Abstract
Females often select their mates on the basis of the size or intensity of sexual ornaments, and it is thought that such traits are reliable indicators of male quality because the costliness of these traits prevents cheating. The immunocompetence handicap hypothesis is a recently proposed mechanistic explanation of these costs and states that males carry ornaments at the expense of their resistance to disease and parasites. The tradeoff between immunocompetence and sexual ornamentation was hypothesized to arise as a consequence of the dual effect of androgens on ornamentation (+) and immune function (−). To test this hypothesis, we compared comb size between male domestic chickens Gallus domesticus of lines divergently selected for antibody responses to sheep erythrocytes (three lines: selected for low response or high response and a control line). The importance of comb size in inter- and intrasexual selection is well established, and comb size is strongly dependent on testosterone level. Comb size was larger in the males of the low line than in the high line, and comb size of control males was intermediate, indicating a tradeoff between ornamentation and immunocompetence. Testosterone (T) levels varied in a similar fashion (TLow > TControl > THigh), suggesting that this hormone could mediate the tradeoff between ornamentation and immunocompetence. These results support the idea that a tradeoff with immune function may constrain the expression of secondary sexual ornaments.
Keywords: immunocompetence handicap hypothesis, sheep erythrocyte, sexual ornaments, Gallus domesticus, testosterone
Animals often select their mates on the basis of the size of sexual ornaments or intensity of sexual displays (1). The finding that mating success can be improved by carrying larger ornaments has raised the question of what constrains the expression of these traits. Carrying an ornament has been shown to incur a fitness cost (2–4), and if the costs incurred by carrying a larger ornament depend on the quality of its bearer, such traits can be reliable indicators of phenotypic quality (5, 6).
Folstad and Karter (7) recently proposed the immunocompetence handicap hypothesis as a general proximate mechanism to explain the costs of ornamentation; this hypothesis states that males carry ornaments at the expense of their resistance to disease and parasites. This tradeoff was originally hypothesized to arise as a consequence of the dual effect of androgens on ornamentation and immune function (higher androgen levels result in larger ornaments, but suppress immune function), but could also be the consequence of variation in resource allocation in the absence of direct effects of sex hormones (8).
The immunocompetence handicap hypothesis was based on a number of well established physiological relationships between condition, testosterone, sexual ornamentation, and the immune system, but when initially proposed, there were no studies that directly related immune function to sexual ornamentation (7). Since then, several attempts have been made to test this hypothesis by using different approaches. Some studies have manipulated testosterone levels (9–12) and studied the consequences for immune function or parasite burden. Although testosterone administration can be used to investigate the effects of this hormone on ornamentation and immune function, this experiment does not test directly whether there is a tradeoff between sexual ornamentation and immunocompetence. Other studies used natural variation to investigate the relationship between immune function and ornamentation and generally reported reduced immune function in individuals with larger or brighter ornaments (13–16). However, such correlations cannot be taken as evidence for a tradeoff between these traits, because reduced immune function in individuals with large ornaments could reflect reduced exposure to disease (for example, dominant individuals may succeed in foraging in patches with low parasite prevalence) or more efficient immune function in the past.
The demonstration of a tradeoff requires experiments in which a trait involved in the tradeoff is manipulated (17, 18). With respect to the immunocompetence hypothesis, one such study has been published, which reported reduced humoral immune function in male swallows Hirundo rustica with elongated tails (15) [tail length in swallows is a sexually selected trait (19)], suggesting a tradeoff between immunocompetence and sexual ornamentation. Here we report a study in which we use the converse approach, a manipulation of immune function, to test the immunocompetence handicap hypothesis. We compared comb size of domestic fowl Gallus domesticus between males belonging to lines divergently selected on their response to sheep erythrocytes (20). Domestic fowl is a suitable system to test this hypothesis, because the importance of comb size in inter- and intrasexual selection is well established (13, 21–23), and comb size is strongly dependent on testosterone level (13, 24, 25). Based on the immunocompetence handicap hypothesis, we predicted a negative association between immune function and comb size among selection lines. We restricted our analyses to the selection-line level, because we have no predictions for variation within selection lines, since this concerns spontaneous natural variation rather than experimental artificial variation (17, 26). For example, the immunocompetence handicap hypothesis predicts a negative relationship between comb size and immunocompetence, but within a line both comb size and immunocompetence are likely to be positively correlated with fitness and hence with each other. As a result of such a mixture of effects, negative, zero, or positive correlations can be found, but none of these would constitute evidence for or against the existence of a tradeoff. This point has been discussed at length in the life history literature (17, 18, 26–29) in the context of demonstration of the costs of reproduction (30).
