29.8 | 1.6 | 60.8 | nd |
| Lipoprotein(a)‡ | 50 | 14 | 4 | 32 | nd |
| LDL‡ | 54 | 10 | 8 | 28 | nd |
| VLDL‡ | 13 | 7.6 | 59.8 | 19.6 | nd |
| Chylomicrons‡ | 3 | 2 | 88 | 7 | nd |
A high quantity of apolar lipids in compact bone suggests the presence of lipoproteins or lipid droplets.
(35), the range covers both cytoplasmic membrane and intracellular membranes.
(40).
nd, not determined, although FA is known to be present in a small percentage in lipoproteins and cellular membranes.
FIGURE 6.
Lipid dyes stain the demineralized bones. Both oil red O (panel B) and Sudan black (panel C) appear to stain the demineralized bones. Panel A is the control, demineralized bone without stain. The microtomed slices were incubated with the dye for 10 min at 60°C. Stained particles by the dyes were observed under a light microscope (magnification, 400×).
Oxidation, acidic pH, Ca2+, or Cu2+ may have resulted in a change in the surface chemical potential of the LDL particles, which may have led to the aggregation of monomeric LDL into nucleation units (Fig. 7 a). The aggregation of LDL resembles the well-understood process of chemical colloidal aggregation. Colloidal aggregation can be induced by a change in the surface electrical properties of the particles.
FIGURE 7.
Aggregation of LDL. LDL (0.2 mg/ml in phosphate buffer, pH 7.2) oxidized with H2O2 (15 mM) was analyzed with digital video microscopy. LDL is invisible under digital video microscopy. (a) After 12 h of reaction, nucleation units are found. (b) After 24 h of reaction, nucleation units chained linearly. (c) After 24 h of reaction, the linear aggregates grow into fractals. Scale bar of 8 μm is for a–c. Similar results were observed when Ca2+(5 mM) was used to initiate the aggregation process (37).
DISCUSSION
An understanding of the mechanism of bone formation at the molecular level holds the key to our effort to develop a countermeasure against disuse-induced bone loss. Knowledge of the building blocks involved in bone formation provides guidance in the study at the cellular or animal level of osteogenesis and osteoporosis. Despite its importance to biology, medicine and space exploration, calcified tissue seems to be a difficult subject to study. Our work demonstrates that the application of AFM to bone research is capable of providing new insights. Our results show that calcium phosphate crystals are not in direct contact with collagen bundles. A layer of round lipid particles binds to the collagen bundles and may mediate mineral deposition. These lipid particles can be removed with an organic solvent, but not with a mild acid. Results from the Western blot of the extracted proteins and from immunohistochemical labeling of demineralized tissue suggest the presence of lipoproteins in bones. Dye molecules that stain lipid droplets were found capable of staining the demineralized bones.
The discovery of a layer of round lipid particles coating collagen bundles provides new information for the elucidation of the mechanism of collagen calcification in compact bone. From a physical chemistry perspective, collagen, having a basic isoelectric point and carrying positive charges under physiological pH, repels positively charged calcium ions in solution (42). Calcium phosphate forms complexes with collagen only at an extremely high pH (above 12). Since biological membranes generally carry a negative surface potential due to the presence of charged phospholipids, its deposition on collagen changes the polarity of the fibers' surface potential in favor of calcium adsorption. The positive surface chemical potential of the collagen bundles supports the adsorption of negatively charged lipoproteins, which, in turn, supports the adsorption of positively charged calcium ions. Depending on the strength of the potential and the charge of the counter ion, the ion concentration near the surface can be several orders of magnitude higher than that in the bulk solution. Such a chemical potential promotes both the negatively charged membrane onto the positively charged collagen bundles and the positively charged calcium ions onto the membrane surface deposited on the bundle (Fig. 8). Extracellular calcium concentration is near saturation at 2.2–2.6 mM. Cytoplasmic calcium concentration is <1.5 μM. A small change in the extracellular matrix, such as lipid deposition onto a collagen surface, may lead to calcium deposition. Calcification occurs in many other tissues extracellularly, and can result in kidney stones and atherosclerotic plaques.
FIGURE 8.
