Abstract
The ability of cells to mount localized responses to external or internal stimuli is critically dependent on organization of lipids and proteins in the plasma membrane. Involvement of the actin cytoskeleton in membrane organization has been documented, but an active role for actin networks that directly links internal organization of the cytoskeleton with membrane organization has not yet been identified. Here we show that branched actin networks formed on model lipid membranes enriched with the lipid second messenger PIP2 trigger both temporal and spatial rearrangement of membrane components. Using giant unilamellar vesicles able to separate into two coexisting liquid phases, we demonstrate that polymerization of dendritic actin networks on the membrane induces phase separation of initially homogenous vesicles. This switch-like behavior depends only on the PIP2-N-WASP link between the membrane and actin network, and we find that the presence of a preexisting actin network spatially biases the location of phase separation. These results show that dynamic, membrane-bound actin networks alone can control when and where membrane domains form and may actively contribute to membrane organization during cell signaling.
INTRODUCTION
Spatial organization of the plasma membrane and associated membrane proteins underlies many important cellular processes, including cell-cell communication, polarization, and motility (1–4). For example, formation of the immunological synapse requires clustering of T cell receptors and MHC-peptide at the junction between a T cell and antigen presenting cell (5), and antigen-mediated oligomerization of IgE receptors in mast cells drives their association with lipid rafts (6). Local interaction of lipids or proteins via clustering or oligomerization has been shown to trigger a cascade of events that affect partitioning of membrane molecules into membrane domains (7–10). However, a mechanism by which the cell could actively control this partitioning is not well understood, though the cell's actin cytoskeleton is believed to play an important role (6,11).
The actin cytoskeleton is a dynamic network of filaments that can rapidly reorganize in response to external or internal stimuli (12,13). An important role for the actin cytoskeleton in membrane organization is suggested by the observation that signaling from IgE receptors and formation of the immunological synapse both require an intact actin cytoskeleton (14–17). One of the most prominent physical and functional links between the plasma membrane and the actin cytoskeleton is the lipid second messenger phosphatidyl-inositol 4,5 bisphosphate (PIP2) through the actin nucleation promoting factor neural Wiscott-Aldrich syndrome protein (N-WASP) (18,19). Transient binding of N-WASP by PIP2 and GTP-bound Cdc42 controls activation of the branching complex Arp2/3, which is responsible for the formation of dendritic actin networks (20). We hypothesized that membrane components linked through the cell's actin cytoskeleton could influence global membrane organization, providing a mechanism through which changes in cytoskeletal organization could directly alter membrane organization.
Ternary lipid systems that undergo phase separation into coexisting liquid ordered (Lo) and liquid disordered (Ld) domains have served as model systems for investigating membrane organization in recent years (21). One advantage of this system is that temperature can be used to reversibly change the thermodynamic state of the membrane without perturbing membrane composition. Phase separation behavior can be quantified with miscibility transition temperature, denoted as Tmisc. Tmisc corresponds to the temperature above which separated phases are homogenized into a single phase. Recently, Hammond et al. (22) reported that local clustering of GM1 by the pentameric ligand cholera toxin B (CTB) causes a uniform membrane to phase separate into domains. We used a similar in vitro vesicle system to investigate the interplay between membrane organization and dynamic actin networks.
In our work, formation of a localized actin network on model lipid membranes both induces de novo membrane domain formation and stabilizes existing membrane domains by shifting Tmisc of the membrane. Furthermore, the presence of an actin network associated with the membrane was found to spatially bias location of membrane domain formation after homogenization. These results suggest that actin polymerization can act as a “switch” for spatial and temporal organization of membrane components. Upon growth of an Arp2/3-branched actin network on PIP2-containing vesicles, membrane organization can be reversibly changed from a homogeneous distribution of lipids to a phase-separated membrane.