METHODS
Selection lines originated from an Institut Séléction Animal (ISA) Warren cross, selected for 15 nonoverlapping generations on high or low primary antibody response at day 5 after primary intramuscular immunization with sheep erythrocytes at 37 days of age. A random-bred control line was also maintained. Details regarding immunological methods and the selection process are described elsewhere (20, 31). Briefly, in each generation approximately 300 chicks were reared in each line. In each selection line, the 25–40 males and 50–70 females with most extreme antibody titers were selected to produce the next generation, and the control line was random bred. Realized selection differentials were in the order of 1.7–2.1 antibody titers per generation (31). Birds were individually housed in battery cages (floor 46 × 35 cm, height 40 cm) and were approximately 1 yr old when measured.
Blood samples (1 ml) were collected from the wing web within 3 min of removal from the cage. Plasma was stored at −20°C for 3 mos before analysis. Concentrations of testosterone were estimated by a solid-phase 125I RIA method (Coat-A-Count TKTT; Diagnostic Products, Los Angeles) according to the manufacturer by using duplicate aliquots of 50 μl plasma. Calculation of results was performed by applying a spline approximation using riasmart (Packard). The main crossreactivities were 3.3 and 0.5% for dihydrotestosterone and androstenedione, respectively, and <0.1% for other steroids of interest. The limit of quantitation was 0.01 ng/ml, and the interassay coefficient of variation was 12%.
After the blood sample was taken, birds were weighed and morphometric data were collected with vernier callipers (to nearest 0.1 mm) by observers unaware of the selection regime from which a bird stemmed. Comb length (maximum length) and height (above the eye perpendicular to longitudinal axis of head) were measured and multiplied to give an index of comb size in cm2. Combs were clipped at hatch, and combs that were clipped unsuccessfully were not measured (birds with serrated edge of the comb were not measured; n = 5; three high line, one control line, one low line). Combs would have been larger in the absence of clipping, but because the immune system is not yet active at this age (32), and all lines were treated equally, there is no reason to expect that such an effect would differ between selection lines. Furthermore, independent support for the immunocompetence effect on comb size comes from the testosterone data (see Fig. 2, below). Tarsus and head lengths were measured as indicators of structural body size. Tarsus length was measured according to Svensson (33), and head length was measured from bill tip to back of head in a straight line over the eye. Tarsus and head length were correlated (r = 0.44, n = 87, P < 0.001), and these variables were combined in an index of body size (calculated as the mean of head and tarsus length after transformation to standardized distribution [mean = 0.0, SD=1.0]). However, similar results appeared throughout for both size variables.
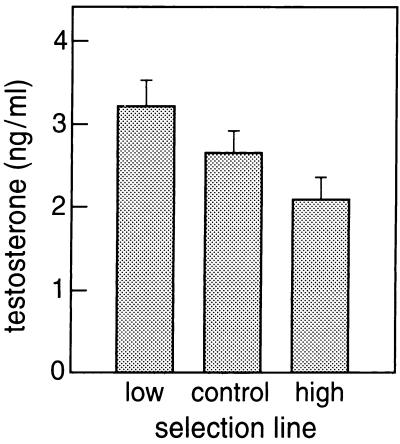
Figure 2.