Illustration of the lipoprotein-mediated formation of calcium phosphate crystals on collagen bundles in compact and trabecular bones. Trabecular and compact bones are lined with bone cells, not endothelial cells, which is different from blood vessels. Lipoproteins after leaving sinusoids in bone marrow have direct contact with collagens under physiologic condition. Bone collagen forms thick bundles packed together. The high density of collagen and the availability of lipoproteins make bone calcification possible. LDL (22 nm in diameter) in the presence of Ca2+ or collagen can aggregate and form solid nucleation units (37). The aggregated LDL, together with IDL, VLDL, and chylomicrons with diameters of 35–120 nm, would be potential candidates for the lipid particles found on collagen bundles because they are within the size range and all possess apolipoprotein B. Collagen has a positive surface potential and lipoproteins have a negative charge; thus lipoproteins are preferred for adsorption onto the bundles' surface, creating a negative surface potential. Calcium ions concentration on negatively charged surfaces will increase (Figs. 2 and 3). Molecular sieve effect in the presence of mechanical force may press water molecules out of the space sealed by collagen bundles as well as push the bundles closer, which would lead to the concentrating of the ions. The initial interaction of calcium ions with phospholipids or fatty acids may have provided the seeding for initial crystal formation. The concentrated calcium and phosphate ions and condensed lipid-collagen bundles provide an environment for the continued crystal formation and growth as well as constraining crystal size and orientation.
We must point out that our model is based on the analysis of compact bone. A number of issues lead us to the new model instead of the matrix vesicle model that is based on the study of cartilage and other bones:
Composition analysis of lipids isolated from compact bones indicated the predominant content of TG and CE. None of these apolar lipids are known to be structural components of plasma membranes or intracellular membranes of any cells that we are aware of. In other words, one does not expect to find any TG or CE on a cellular plasma membrane. In addition, it is not possible to make vesicles with such a high concentration of apolar lipids. A lipid droplet forms if TG or CE are involved.
No other cell that has been reported in the literature is able to secret vesicles into the matrix. Two kinds of particles known to be secreted by cells and consisting of a large quantity of TG or CE in our body are lipoproteins in serum and lipid droplets in milk.
As calcium phosphate concentration gets close to saturation, the membrane surface potential likely collapses and leads to a membrane structural deformation and the generation of structural features other than the round-shaped particle. This is in contrast to what we observed in our AFM images.
Understanding the nature, the origin, and the role of the round lipid particles provides new potential targets for pharmaceutical interventions of bone metabolism. A Western blot examination of extracted bovine bone proteins using anti-LDL antibody suggests the presence of lipoproteins. Immunohistochemical labeling of the demineralized tissue showed their localization along the osteon rings as well as the Haversian canal.
Wherever there is calcification in the body, we find lipids. In bone, calcification is normal and the disease of osteoporosis results when it is impeded. In contrast, pathological calcification can occur in almost any part of the body and contributes to a broad range of diseases, ranging from renal stones to atherosclerosis. An understanding of the critical role of lipids in the deposition of calcium is needed if we are to find optimal treatments for all these conditions, whether they occur on earth or in space.
The bone surface is lined with osteoblasts, and the sinusoids with a fenestrated layer of endothelial cells. As in the liver, the sinusoid in bone has pores large enough to allow lipoproteins to move between vascular and extravascular environment. Secreted collagen bundles are exposed to bone marrow and its constituents, including lipoproteins.
Bone marrow consumes half of the plasma chylomicrons. Uptake of lipoproteins by bone marrow has proved to be unmodulated by lipoprotein lipase, which is different from most other tissues (26). The diameters of the lipid particles found in demineralized bones are in the size range of chylomicrons, VLDL, and IDL, and aggregated LDL. Lipoproteins are known to interact with and deposit on collagen fibers from the study of advanced atherosclerotic plaques. Although the environment in the vessel wall and in the bone matrix is different, essentially the same molecules, namely collagens, lipoproteins, and calcium ions, are involved in the calcification. A typical feature of advanced atherosclerosis is calcification, which shares many histological features with bone and involves many bone-related proteins, such as osteopontin, osteonectin, and osteocalcin (43). Lipid metabolism abnormality has been identified in periosteal vessels after space flight (44). Bone is unique in the large quantity and density of collagen bundles as shown in AFM images (Figs. 2 and 3). Due to the high content of apolar lipids extracted particularly from compact bones (17), we are examining the possible involvement of lipoproteins or lipid droplets thoroughly.
In summary, a new model for collagen calcification in compact bone is introduced for further examination (Fig. 8). The high density of collagen promotes lipoprotein aggregation and deposition. Binding of lipoproteins or lipid droplets to collagen bundles changes the surface chemical potential of the fibers in favor of the adsorption of the calcium ions. Continuous growth of the collagen bundles in lateral dimensions leads to an increased surface chemical potential and then to calcium adsorption. In addition, collagen bundles form a two-dimensional molecular sieve that traps molecules in between. The high density of collagen bundles reduces its permeability to large molecules or ions. In the presence of an external loading force as well as the internal forces of the interacting surface chemical potentials between two collagen bundles, water molecules could be forced out of the space, whereas calcium ions are trapped. The calcium concentration increases, either due to the chemical potential-induced adsorption effect and/or to the molecular sieve effect, lead to the formation of mineral crystals between the two layers of lipid-coated collagen bundles.