MATERIALS AND METHODS
Lipids and proteins
The following lipids were purchased from Avanti Polar Lipids (Alabaster, AL): DPPC, dipalmitoylphosphatidylcholine (di(16:0)PC), DOPC, dioleoylphosphatidylcholine (di(18:1)PC), DOPS, dioleoylphosphatidylserine (di(18:1)PS), Chol, cholesterol, Brain PI(4,5)P2, Fluo-DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein). BODIPY tetramethylrhodamine 1,2-dipalmitoylphosphatidylinositol 4,5 diphosphate, BODIPY tetramethyl rhodamine (TMR) PIP2, was purchased from Echelon Biosciences (Salt Lake City, UT). Actin was purified from rabbit acetone dried powder as described by Spudich and Watt (23). Rhodamine actin was labeled according to Amann et al. (24). His-tagged Rat ΔEVH1 N-WASP was overexpressed in Escherichia coli and purified using Nickel affinity chromatography (25). Arp2/3 complex was purified from bovine brains (26). Protein purities were checked by sodium dodecyl sulfate gel electrophoresis. Protein concentrations were determined by protein absorbance at 280 nm or 290 nm. N-WASP activity was confirmed by bead motility assay using N-WASP-coated microspheres (27). Arp2/3 complex activity was confirmed by pyrene actin polymerization assay (28). Alexa Fluor 546 phalloidin was purchased from Invitrogen (Carlsbad, CA).
Giant unilamellar vesicles formation
Giant unilamellar vesicles (GUVs) were grown using the electroformation technique at 60°C (29). Our model system consists of a lipid composition of 2%:1%:30%:0.6% DPPC/DOPC/Chol/BODIPY TMR PIP2. The ternary lipid system of DPPC/DOPC/Chol at a ratio of 2%:1%:30% has been shown to phase separate into Lo and Ld domains at ∼33° (21). A trace amount of either lis-DPPE or fluo-DOPE (0.5–1.0%) was used as a marker for DOPC-rich Ld domains. To prepare lipid mixtures, stock lipids in chloroform are used to make 2%:1%:30%:0.6% mixture of DPPC/DOPC/Chol/BODIPY TMR PIP2 by mole to yield vesicles that are phase separated at room temperature. Lipid mixture was spread onto an electrically conductive and optically transparent slide coated with indium tin oxide (ITO) (Delta Technologies, Stillwater, MN), dried under vacuum for 30 min before swelling in 350 mOsm sucrose solution under alternate electric field for 3 h in a chamber consisting of two ITO slides spaced between a silicon rubber spacer.
Actin-associated vesicles
PIP2-containing vesicles were incubated with N-WASP for 15 min (20 mM HEPES, pH 7.5, 100 mM NaCl) and then were added to a solution of actin and Arp2/3 complex. The final mixture consists of 6 μM actin, 150 nM Arp2/3 complex, 390 nM N-WASP in polymerization buffer (50 mM KCl, 2 mM MgCl2, 5 mM Tris, pH 7.4, 1 mM ATP, 1 mM dithiothreitol) with adjusted osmolarity. The sample was loaded into a flow cell consisting of a glass slide and a cover glass spaced between two strips of double-sided tape ∼6 mm apart. To remove membrane-associated actin patches, 0.75 mg/ml Proteinase K was added and incubated for 15 min at 37°C. Entangled actin filaments were found to be stable at temperature up to 50°C based on an analysis of Brownian motion of micron-sized polystyrene beads.
Microscopy
GUVs were observed by phase contrast and epifluorescence microscopy using a 40× objective on an inverted microscope (Axiovert 200, Carl Zeiss, Germany). Plain vesicles were diluted with glucose solution of equal osmolarity to allow sedimentation and refractive index gradient for contrast enhancement. Temperature of the sample is controlled by a temperature-controlled stage fitted onto the microscope platform (Instec, Boulder, CO), and sample temperature was calibrated with a digital thermometer to within 0.2°C. At each temperature, vesicles were viewed in phase contrast and fluorescence microscopy at random locations throughout the chamber, and the number of vesicles that were phase separated was counted. Temperature was ramped at a rate of 2°C/min, and the stage and the sample were allowed to equilibrate to the new temperature for at least 15 min. Tmisc and its associated standard deviation were obtained by fitting the data points based on 6–9 temperatures with n = 30–100 to a sigmoidal curve. The standard deviation associated with change in Tmisc was calculated by using the appropriate error propagation.
Laser scanning confocal microscopy was used to image actin network (labeled with Alexa Fluor 546 phalloidin) on the surface of Ld membrane (labeled with fluo-DOPE) using a 60× oil objective (SP2-AOBS, Leica, Germany). Three-dimensional reconstruction was performed by using VolumeJ, a downloadable plug-in for ImageJ (http://rsb.info.nih.gov/ij/). Confocal images are shown in Supplemental Materials.