Testosterone titers (ng/ml; +SE) in chicken lines divergently selected on response to sheep erythrocytes.
Sample sizes in the present study were 28–30 males for each of the three lines. Data were analyzed by using analysis of variance and analysis of covariance. To enhance statistical power, the results of these analyses were combined with the ordered heterogeneity test (34), which yields a new test statistic (rsPc). This test is appropriate because the selection lines are ordered with respect to immunocompetence. Before analysis, testosterone titers were natural logarithm transformed to normalize the distribution.
RESULTS
There was a strong response to selection, as evidenced from the log2 antibody titers, to the challenge with sheep erythrocytes in males used in this study [low line (mean ± SE) (n): 0.00 ± 0.00 (28); control line: 4.11 ± 0.50 (27); high line: 14.31 ± 0.45 (29); F2,83 = 391.5, rsPc > 0.9999, P < 0.0001). Although selection focused only on this aspect of immune function, previous studies showed that humoral responsiveness to a variety of antigens (Escherichia coli, Newcastle disease virus, bronchitis virus, bursal disease virus) varied between selection lines in a similar fashion (20). Furthermore, selection lines differed in size of spleen and bursa (35), cellular immunity in vivo (36), and mortality after infection with Marek’s disease (37). Thus it seems safe to assume that immunocompetence was successfully manipulated.
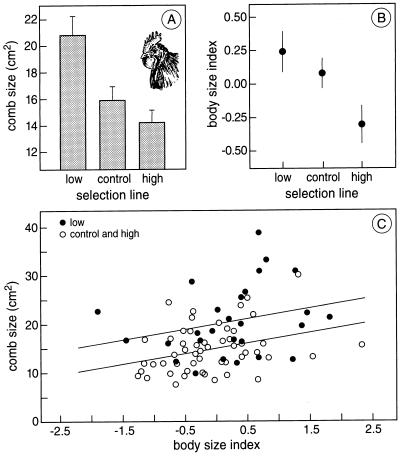
Comb size varied significantly between selection lines (F2, 79 = 9.78, rsPc > 0.9997, P < 0.0001), being larger in males from the low line than in the high line (t53 = 3.96, P < 0.0003), while comb size of males from the control line was intermediate (Fig. 1A). Thus, there was a negative association between immunocompetence and the degree of sexual ornamentation, as predicted by the immunocompetence handicap hypothesis.
Figure 1.
Male comb and body size in chicken lines divergently selected on response to sheep erythrocytes. (A) Comb size (+SE). (B) Body size (±SE), defined as the mean of standardized tarsus and head length. (C) Comb size vs. body size.
Artificial selection on a particular trait often affects many other traits, and the variation in comb size between selection lines could reflect a general effect on body size, rather than a specific effect on sexual ornamentation. Indeed, body size varied between selection lines (F2, 84 = 4.03, rsPc = 0.98, P < 0.01), males from the low line being larger than males from the high line (t57 = 2.55, P < 0.02), whereas size of males from the control line was intermediate (Fig. 1B). However, further analysis showed that selection lines differed in comb size even when body size was controlled for statistically (Fig. 1C; results of ANCOVA: selection line: F2, 78 = 7.22, rsPc = 0.999, P < 0.001; size: F1, 78 = 6.27, P < 0.02; using allometric scaling yields the same result). Using body mass instead of body size yields the same results (data not shown). Body mass also varied between selection lines [low line (mean ± SE in g) (n): 3,465 ± 49 (28); control line: 3,333 ± 56 (27); high line: 3,305 ± 47 (29); F2,84 = 2.87, rsPc = 0.939, P < 0.03), but body condition (as indicated by residuals of a regression of mass on body size) did not differ between selection lines (F2, 83 = 1.61, rsPc = 0.40, P > 0.3). Thus we conclude that artificial selection on immunocompetence affected sexual ornamentation independent of selection effects on body size or condition.