We should point out that our study has limited resolution. More studies are needed for an unambiguous identification of whether lipoproteins or lipid droplets, secreted by osteoblasts and chondrocytes, coat collagen bundles. Current evidence favors the hypothesis that these lipid particles are lipoproteins and/or their aggregates. This is due to their homogeneity in size, availability, and known interaction with collagen. Involvement of lipid droplets, secreted by osteoblasts, in collagen mineralization was proposed (45) to help explain the high apolar lipids composition extracted from bones. However, lipid droplets in general are known to fuse with each other easily and to have a high TG content and various diameters up to microns, which is in conflict with those shown in Fig. 2. Lipid droplets are often thought to serve as inert energy storage sites (46), not as structural components.
Demineralized bones were reportedly remineralized when implanted in rat muscle in the absence of osteoblasts (47), suggesting that bone-forming cells are dispensable. On the other hand, some serum components are reportedly found essential to the remineralization of such demineralized bones (48). Of course, one could argue that once the nucleation units are formed, as in the demineralized bones, continuous growth of mineral crystals occurs even in the absence of bone cells and matrix vesicles. However, from a physical chemistry perspective, any solid particles could seed calcium phosphate crystal formation as long as the solution is saturated with calcium phosphate. Theoretically, those lipid particles coated on collagen bundles could serve well to seed and then initiate the mineral crystal formation in compact bone. Thus, as the structural components of bones, the round lipid coat likely mediates the deposition of minerals on collagen bundles in compact bones.
During spaceflight astronauts experience loss of calcium from weight-bearing bones, loss of body fluid, a reduction in red cell mass, and a shift of osteoblast and osteoclast density. Since all are connected to bone marrow, it remains unknown whether these changes are all related to each other and whether a shift in bone marrow chemistry during a space trip is responsible for all of these. This dissolved bone calcium is excreted by the kidneys, increasing the risk of renal stones. Thus, controlling the dissolution and precipitation of calcium is essential to developing countermeasures for astronauts.
Acknowledgments
We appreciate the support of Dr. Samuel T. Durrance, Director of the Florida Space Research Institute. We are very thankful to Dr. Michael Grace for the use of his laboratory for tissue slices and for some of the materials. We thank Dawn Traynor and Holly Mills for their assistance in editing the manuscript and preparing the figures. We appreciate the helpful discussions with Drs. Duan P. Chen of Rush Medical University, Ming Zhang of Florida Institute of Technology, Godfrey S. Getz of The University of Chicago, and Dan Woodard of Kennedy Space Center.
The work was funded by the Florida Space Research Institute.
Abbreviations used: TG, triacylglycerol; CE, cholesterol ester; AFM, atomic force microscopy; apoB, apolipoprotein B; FA, fatty acid; HCl, hydrochloric acid; LDL, low-density lipoprotein; PBS, phosphate buffer saline; VLDL, very low density lipoprotein; Lp(a), lipoprotein (a); PL, phospholipids.
References
- 1.Schneider, V. S., V. A. Oganov, A. LeBlanc, A. Rakhmanov, A. Bakulin, A. I. Grigoriev, and L. Varonin. 1992. Space flight bone loss and change in fat and lean body mass. J. Bone Miner. Res. 7(Suppl.):S122. [Google Scholar]
- 2.Oganov, V. S., A. I. Grigoriev, L. I. Voronin, A. S. Rakhmanov, A. V. Bakulin, V. S. Schneider, and A. D. LeBlanc. 1992. Bone mineral density in the cosmonauts after 4.5–6 month flights on board MIR orbital station. Aviakosm. Ekolog. Med. 26:20–24. [PubMed] [Google Scholar]
- 3.Vico, L., M. H. Lafage-Proust, and C. Alexandre. 1998. Effects of gravitational changes on the bone system in vitro and in vivo. Bone. 22:95S–100S. [DOI] [PubMed] [Google Scholar]