High resolution scanning electron microscopy was used to image the dense ultrastructure of actin network nucleated from the membrane surface (Hitachi, Pleasanton, CA). The sample was deposited on polylysine-coated silicon substrate, fixed with 2% glutaraldehyde, permeablized partially with 0.1% Triton X-100, and postfixed with 1% OsO4. The sample was then dried with ethanol dehydration followed by critical point drying using liquid CO2. Dried sample was coated with 2 nm of iridium using a high resolution sputter coater (BAL-TEC, Tuscan, AZ).
RESULTS
Actin networks assemble on PIP2-enriched domains
GUVs were made by electroformation from a mixture of saturated lipid dipalmitylphosphatidylcholine (DPPC), unsaturated lipid dioleoylphosphatidylcholine (DOPC), and cholesterol (Chol) (see Materials and Methods). BODIPY TMR phosphatidylinositol 4,5 bisphosphate (TMR-PIP2) was added to stimulate actin network growth, and a trace amount of fluorescent phospholipid probe dioleoylphosphatidylethanolamine-N-carboxyfluorescein (fluo-DOPE) or 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (lis-DPPE) was added to identify phase separation.
On phase-separated vesicles, the purified proteins N-WASP, Arp2/3 complex, and actin formed dendritic actin networks only on the domains enriched in TMR-PIP2, creating an actin-associated domain shown schematically in Fig. 1 A. Networks were formed on the outside of vesicles for experimental simplicity. High resolution scanning electron microscopy confirmed that the actin polymerized on the vesicle consisted of a dense network of filaments with similar spacing to actin networks in lamellipodia (Fig. 1 B and Supplemental Materials). Sufficiently dense actin networks can be identified by phase contrast microscopy and appear dark due to their optical phase density (Fig. 1 C i). The optical phase-dense appearance is consistent with images of the actin-propelled pathogen Listeria monocytogenes (30) and actin-propelled microspheres coated in nucleation promoting factors (31), and it serves as a convenient indicator of actin network growth. Presence of the actin network was further confirmed fluorescently through use of rhodamine actin and fluorescent phalloidin (see Supplemental Materials Fig. S1). Epifluorescence microscopy of fluo-DOPE (Fig. 1 C ii) and TMR-PIP2 (Fig. 1 C iii) showed that TMR-PIP2 colocalized with fluo-DOPE. Fig. 1 C iv shows an overlay of Fig. 1 C i–iii where phase-dense actin has been pseudocolored in blue to show localized growth of actin on the phase-separated vesicle.
FIGURE 1.
Assembly of actin networks on phase-separated GUVs. (A) Illustration of membrane-associated actin on a GUV (only the outer phospholipid bilayer is shown, not drawn to scale). The lipid PIP2 (in red), which binds to N-WASP (in blue) and then activates the Arp2/3 complex (in yellow) to initiate growth of a dendritic actin network at the surface of the vesicle. (B) Scanning electron micrograph of an actin network grown at the surface of a vesicle. (C) Phase-separated vesicle with membrane-associated actin. (i) Optical phase contrast image showing the outline of the vesicle and phase-dense actin network. (ii) Epifluorescence image of fluo-DOPE that labels the Ld domain. (iii) Epifluorescence image of BODIPY TMR PIP2 showing localization of PIP2 within the Ld domain. (iv) Overlay of fluo-DOPE, TMR-PIP2, and optical phase contrast (optical phase-dense object is pseudocolored in blue) images showing colocalization of the actin network on the Ld domain. Scale bars are 10 μm.
Based on the smaller fraction of bright phase than dark phase in the vesicle coinciding with the smaller proportion of unsaturated lipid than saturated lipid present in our model system, we believe that fluo-DOPE labels the Ld domain, and therefore TMR-PIP2 partitions to the Ld domain. However, the designation of the domain associated with PIP2 is not central to our results. The spatial organization of PIP2 in plasma membranes is still under debate (32,33), and at least one discrepancy in the phase behavior of proteins between model and native membranes has been reported (34). In our experiments, phase-separated membranes enriched in TMR-PIP2 facilitate polymerization of dendritic actin networks on a single domain so that the influence of an associated actin network on phase behavior can be studied.