According to the immunocompetence hypothesis, the tradeoff between immunocompetence and sexual ornamentation is caused by a dual effect of testosterone on immune function and ornamentation (7). Other compounds could also play this role, and corticosterone has been suggested as an alternative (38), but previous work has shown that corticosterone level does not vary between the selection lines used in our study (39). Testosterone varied significantly between selection lines (F2, 77 = 4.93, rsPc = 0.99, P < 0.005), males from the low line having higher levels of testosterone than males from the high line (t50 = 3.08, P < 0.004), whereas testosterone level of males from the control line was intermediate (Fig. 2). Thus testosterone may regulate resource allocation to immune function and sexual ornamentation, as suggested by the immunocompetence handicap hypothesis.
DISCUSSION
The central prediction of Folstad and Karter’s (7) immunocompetence handicap hypothesis is that a tradeoff exists between sexual ornamentation and immune function. We found a negative association between manipulated levels of immunocompetence and comb size, in agreement with this prediction. Thus females selecting males with extravagant ornaments may thereby select males that either can afford to reduce their investment in immune function, for example because their genome is relatively well suited to the currently prevailing parasites (40, 41), and/or males that have relatively abundant resources available and can therefore afford to keep both their ornamentation and their immune system functioning at high levels (26, 42).
Repeated selection experiments sometimes yield slightly different results, and in our study, for financial reasons, there were no replicates of the different selection regimes. It can be argued that replication is required before any firm conclusions can be drawn. It is therefore important to note that similar results were obtained in lines of broiler chicks divergently selected on their response to E. coli (43). These results were discussed in a different context, and data were collected on chicks up to 30 days old (long before they are sexually active), but also in this experiment high-response chicks had smaller combs and lower testosterone levels than low-response chicks. These observations support the conclusions from the present study.
The existence of a tradeoff between sexual ornamentation and immune function suggests these traits draw resources from the same pool, but which resources are involved remains to be investigated. Maintaining circulating populations of leukocytes and antibodies is, however, a continuous resource drain, and repair costs associated with immunopathology have recently been suggested as an additional drain on resources (42, 44). Carrying a large comb costs energy simply through its effect on body mass, and in addition heat lost through this ornament may be important (unpublished observation, J. Tinbergen and S.V. with thermal camera). Thus the tradeoff between sexual ornamentation and immune function may at least be partly energy based, although it is important to note that even the magnitude of the energy costs of these traits is largely unknown (but see refs. 45 and 46). Obviously, other resources, such as carotenoids (47, 48), could also be important.
The immunocompetence handicap hypothesis is based on the assumption that testosterone suppresses immune function, and the negative association between immunocompetence and testosterone level in our study (Fig. 2) is in accordance with this assumption. However, this assumption was largely based on mammalian studies (7), and several recent studies in different bird species failed to find an immunosuppressive effect of exogenously administrated testosterone (11, 12, 43). Furthermore, parasite prevalence does not differ between male and female birds (49), while males typically have higher testosterone levels. Thus it appears that for adult birds the evidence for immunomodulation by testosterone is weak (at least with respect to the humoral immune response).
Effects in the opposite direction, of immunological products on endocrine state, have been extensively studied in mammals (50). It is interesting to note that our study, in conjunction with the study of Leitner (43), provides experimental evidence for such feedback in birds, because testosterone levels responded to manipulation of immunocompetence. This finding is in agreement with the observation that testosterone and ornamentation decrease with increasing parasite load in domestic fowl and other species (48, 51–56). Taken together, these observations suggest that testosterone in birds plays only some of the roles assumed in the original immunocompetence hypothesis, in the sense that it modulates the expression of sexual ornaments but in turn is modulated by immune function, rather than vice versa as originally assumed (7).