- 4.Aerssens, J., J. Dequeker, and J. M. Mbuyi-Muamba. 1994. Bone tissue composition: biochemical anatomy of bone. Clin. Rheumatol. 13(Suppl. 1):54–62. [PubMed] [Google Scholar]
- 5.Anderson, H. C. 1969. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 41:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonucci, E. 1967. Fine structure of early cartilage calcification. J. Ultrastruct. Res. 20:33–50. [DOI] [PubMed] [Google Scholar]
- 7.Ali, S. Y., S. W. Sajdera, and H. C. Anderson. 1970. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc. Natl. Acad. Sci. USA. 67:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennever, J., L. J. Riggan, and J. J. Vogel. 1984. Proteolipid and collagen calcification, in vitro. Cytobios. 39:151–157. [PubMed] [Google Scholar]
- 9.Iannotti, J. P., S. Naidu, Y. Noguchi, R. M. Hunt, and C. T. Brighton. 1994. Growth-plate matrix vesicle biogenesis: The role of intracellular calcium. Clin. Orthop. 306:222–229. [PubMed] [Google Scholar]
- 10.Landis, W. J., and F. H. Silver. 2002. The structure and function of normally mineralizing avian tendons. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133:1135–1157. [DOI] [PubMed] [Google Scholar]
- 11.Landis, W. J., M. J. Song, A. Leith, L. McEwen, and B. F. McEwen. 1993. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 110:39–54. [DOI] [PubMed] [Google Scholar]
- 12.Du, C., F. Z. Cui, W. Zhang, Q. L. Feng, X. D. Zhu, and K. De Groot. 2000. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 50:518–527. [DOI] [PubMed] [Google Scholar]
- 13.Boskey, A. L., B. D. Boyan, and Z. Schwartz. 1997. Matrix vesicles promote mineralization in a gelatin gel. Calcif. Tissue Int. 60:309–315. [DOI] [PubMed] [Google Scholar]
- 14.Anderson, H. C. 2003. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5:222–226. [DOI] [PubMed] [Google Scholar]
- 15.Genge, B. R., L. N. Wu, H. D. Adkisson, and R. E. Wuthier. 1991. Matrix vesicle annexins exhibit proteolipid-like properties. J. Biol. Chem. 266:10678–10685. [PubMed] [Google Scholar]
- 16.Shapiro, I. M. 1970. The phospholipids of mineralized tissues. Calcif. Tissue Res. 5:21–29. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro, I. M. 1971. The neutral lipids of bovine bone. Arch. Oral Biol. 16:411–421. [DOI] [PubMed] [Google Scholar]
- 18.Tirziu, D., A. Dorbrian, C. Tasca, M. Simionescu, and N. Simionescu. 1995. Initimal thickenings of human aorta contain modified reassembled lipoproteins. Atherosclerosis. 112:101–114. [DOI] [PubMed] [Google Scholar]
- 19.Tertov, V. V., A. N. Orekhov, I. A. Sobenin, Z. A. Gabbasov, E. G. Popov, A. A. Yaroslavov, and V. N. Smirnov. 1992. Three types of naturally occurring modified lipoproteins induce intracellular lipid accumulation due to lipoprotein aggregation. Circ. Res. 71:218–228. [DOI] [PubMed] [Google Scholar]
- 20.Guyton, J. R., and K. F. Klemp. 1996. Development of the lipid-rich core in human atherosclerosis. Arterioslerosis, Thrombosis, and Vascular Biology. 16:4–11. [DOI] [PubMed] [Google Scholar]
- 21.Schaffner, T., K. Taylor, E. J. Bartucci, K. Fischer-Dzoga, J. H. Beeson, S. Glagov, and R. W. Wissler. 1980. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am. J. Pathol. 100:57–73. [PMC free article] [PubMed] [Google Scholar]
- 22.Frank, J. S., and A. M. Fogelman. 1989. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freeze-etching. J. Lipid Res. 30:967–978. [PubMed] [Google Scholar]
- 23.Guyton, J. R., and K. F. Klemp. 1989. The lipid-rich core region of human atherosclerotic fibrous plaques. Prevalence of small lipid droplets and vesicles by electron microscopy. Am. J. Pathol. 134:705–717. [PMC free article] [PubMed] [Google Scholar]
- 24.Tintut, Y., and L. L. Demer. 2001. Recent advances in multifactorial regulation of vascular calcification. Curr. Opin. Lipidol. 12:555–560. [DOI] [PubMed] [Google Scholar]
- 25.Mody, N., Y. Tintut, K. Radcliff, and L. L. Demer. 2003. Vascular calcification and its relation to bone calcification: possible underlying mechanisms. J. Nucl. Cardiol. 10:177–183. [DOI] [PubMed] [Google Scholar]