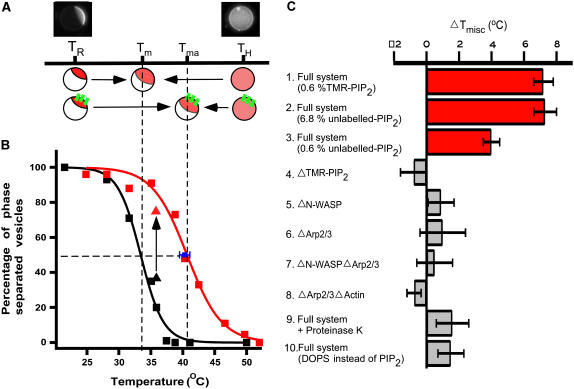
Miscibility transition temperature increases in actin-associated membrane
We first examined the effect of a dynamic actin network on membrane domain formation by determining Tmisc (Fig. 2 A). Phase-separated vesicles with associated actin networks were prepared below Tmisc, and temperature was increased until the lipids homogenized according to visualization of fluo-DOPE. Since lipid composition of individual vesicles varies slightly during preparation (35), we defined Tmisc as the temperature at which 50% of vesicles in a population were phase separated by random sampling at each temperature. Vesicles associated with actin networks (Fig. 2 B, red squares; n = 30–33) were found to have Tmisc that was 7.2°C ± 0.6°C (n = 30–33) higher than in the absence of actin (Fig. 2 B, black squares; n = 30–100), signifying an increase in the enthalpy of mixing and stabilization of existing membrane domains associated with actin.
FIGURE 2.
Shift in Tmisc due to membrane-associated actin networks. (A) Illustration of domain stabilization by actin networks. Tma and Tm denote Tmisc for the vesicles with and without actin, respectively. TR and TH indicate room temperature and high temperature, respectively. (B) Tmisc increases for GUVs associated with an actin network (red squares) compared to those without (black squares). Tm and Tma were determined to be 33.4°C ± 0.5°C and 40.6°C ± 0.3°C by fitting the data points to a sigmoidal curve (black and red line). When actin was polymerized at TH, Tmisc of individual vesicles (n = 3) was found to be 40.3°C ± 0.8°C (blue square) by lowering temperature. (C) Dependence of Tmisc shift on model system composition. A substantial increase in Tmisc was not observed when N-WASP or TMR-PIP2 were omitted from the model system (bars 4 and 5), indicating that PIP2-N-WASP linked through actin filaments is responsible for the observed increase in Tmisc. When the actin network was removed from membrane by incubation with Proteinase K or when TMR-PIP2 was replaced by DOPS, a substantial increase in Tmisc was not observed (bars 9 and 10). However, when TMR-PIP2 was replaced with unlabeled-PIP2, a significant increase in Tmisc was measured (bars 2 and 3). Phase separation of membrane was determined by lis-DPPE fluorescence for bars 2, 3, and 10, and fluo-DOPE for all other experiments. Tmisc was determined as in B.
We next examined the effect on phase separation behavior of actin networks formed on initially homogenized membranes. Vesicles were prepared above Tmisc and components of the actin network were added. Distinct actin patches were observed to assemble on membranes that had an initially homogeneous distribution of TMR-PIP2. Tmisc was then quantified by observing individual vesicles while temperature was decreased. The vesicles exhibited an increase in Tmisc of 6.9°C ± 0.8°C (n = 3), the same as actin-associated vesicles that were initially phase separated (Fig. 2 B, blue square). We also measured Tmisc using unlabeled PIP2 vesicles (at 6.8%) and found a similar increase in Tmisc of 7.3°C ± 0.7°C (n = 35–40, Fig. 2 C, bar 2). Interestingly, at 0.6% PIP2 we found a smaller but significant increase in Tmisc of 4.1°C ± 0.4°C (n = 35–40, Fig. 2 C, bar 3), which was consistent with the decreased nucleation activity of unlabeled PIP2 compared to TMR-PIP2 (see Discussion and Supplemental Materials). The increase in Tmisc found with increasing PIP2 concentrations suggests that the stabilizing effects of vesicle-associated actin networks may increase with the number of linkages between the membrane and actin; however, the dependence on specific components in the system and the resulting behavior are expected to be complex.