Acknowledgments
We thank Jan Komdeur, Kate Lessells, Anders Pape Møller, Miguel Rodríguez-Gironés, George Williams, Marlene Zuk, and anonymous reviewers for comments on the manuscript and Bernd Riedstra for assistance with measuring the birds. Dick Visser prepared the figures. S.V. was supported by Stichting Levenswetenschappen of the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO–SLW) grant 803-30.165.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 2.Møller A P. Nature (London) 1989;339:132–135. [Google Scholar]
- 3.Mappes J, Alatalo R V, Kotiaho J, Parri S. Proc R Soc London Ser B. 1996;263:785–789. [Google Scholar]
- 4.Grether G F. Proc R Soc London Ser B. 1997;264:207–210. [Google Scholar]
- 5.Zahavi A. J Theor Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- 6.Grafen A. J Theor Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- 7.Folstad I, Karter A J. Am Nat. 1992;139:603–622. [Google Scholar]
- 8.Wedekind C, Folstad I. Am Nat. 1994;143:936–938. [Google Scholar]
- 9.Saino N, Møller A P, Bolzern A M. Behav Ecol. 1995;6:397–404. [Google Scholar]
- 10.Salvador A, Veiga J P, Martin J, Lopez P, Abelenda M, Puerta M. Behav Ecol. 1996;7:145–150. [Google Scholar]
- 11.Ros A F H, Groothuis T G G, Apanius V. Am Nat. 1997;150:201–219. doi: 10.1086/286063. [DOI] [PubMed] [Google Scholar]
- 12.Hasselquist D, Marsh J A, Sherman P W, Wingfield J C. Behav Ecol Sociobiol. 1999;45:167–175. [Google Scholar]
- 13.Zuk M, Johnsen T S, Maclarty T. Proc R Soc London Ser B. 1995;260:205–210. [Google Scholar]
- 14.Møller A P, Kimball R T, Erritzoe J. Behav Ecol Sociobiol. 1996;39:317–322. [Google Scholar]
- 15.Saino N, Møller A P. Behav Ecol. 1996;7:227–232. [Google Scholar]
- 16.Skarstein F, Folstad I. Oikos. 1996;76:359–367. [Google Scholar]
- 17.Grafen A. In: Reproductive Success. Clutton-brock T H, editor. Chicago: Univ. Chicago Press; 1988. pp. 454–471. [Google Scholar]
- 18.Lessells C M. In: Behavioural Ecology; An Evolutionary Approach. Krebs J R, Davies N B, editors. Oxford: Blackwell Scientific; 1991. pp. 32–68. [Google Scholar]
- 19.Møller A P. The Barn Swallow and Sexual Selection. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 20.Parmentier H K, Nieuwland M G B, Rijke E, de Vries Reilingh G, Schrama J W. Avian Dis. 1996;40:634–644. [PubMed] [Google Scholar]
- 21.Zuk M, Thornhill R, Ligon J D, Johnson K, Austad S, Ligon S H, Thornhill N W, Costin C. Am Nat. 1990;136:459–473. [Google Scholar]
- 22.Ligon J D, Thornhill R, Zuk M, Johnson K. Anim Behav. 1990;40:367–373. [Google Scholar]
- 23.von Schantz T, Tufvesson M, Goransson G, Grahn M, Wilhelmson M, Witzell H. Heredity. 1995;75:518–529. [Google Scholar]
- 24.Zeller F J. J Reprod Fertil. 1971;25:125–127. doi: 10.1530/jrf.0.0250125. [DOI] [PubMed] [Google Scholar]
- 25.Fennell M J, Scanes C G. Poult Sci. 1992;71:539–547. doi: 10.3382/ps.0710539. [DOI] [PubMed] [Google Scholar]
- 26.van Noordwijk A J, de Jong G. Am Nat. 1986;128:137–142. [Google Scholar]
- 27.Partridge L, Harvey P H. Nature (London) 1985;316:20–21. [Google Scholar]
- 28.Gustafsson L, Sutherland W J. Nature (London) 1988;335:813–817. [Google Scholar]
- 29.Tinbergen J M, Daan S. Behavior. 1990;114:161–190. [Google Scholar]
