Abstract
Voltage-sensor (VS) domains cause the pore of voltage-gated ion channels to open and close in response to changes in transmembrane potential. Recent experimental studies suggest that VS domains are independent structural units. This independence is revealed dramatically by a voltage-dependent proton-selective channel (Hv), which has a sequence homologous to the VS domains of voltage-gated potassium channels (Kv). Here we show by means of molecular dynamics simulations that the isolated open-state VS domain of the KvAP channel in a lipid membrane has a configuration consistent with a water channel, which we propose as a common feature underlying the conductance of protons, and perhaps other cations, through VS domains.
Voltage-gated ion channels open and close in response to changes in transmembrane potential due to the motion of their voltage-sensor (VS) domains. Long known exclusively as regulatory accessories for ion-channel pore domains, VS domains were recently identified in voltage-activated phosphatases (1), and more recently Ramsey et al. (2) and Sasaki et al. (3) discovered a novel VS protein that functions as a voltage-gated proton channel. Named Hv1 by Ramsey et al. (2), the protein has high sequence similarity to the VS domains of voltage-gated potassium channels (Kv). This finding is consistent with earlier reports of ion conductance through mutated VS domains: protons in a Shaker VS (4) and alkali metal cations in Shaker (5) and Nav1.2a VS mutants (6). A key unresolved issue, which we address here, is the structural basis for conductance by VS domains. Specifically, we show by means of molecular dynamics (MD) simulations that the isolated open-state VS domain of the KvAP channel (7) in a lipid membrane assumes a configuration consistent with a water channel, which we propose as a common feature underlying the conductance of protons, and perhaps other cations, through VS domains.
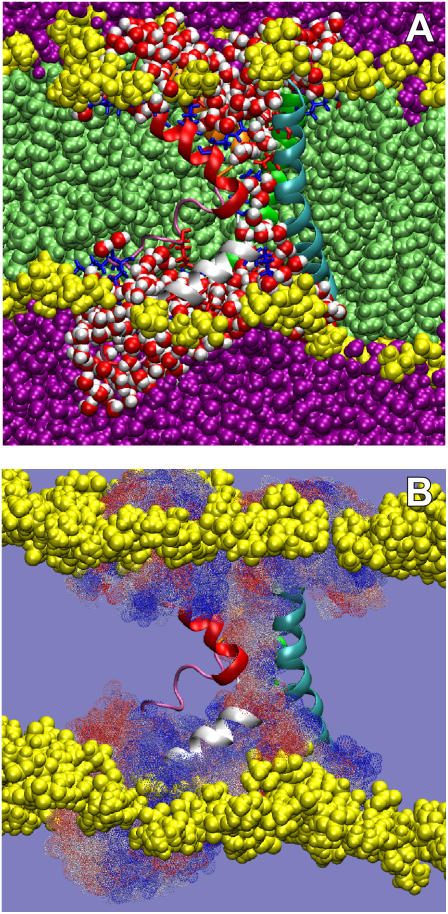
We performed a 34 ns MD simulation of the open-state configuration of the whole KvAP VS domain in a 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) bilayer in excess water, arranged according to the model for the structure of KvAP in a lipid membrane proposed by Lee et al. (7) (see Supplementary Material). No constraints were applied at any time during the simulation. The equilibrated configuration of the isolated VS was remarkably stable in the bilayer during the simulation (Supplementary Material, Fig. S1). Snapshots from the equilibrated simulation (Fig. 1 A) show that the helix packing of the VS in its open state forms a water-filled hourglass-like structure that spans the hydrocarbon core of the lipid bilayer. The hourglass is anchored to the external membrane interface by salt bridges between the outer voltage-gating arginines (R117 and R121) and lipid phosphates and between the inner voltage-gating arginines (R123 and R126) and acidic groups on the S3 and S2 helices, E107 and E45, respectively (see Supplementary Fig. S2). At the internal surface, the hourglass is similarly stabilized by the interactions of basic residues with lipid phosphates and acidic residues. Aromatic residues, especially on the inner surface, apparently provide additional stabilization. These interactions stabilize the VS helices in the lipid bilayer to create the water-filled vestibules or crevices that penetrate to the membrane midplane from both membrane surfaces. A similar architecture has been reported for VS domains in a simulation of a model Kv1.2 potassium channel (8) and in a simulation of the KvAP VS in a detergent micelle (9).