PIP2-N-WASP link is necessary for the observed increase in Tmisc
To determine which proteins and lipids were responsible for the observed increase in Tmisc, we systematically omitted components of the system and quantified Tmisc as before (Fig. 2 C). A mixture without either PIP2 or N-WASP had little effect on Tmisc compared to the original ternary lipid vesicles (bars 4 and 5). Vesicles in polymerized actin filaments in the absence of Arp2/3 complex also behaved similarly, with or without N-WASP (bars 6 and 7), compared to vesicles without added proteins. To exclude the possibility that N-WASP binding to PIP2 affects phase transition behavior, we incubated the PIP2 vesicle with N-WASP but no other components of the actin network and found no change in Tmisc (bar 8). Digestion of membrane-associated actin networks by incubation with Proteinase K (bar 9) also resulted in comparable Tmisc with respect to the original vesicles. Finally, to ensure N-WASP is activated in a physiologically relevant manner through binding to PIP2, we found that replacing PIP2 with charged lipid DOPS in sufficient concentration to achieve the same surface charge as PIP2 does not alter Tmisc in the presence of purified proteins (bar 10). These experiments demonstrate that the interaction between PIP2 and N-WASP bound to actin filaments is necessary and sufficient for altering membrane organization.
Actin networks serve as a membrane domain switch
The shift in Tmisc of membrane associated with an actin network relative to membrane not associated with an actin network suggests that actin polymerization can induce formation of membrane domains. To demonstrate this, we held a population of homogeneous vesicles at a temperature slightly above Tmisc and added the actin network components, maintaining a constant temperature of 36°C. We observed that 74.3% ± 5.1% (n = 74) of the vesicles were phase separated after addition of actin compared to 38.0% ± 4.9% (n = 100) of vesicles before. This shift, shown by the arrow pointing vertically upward from a black triangle to a red triangle in Fig. 2 B, demonstrates that the associated actin network had switched the phase behavior of the vesicle population without changes in temperature or membrane composition.
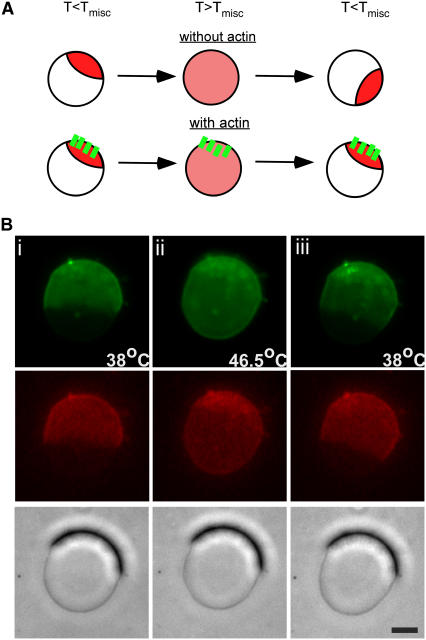
To investigate whether a preexisting actin network can spatially direct domain arrangement on membranes, we tracked the formation of membrane domains on individual actin-associated vesicles as they were cycled above and below Tmisc (Fig. 3 A). Upon lowering temperature to induce phase separation, we found that membrane domains repeatedly reformed at locations that colocalized with actin networks (n = 6) (Fig. 3 B). By comparison, vesicles without membrane-associated actin networks yielded domains in random positions after temperature cycling above and below Tmisc (data not shown).
FIGURE 3.
Spatially biased formation of phase-separated domains. (A) Illustration of domain reformation at existing membrane-associated actin networks after temperature cycling above and below Tmisc. (B) Optical phase contrast, epifluorescence of fluo-DOPE, and TMR-PIP2 images of a GUV during temperature cycling above and below Tmisc. After temperature cycling, fluorescent phase-separated lipid domains reformed at the same location as existing phase-dense membrane-associated actin networks with temperature cycling. Scale bar is 10 μm.
DISCUSSION
Mechanism of domain stabilization
The results presented in this work demonstrate that dendritic actin networks associated with model lipid bilayers through a PIP2-N-WASP link can both stabilize and induce phase-separated domains. Although changes in Tmisc have been observed upon cross-linking or oligomerization of lipids, the mechanism of actin-induced phase separation is distinct. Cross-linking a lipid raft component GM1 by CTB has been shown to increase the Tmisc of GM1-containing vesicles after CTB binding by an average of ∼6°C (22). Although stabilization of phase-separated domains is also shown in our experiments, membrane components organized by association with actin must be linked by long-range (i.e., nonadjacent) connections through the filament network rather than by local (i.e., adjacent) connections such as through CTB binding.