- 30.Williams G C. Am Nat. 1966;100:687–690. [Google Scholar]
- 31.Pinard M H, Van Arendonk J A M, Nieuwland M G B, Van Der Zijpp A J. J Anim Sci. 1992;70:2986–2993. doi: 10.2527/1992.70102986x. [DOI] [PubMed] [Google Scholar]
- 32.Apanius V. In: Avian Growth and Development: Evolution Within the Altricial-Precocial Spectrum. Starck J M, Ricklefs R E, editors. Chicago: Univ. Chicago Press; 1997. [Google Scholar]
- 33.Svensson L. Identification Guide to European Passerines. 3rd Ed. Stockholm: Naturhistoriska Riksmuseet; 1985. [Google Scholar]
- 34.Rice W R, Gaines S D. Proc Natl Acad Sci USA. 1994;91:225–226. doi: 10.1073/pnas.91.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmentier H K, Kreukniet M B, Goeree B, Davison T F, Jeurissen S H M, Harmsen E G M, Nieuwland M G B. Vet Immunol Immunopathol. 1995;48:155–168. doi: 10.1016/0165-2427(94)05411-k. [DOI] [PubMed] [Google Scholar]
- 36.Parmentier H K, Schrama J W, Meijer F, Nieuwland M G B. Poultry Sci. 1993;72:1679–1692. doi: 10.3382/ps.0721679. [DOI] [PubMed] [Google Scholar]
- 37.Pinard M H, Janss L L G, Maatman R, Noordhuizen J P T M, Van Der Zijpp A J. Poultry Sci. 1993;72:391–402. doi: 10.3382/ps.0720391. [DOI] [PubMed] [Google Scholar]
- 38.Møller A P. Trends Ecol Evol. 1995;11:121. [Google Scholar]
- 39.Donker R A. Ph.D. thesis. The Netherlands: Agricultural University of Wageningen; 1989. [Google Scholar]
- 40.Hamilton W D, Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 41.Von Schantz T, Wittzell H, Goransson G, Grahn M, Persson K. Proc R Soc London Ser B. 1996;263:265–271. doi: 10.1098/rspb.1996.0041. [DOI] [PubMed] [Google Scholar]
- 42.Westneat D F, Birkhead T R. Proc R Soc London Ser B. 1998;265:1065–1073. [Google Scholar]
- 43.Leitner G, Landsman T, Blum O, Zaltsmann N, Heller E D. Poultry Sci. 1996;75:1373–1382. doi: 10.3382/ps.0751373. [DOI] [PubMed] [Google Scholar]
- 44.Råberg L, Grahn M, Hasselquist D, Svensson E. Proc R Soc London Ser B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demas G E, Chefer V, Talan M I, Nelson R J. Am J Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- 46.Svensson E, Råberg L, Koch C, Hasselquist D. Funct Ecol. 1998;12:912–919. [Google Scholar]
- 47.Folstad I, Hope A M, Karter A, Skorping A. Oikos. 1994;69:511–515. [Google Scholar]
- 48.Shykoff J A. Naturwissenschaften. 1996;83:113–121. doi: 10.1007/BF01142175. [DOI] [PubMed] [Google Scholar]
- 49.McCurdy D G, Shutler D, Mullie A, Forbes M R. Oikos. 1998;82:303–312. [Google Scholar]
- 50.Besedovsky H O, del Rey A. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 51.Milinski M, Bakker T C M. Nature (London) 1990;344:330–333. [Google Scholar]
- 52.Zuk M, Thornhill R, Ligon J D, Johnson K. Am Zool. 1990;30:235–244. [Google Scholar]
- 53.Wedekind C. Proc R Soc London Series B. 1992;247:169–174. [Google Scholar]
- 54.Buchholz R. Anim Behav. 1995;50:929–943. [Google Scholar]
- 55.Hillgarth N, Wingfield J C. In: Host–Parasite Evolution: General Principles and Avian Models. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 78–104. [Google Scholar]
- 56.Thompson C W, Hillgarth N, Leu M, McClure H E. Am Nat. 1997;149:270–294. [Google Scholar]