FIGURE 1.
MD simulation of the KvAP VS domain in a POPC bilayer. (A) Cutaway view of the simulation system showing water penetration, bilayer distortion, and contacts between lipid headgroups and the ends of the VS domain. Color scheme: waters within 4 Å of protein, red/white spheres; other waters, purple; phosphocholine headgroups, yellow; acyl chains, light green; basic residues, blue stick representation; and acidic residues, red in stick representation. VS color code is S1, cyan; S2, green; S3a, mauve; S3b orange; S4, red; and S4-S5 linker, white. (B) Summary of water trajectories passing through the sensor “pore” over the last 16 ns of simulation. Each dotted surface represents a location visited by at least one water molecule during the simulation (blue, early trajectory; white, midtrajectory; red, late trajectory).
Overall, this configuration arises because the lipid bilayer around the VS domain adopts a conformation that maximizes the interaction of the headgroup interfaces with the end surfaces of the VS, which are highly populated with charged and polar residues, including a number of buried salt bridges (Fig. S2). Thus, the water-filled hourglass configuration is at least partially a result of the hydration process that confers stability to the VS in the lipid bilayer. The distorted bilayer interfaces, which are separated by 20–25 Å, allow the hydration of the internal salt bridges (Fig. S2).
The possibility that the VS hourglass configuration is suited to ion transport along the transmembrane direction is apparent in Fig. 1 B, which shows a superposition of all the positions within the hourglass occupied by water molecules during the last 16 ns of the simulation. The two water-filled vestibules of the hourglass meet in the membrane midplane at a central salt bridge R133-D62, and, with it, form a continuous molecular surface (see Fig. S2F). Accordingly, Fig. 2 A reveals an uninterrupted hydrogen-bond network that extends from one membrane interface to the other. But, as shown in Fig. 2 B, the R133-D62 salt bridge apparently interrupts the continuity of the water chain. Sands et al. (9) reached a similar conclusion based on measurements of the pore-size profile along the VS axis in their simulation of KvAP VS in a micelle environment. This salt bridge, which connects S2 and S4, is a common feature in KvAP crystal structures (7,10), including that of the VS expressed by itself (10). It is believed to be essential to the structural integrity of KvAP and other Kv channels, because it is a highly conserved feature (see Fig. S3). Residue R133 corresponds to an arginine position that appears to be essential for channel function (11), and residue D62 to an acidic residue position in Kv channels that, in conjunction with the basic residues in S4, is believed to play an important role in the gating process (12).
FIGURE 2.
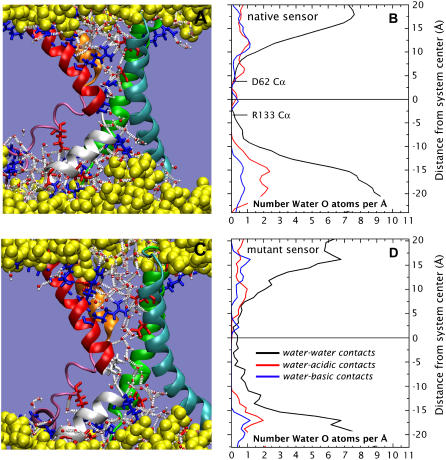
Unblocking the water pore. (A) Snapshot showing an example of the hydrogen-bond network between water and charged residues in the native VS structure. The hydrogen bonds are indicated as white dotted lines. (B) The time-averaged transmembrane distribution of water for the native protein in the POPC bilayer. (C) Snapshot from a 2 ns simulation of the sensor in which R133 has been replaced with an isoleucine, showing the unblocked passage of water through the VS. (D) The time-averaged transmembrane distribution of water for the R133I VS protein in the POPC bilayer.