Links between the actin cytoskeleton and PIP2 have been shown to be important in a number of studies. For example, Kwik et al. (36) found that organization of cellular actin was dependent on both PIP2 and cholesterol, a component critical for lipid rafts. Furthermore, force measurements on tethers pulled from cell membrane showed that PIP2 regulates the adhesion energy between the membrane and cytoskeleton (37). The only component in our simplified system that is able to bind to PIP2 is N-WASP, but binding of PIP2 to N-WASP alone was unable to increase Tmisc (Fig. 2 C, bar 8). This suggests that the presence of an interconnected actin network is essential for mediating the observed shift in phase behavior. In contrast to ligands that cross-link neighboring molecules on a membrane (22), a branched actin network is able to connect distant PIP2 molecules to stabilize membrane domains.
This proposed “networking” mechanism for organizing membrane components is expected to exhibit different behavior from local cross-linking. For instance, because local cross-links of membrane components, such as CTB-GM1, act independently, we would not expect to see a spatial biasing of domain reformation when temperature is lowered below Tmisc on CTB-GM1 vesicles. In contrast, we do observe spatial biasing in membrane associated with actin networks. This spatial biasing may be explained by a preferential localization of PIP2-N-WASP with preexisting actin networks that in turn localizes associated lipid molecules in the Ld phase.
Actin polymerization on model lipid membrane
We found that vesicles containing unlabeled PIP2 exhibit increased Tmisc when associated with actin networks (Fig. 2 C, bars 2 and 3), but we chose to use TMR-PIP2-containing vesicles for most experiments to enable fluorescence imaging of both PIP2 and DOPE at the same time as phase microscopy imaging of phase-dense actin networks. The fluorescent group of TMR-PIP2 is attached to the hydrophobic lipid tail, and this modification is known to preserve the activity of PIP2 as shown by fluorescence resonance energy transfer experiments in a model system (19). We speculate the difference between unlabeled PIP2 and TMR-PIP2 lies in their capacity to activate N-WASP. In vivo, N-WASP can be activated through the release of autoinhibitory interactions by binding to coactivators PIP2 and GTP-bound Cdc42 (38). It has previously been found that PIP2 alone (in the absence of Cdc42) can activate N-WASP activity when reconstituted in small lipid vesicles (kact = 8 μM) (39). In a cell of 10 μm in diameter, a typical PIP2 concentration of 1% has an effective concentration of ∼14 μM on the membrane surface, which is sufficient to activate N-WASP without Cdc42. We found that in the absence of Cdc42, vesicles containing 0.6% TMR-PIP2 could activate N-WASP sufficiently to produce actin networks with a density similar to those found in lamellipodia, and the presence of phase-dense dendritic actin networks could be detected within a minute of incubating the vesicles with actin and Arp2/3 complex. When unlabeled PIP2 was used in place of TMR-PIP2, rhodamine-labeled actin confirmed that a network was polymerized on the membrane surface, though more slowly and less dense than TMR-PIP2-containing vesicles. As a result, we could not easily detect the presence of actin networks on the surface of the vesicles using phase contrast microscopy. How TMR-PIP2 mechanistically enhances activation of N-WASP compared to unlabeled PIP2 is unclear. It has been suggested that PIP2 binds to the basic domain of N-WASP in a multi-valent, cooperative manner (25). Thus, one possible explanation for the enhanced activity of TMR-PIP2 could be its ability to cluster due to hydrophobic stacking of the BODIPY-TMR dye, though we note that TMR-PIP2 alone had no significant effect on the miscibility transition temperature. Alternatively, the fluorescent group may cause extension of the headgroup, which would facilitate destabilization of inactivated N-WASP similar to the combined effect of PIP2 and Cdc42 in vivo.
It is worth noting that at temperatures above Tmisc, the lipid composition may not be truly homogeneous. It has been shown that domains with dimensions below the optical resolution exist above Tmisc (40,41), and it is possible that the spatial biasing of domain formation occurs through coalescence of small domains into a larger liquid lipid phase. At temperatures above Tmisc, a slightly heterogeneous distribution of lipids may have a catalytic effect on large-scale phase separation when temperature is lowered. In addition, our model lipid bilayer composition does not accurately match that of the inner surface of the plasma membrane. Since membrane composition is likely to vary between cell types and at different times in the life cycle of the cell, there is no standard way to model composition of the inner leaflet of plasma membrane. It remains to be understood how actin networks that stabilize inner membrane lipids could influence organization of outer membrane lipids through transbilayer coupling.