- 26.Hussain, M. M., I. J. Goldberg, K. H. Weisgraber, R. W. Mahley, and T. L. Innerarity. 1997. Uptake of chylomicrons by the liver, but not by the bone marrow, is modulated by lipoprotein lipase activity. Arteriosclero, Thromb, and Vasc. Biol. 17:1407–1413. [DOI] [PubMed] [Google Scholar]
- 27.Kruger, M. C., and D. F. Horrobin. 1997. Calcium metabolism, osteoporosis and essential fatty acids: a review. Prog. Lipid Res. 36:131–151. [DOI] [PubMed] [Google Scholar]
- 28.Hamrick, M. W. 2004. Leptin, bone mass, and the thrifty phenotype. J. Bone Miner. Res. 19:1607–1611. [DOI] [PubMed] [Google Scholar]
- 29.Beisiegel, U., and A. A. Spector. 2002. Bone: a forgotten organ in lipidology? Curr. Opin. Lipidol. 13:239–240. [DOI] [PubMed] [Google Scholar]
- 30.Binnig, G., C. F. Quate, and C. Gerber. 1986. Atomic force microscopy. Phys. Rev. Lett. 56:930–933. [DOI] [PubMed] [Google Scholar]
- 31.Xu, S. 1998. Apolipoprotein(a) binds to low-density lipoprotein at two distant sites in lipoprotein(a). Biochem. 37:9284–9294. [DOI] [PubMed] [Google Scholar]
- 32.Xu, S., B. Bevis, and M. F. Arnsdorf. 2001. The assembly of amyloidogenic yeast Sup35 as assessed by scanning (atomic) force microscopy: an analogy to linear colloidal aggregation? Biophys. J. 81:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, S., D. Wu, M. Arnsdorf, R. Johnson, G. S. Getz, and V. G. Cabana. 2005. Chemical colloids versus biological colloids: a comparative study for the elucidation of the mechanism of protein fiber formation. Biochemistry. 44:5381–5389. [DOI] [PubMed] [Google Scholar]
- 34.Xu, J., J. Y. Rho, S. R. Mishra, and Z. Fan. 2003. Atomic force microscopy and nanoindentation characterization of human lamellar bone prepared by microtome sectioning and mechanical polishing technique. J. Biomed. Mater. Res. A. 67:719–726. [DOI] [PubMed] [Google Scholar]
- 35.Hassenkam, T., G. E. Fantner, J. A. Cutroni, J. C. Weaver, D. E. Morse, and P. K. Hansma. 2004. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 35:4–10. [DOI] [PubMed] [Google Scholar]
- 36.Gartner, L. P., and J. L. Hiatt. 1987. Atlas of Histology. Williams & Wilkins, Baltimore.
- 37.Zubay, G. 1983. Biochemistry. Addison-Wesley Publishing Company, Menlo Park.
- 38.Wuthier, R. E. 1975. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim. Biophys. Acta. 409:128–143. [DOI] [PubMed] [Google Scholar]
- 39.Genge, B. R., L. N. Wu, and R. E. Wuthier. 2003. Separation and quantification of chicken and bovine growth plate cartilage matrix vesicle lipids by high-performance liquid chromatography using evaporative light scattering detection. Anal. Biochem. 322:104–115. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg, B. 1985. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 56:93–110. [DOI] [PubMed] [Google Scholar]
- 41.Xu, S., and B. Lin. 2001. The mechanism of oxidation-induced low-density lipoprotein aggregation: an analogy to colloidal aggregation and beyond? Biophys. J. 81:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Israelachvili, J. 1994. Intermolecular and Surface Forces. Academic Press, San Diego.
- 43.Bini, A., K. G. Mann, B. J. Kudryk, and F. J. Schoen. 1999. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 19:1852–1861. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, T., S. Ueda, K. Takahashi, and R. O. Scow. 1994. pH-dependent multilamellar structures in fetal mouse bone: possible involvement of fatty acids in bone mineralization. Am. J. Physiol. Cell Physiol. 266:590–600. [DOI] [PubMed] [Google Scholar]
- 45.Van Meer, G. 2001. Caveolin, cholesterol, and lipid droplets? J. Cell Biol. 152:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita, K., and T. Takagi. 1992. Ultrastructural observation of calcification preceding new bone formation induced by demineralized bone matrix gelatin. Acta Anat. (Basel). 143:261–267. [DOI] [PubMed] [Google Scholar]
- 47.Hamlin, N. J., and P. A. Price. 2004. Mineralization of decalcified bone occurs under cell culture conditions and requires bovine serum but not cells. Calcif. Tissue Int. 75:231–242. [DOI] [PubMed] [Google Scholar]
- 48.Doty, S. B., E. R. Morey-Holton, G. N. Durnova, and A. S. Kaplansky. 1992. Morphological studies of bone and tendon. J. Appl. Physiol. 73:10–13. [DOI] [PubMed] [Google Scholar]