What bearing does the structure of the KvAP VS have on the possible structure and function of Hv1 sensor channels? The putative transmembrane region of the Hv1 sequence in homologous proteins presents a high degree of similarity with the VS sequence of Kv channels (see Fig. S3). However, R133 in the salt bridge, which breaks the water chain, is replaced by an isoleucine residue in Hv1. Similarly, a highly conserved serine in Hv1 (2,3) replaces D62 of KvAP (Fig. S3). We first constructed the R133I mutant, protonated D62 (to maintain system charge neutrality), and continued the simulation. Fig. 2, C and D, show a well-defined string of waters passing through the mutant VS after only 2 ns of continued simulation. We then constructed the double mutant R133I-D62S, which we simulated for 3 ns. No substantial differences in the behavior of the string of waters in the double mutant relative to the R133I mutant were observed (see Fig. S4). This string of waters in the mutants leads to two conclusions. First, the native Hv1 sequence supports a transmembrane chain of waters, which is a necessary first step for the formation of a proton-conducting water wire. Second, salt bridges can interrupt transmembrane water chains.
The main structural features reported here for the VS of KvAP are likely to be encountered in any related system. The passive conductance of protons (4) and cations (5,6) through the VS seems to be a natural consequence of the hydration state of VS in the bilayer. However, the specific structural details underlying transport will certainly vary among different systems. For example, a mutation of the first S4 arginine (equivalent of R117 in KvAP) is necessary for passive cation transport through the VS of the Shaker K+ channel (5), whereas S4 Arg-to-His mutations are necessary for proton transport (4). Hv1 sensor channels, on the other hand, do not require His proton acceptors (2). The importance of specific structural features for enabling VS conductance is emphasized by the observation in Shaker VS mutants that the sensor must be in the closed state for passive cation transport to occur (5).
An important conclusion from our simulation and the identification of the Hv1 proton channel as a VS protein is that VS domains are independent structural and functional units (1,7). As has been suggested before (7), and is evident in our simulations, the stability and functionality of VS domains in voltage-dependent cation channels must rely on their own set of interactions with the lipid membrane, largely independent of the channel pore domain.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Dr. Rod MacKinnon for advice on positioning the sensor in the membrane and Joseph Farran of the University of California, Irvine, Medium Performance Computer Center for outstanding technical support.
This research was supported by grants from the National Institute of General Medical Sciences (GM-68002 and GM-74637) and the National Center for Research Resources (RR-14812) to S.H.W., and the National Science Foundation (CHE-0417158) to D.J.T. JAF is supported by a National Research Service Award from the National Library of Medicine.
References
- 1.Murata, Y., H. Iwasaki, M. Sasaki, K. Inaba, and Y. Okamura. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey, I. S., M. M. Moran, J. A. Chong, and D. E. Clapham. 2006. A voltage-gated proton-selective channel lacking the pore domain. Nature. 440:1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki, M., M. Takagi, and Y. Okamura. 2006. A voltage-sensor domain protein is a voltage-gated proton channel. Science. 312:589–592. [DOI] [PubMed] [Google Scholar]
- 4.Starace, D. M., and F. Bezanilla. 2004. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 427:548–553. [DOI] [PubMed] [Google Scholar]
- 5.Tombola, F., M. M. Pathak, and E. Y. Isacoff. 2005. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 45:379–388. [DOI] [PubMed] [Google Scholar]
- 6.Sokolov, S., T. Scheuer, and W. A. Catterall. 2005. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 47:183–189. [DOI] [PubMed] [Google Scholar]
- 7.Lee, S.-Y., A. Lee, J. Chen, and R. MacKinnon. 2005. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci. USA. 102:15441–15446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treptow, W., and M. Tarek. 2006. Environment of the gating charges in the Kv1.2 Shaker potassium channel. Biophys. J. 90:L64–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sands, Z. A., A. Grottesi, and M. S. P. Sansom. 2006. The intrinsic flexibility of the Kv voltage sensor and its implications for channel gating. Biophys. J. 90:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, Y. X., A. Lee, J. Y. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal, S. K., and R. MacKinnon. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177. [DOI] [PubMed] [Google Scholar]
- 12.Seoh, S. A., D. Sigg, D. M. Papazian, and F. Bezanilla. 1996. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 16:1159–1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.