CONCLUSIONS
The plasma membrane of cells is a complex structure that is specifically organized for a variety of functional purposes. Cytoplasmic linkages to the plasma membrane through the cytoskeleton have been shown to provide barriers to diffusion (42,43), to stabilize signaling events in some cell types (6), and to inhibit receptor signaling in other cases (44). Our simplified system, based on reconstitution of actin networks on model lipid bilayers, illustrates a general mechanism for how cellular membrane components may be organized by a dynamic actin cytoskeleton. In contrast to the view of actin networks as passively restricting diffusion of lipids and proteins in the membrane, we propose that actin polymerization and depolymerization can actively organize membrane domains. By affecting lipid-lipid interactions through cytoskeleton-linked lipid-protein interaction, actin assembly can promote formation of membrane domains and actin disassembly can destabilize them, thus acting as a switch. Since membrane-associated actin networks favor domain formation, additional signaling molecules may be localized during network growth, leading to positive feedback and amplification. Conversely, disassembly of the network can lead to homogenization of domain components that erases membrane organization. In addition to the previously demonstrated effects of clustering and oligomerization of membrane components by binding to ligands/antibodies on the extracellular side of the membrane (45), our results suggest that the actin cytoskeleton can directly influence membrane organization from the cytosolic side. By controlling actin networking at the membrane, it may be possible for the cell to finely control membrane organization with high spatial and temporal precision.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
We thank M. M. van Duijn, J. W. Shaevitz, S. H. Parekh, and the rest of the Fletcher lab. We also thank S. L. Veatch, D. Mullins, W. Lim, and J. Taunton for helpful discussions, C. Co and D. Wong for help with protein purification, and J. Jans for help with confocal microscopy.
This work is supported in part by a fellowship from Natural Sciences and Engineering Research Council of Canada (A.L.), a National Science Foundation CAREER Award, and support from the National Institutes of Health (D.A.F.).
References
- 1.Blanchard, N., and C. Hivroz. 2002. The immunological synapse: the more you look the less you know. Biol. Cell. 94:345–354. [DOI] [PubMed] [Google Scholar]
- 2.Dustin, M. L. 2002. Shmoos, rafts, and uropods—the many facets of cell polarity. Cell. 110:13–18. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Mouton, C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and A. C. Martinez. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA. 98:9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Mouton, C., R. A. Lacalle, E. Mira, S. Jimenez-Baranda, D. F. Barber, A. C. Carrera, A. C. Martinez, and S. Manes. 2004. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J. Cell Biol. 164:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- 6.Holowka, D., J. A. Gosse, A. T. Hammond, X. Han, P. Sengupta, N. L. Smith, A. Wagenknecht-Wiesner, M. Wu, R. M. Young, and B. Baird. 2005. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim. Biophys. Acta. 1746:252–259. [DOI] [PubMed] [Google Scholar]
- 7.Harder, T., and K. Simons. 1999. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol. 29:556–562. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell, J. S., O. Kanca, and B. W. McIntyre. 2002. Lipid microdomain clustering induces a redistribution of antigen recognition and adhesion molecules on human T lymphocytes. J. Immunol. 168:2737–2744. [DOI] [PubMed] [Google Scholar]
- 9.Gekara, N. O., T. Jacobs, T. Chakraborty, and S. Weiss. 2005. The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell. Microbiol. 7:1345–1356. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. [DOI] [PubMed] [Google Scholar]
- 11.Plowman, S. J., C. Muncke, R. G. Parton, and J. F. Hancock. 2005. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 102:15500–15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diez, S., G. Gerisch, K. Anderson, A. Muller-Taubenberger, and T. Bretschneider. 2005. Subsecond reorganization of the actin network in cell motility and chemotaxis. Proc. Natl. Acad. Sci. USA. 102:7601–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard, T. D., and G. G. Borisy. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. [DOI] [PubMed] [Google Scholar]
- 14.Dustin, M. L., and J. A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. [DOI] [PubMed] [Google Scholar]
- 15.Valensin, S., S. R. Paccani, C. Ulivieri, D. Mercati, S. Pacini, L. Patrussi, T. Hirst, P. Lupetti, and C. T. Baldari. 2002. F-actin dynamics control segregation of the TCR signaling cascade to clustered lipid rafts. Eur. J. Immunol. 32:435–446. [DOI] [PubMed] [Google Scholar]
- 16.Meiri, K. F. 2005. Lipid rafts and regulation of the cytoskeleton during T cell activation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowka, D., E. D. Sheets, and B. Baird. 2000. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. J. Cell Sci. 113:1009–1019. [DOI] [PubMed] [Google Scholar]
- 18.Sheetz, M. P. 2001. Cell control by membrane-cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2:392–396. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin, S., and D. Murray. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 438:605–611. [DOI] [PubMed] [Google Scholar]
- 20.Blanchoin, L., K. J. Amann, H. N. Higgs, J. B. Marchand, D. A. Kaiser, and T. D. Pollard. 2000. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 404:1007–1011. [DOI] [PubMed] [Google Scholar]
- 21.Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond, A. T., F. A. Heberle, T. Baumgart, D. Holowka, B. Baird, and G. W. Feigenson. 2005. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 102:6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spudich, J. A., and S. Watt. 1971. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246:4866–4871. [PubMed] [Google Scholar]
- 24.Amann, K. J., and T. D. Pollard. 2001. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc. Natl. Acad. Sci. USA. 98:15009–15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papayannopoulos, V., C. Co, K. E. Prehoda, S. Snapper, J. Taunton, and W. A. Lim. 2005. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol. Cell. 17:181–191. [DOI] [PubMed] [Google Scholar]
- 26.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M. F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiesner, S., E. Helfer, D. Didry, G. Ducouret, F. Lafuma, M. F. Carlier, and D. Pantaloni. 2003. A biomimetic motility assay provides insight into the mechanism of actin-based motility. J. Cell Biol. 160:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch, M. D., J. Rosenblatt, J. Skoble, D. A. Portnoy, and T. J. Mitchison. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 281:105–108. [DOI] [PubMed] [Google Scholar]
- 29.Angelova, M. I., S. Soleau, P. Meleard, J.-F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields: kinetics and applications. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 30.Loisel, T. P., R. Boujemaa, D. Pantaloni, and M. F. Carlier. 1999. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 401:613–616. [DOI] [PubMed] [Google Scholar]
- 31.Bernheim-Groswasser, A., S. Wiesner, R. M. Golsteyn, M. F. Carlier, and C. Sykes. 2002. The dynamics of actin-based motility depend on surface parameters. Nature. 417:308–311. [DOI] [PubMed] [Google Scholar]
- 32.Golub, T., S. Wacha, and P. Caroni. 2004. Spatial and temporal control of signaling through lipid rafts. Curr. Opin. Neurobiol. 14:542–550. [DOI] [PubMed] [Google Scholar]
- 33.van Rheenen, J., E. M. Achame, H. Janssen, J. Calafat, and K. Jalink. 2005. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 24:1664–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacia, K., P. Schwille, and T. Kurzchalia. 2005. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA. 102:3272–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veatch, S. L., and S. L. Keller. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 36.Kwik, J., S. Boyle, D. Fooksman, L. Margolis, M. P. Sheetz, and M. Edidin. 2003. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA. 100:13964–13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raucher, D., T. Stauffer, W. Chen, K. Shen, S. Guo, J. D. York, M. P. Sheetz, and T. Meyer. 2000. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 100:221–228. [DOI] [PubMed] [Google Scholar]
- 38.Rohatgi, R., H. Y. Ho, and M. W. Kirschner. 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150:1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prehoda, K. E., J. A. Scott, R. D. Mullins, and W. A. Lim. 2000. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 290:801–806. [DOI] [PubMed] [Google Scholar]
- 40.Silvius, J. R. 2003. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys. J. 85:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisswange, I., T. Bretschneider, and K. I. Anderson. 2005. The leading edge is a lipid diffusion barrier. J. Cell Sci. 118:4375–4380. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie, K., and A. Kusumi. 2004. Role of the membrane skeleton in creation of microdomains. Subcell. Biochem. 37:233–245. [DOI] [PubMed] [Google Scholar]
- 44.Hao, S., and A. August. 2005. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol. Biol. Cell. 16:2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257–283. [DOI] [PubMed] [Google Scholar]