Abstract
Peripheral nerve injury induces upregulation of the calcium channel α2δ-1 structural subunit in dorsal root ganglia (DRG) and dorsal spinal cord of spinal nerve-ligated rats with neuropathic pain, suggesting a role of the calcium channel α2δ-1 subunit in central sensitization. To investigate whether spinal dorsal horn α2δ-1 subunit upregulation derives from increased DRG α2δ-1 subunit and plays a causal role in neuropathic pain development, we examined spinal dorsal hornα2δ-1 subunit expression with or without dorsal rhizotomy in spinal nerve-ligated rats and its correlation with tactile allodynia, a neuropathic pain state defined as reduced thresholds to non-noxious tactile stimulation. We also examined the effects of intrathecal α2δ-1 antisense oligonucleotides on α2δ-1 subunit expression and neuropathic allodynia in the nerve-ligated rats. Our data indicated that spinal nerve injury resulted in time-dependentα2δ-1 subunit upregulation in the spinal dorsal horn that correlated temporally with neuropathic allodynia development and maintenance. Dorsal rhizotomy diminished basal level expression and blocked injury-induced expression of the spinal dorsal hornα2δ-1 subunit and reversed injury-induced tactile allodynia. In addition, intrathecal α2δ-1 antisense oligonucleotides blocked injury-induced dorsal horn α2δ-1 subunit upregulation and diminished tactile allodynia. These findings indicate that α2δ-1 subunit basal expression occurs presynaptically and postsynaptically in spinal dorsal horn. Nerve injury induces mainly presynaptic α2δ-1 subunit expression that derives from increased α2δ-1 subunit in injured DRG neurons. Thus, changes in presynaptic α2δ-1 subunit expression contribute to injury-induced spinal neuroplasticity and central sensitization that underlies neuropathic pain development and maintenance.
Keywords: central sensitization, spinal cord, allodynia, neuropathic pain, nerve injury, calcium channels
Introduction
Spinal sensitization derived from altered gene expression in the spinal dorsal horn is an important component in nerve injury-induced pain. Altered gene expression can result in spinal circuit reorganizations and changes in the excitatory or inhibitory tones that lead to neuropathic pain development. Among genes that undergo upregulation or downregulation after injury, changes in the voltage-gated calcium channel (VGCC) α2δ-1 subunit in dorsal root ganglion (DRG) neurons correlate with the onset and duration of tactile allodynia, a neuropathic pain state in which innocuous tactile stimulation elicits pain behavior (Luo et al., 2001; Newton et al., 2001; Wang et al., 2002; Valder et al., 2003). Because central axons of DRG neurons form synapses with dorsal horn neurons that are critical for sensory processing, injury-induced α2δ-1 subunit expression in DRG neurons may contribute to spinal neuroplasticity in neuropathic pain.
The VGCC consists of the channel-forming α1 and β, γ, and α2δ subunits. The α2δ subunit is a heavily glycosylated structural subunit containing covalently linked α2 and δ peptides that are encoded by the same gene (De Jongh et al., 1990). The α2δ subunit is important for functional assembly and expression of the VGCC (Mori et al., 1991; Williams et al., 1992; Brust et al., 1993; Gurnett et al., 1996; Kang et al., 2002). So far, four α2δ genes have been identified that encode the α2δ-1, α2δ-2, α2δ-3, and α2δ-4 subunits, respectively (Ellis et al., 1988; Klugbauer, 1999; Qin et al., 2002). These subunits have distinct tissue-specific expression patterns suggesting diversified functions associated with each individual α2δ subunit (Marais et al., 2001). This is also true for sensory processing, because α2δ-1 and α2δ-2 subunit mRNA is highly expressed in small DRG neurons but at a lower level in large DRG neurons, whereas α2δ-3 mRNA is relatively abundant in large DRG neurons but scarce in small sensory neurons (Yusaf et al., 2001). Expression and distribution of the calcium channel α2δ subunits in spinal cord is essentially unknown. Binding studies have shown that the α2δ-1 and α2δ-2, but not the α2δ-3, subunits bind with high affinities to gabapentin (Marais et al., 2001), a drug that has antineuropathic pain efficacy with unknown mechanisms (Taylor et al., 1998; Laird and Gidal, 2000).
Although calcium channel α2δ-2 subunit levels in spinal cord and DRG under a neuropathic pain condition remain to be determined, we observed enhanced dorsal horn and DRG α2δ-1 subunit expression in a rat neuropathic pain model (Luo et al., 2001, 2002). However, how these changes relate to neuropathic pain development and maintenance are not clear. Injury-induced spinal α2δ-1 subunit could derive from increased presynaptic expression of the DRG α2δ-1 subunit and/or trans-synaptic induction in dorsal horn cells, which affect different aspects of pain transduction, such as neurotransmitter release or postsynaptic neuronal excitability, respectively. Accordingly, we examined spinal cord α2δ-1 subunit expression in a rat neuropathic pain model derived from tight ligation of the L5/L6 spinal nerves, its relationship to injury-induced DRG α2δ-1 subunit expression, and its contribution to neuropathic allodynia.
Materials and Methods
Materials. The monoclonal antibodies against rabbit calcium channel α2 subunit were from Sigma (St. Louis, MO), and those against endothelial nitric oxide synthase (eNOS) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were from BD Biosciences (San Diego, CA) and Ambion (Austin, TX), respectively. Membrane extracts of rat brain and human endothelial cells were used as positive controls for the α2δ-1 subunit and eNOS, respectively. Tris-acetate gels (NuPAGE) and buffers were obtained from Invitrogen (Carlsbad, CA). Horseradish peroxidase-labeled secondary antibodies (mouse IgG) and its substrate and enhancer solutions were from Pierce (Rockford, IL). Other chemicals were from Sigma.
Animals. Male rats (Harlan Sprague Dawley; Harlan, Indianapolis, IN) were housed in separate cages and exposed to a 12 hr light/dark cycle with food and water ad libitum. All animal care and experiments were performed according to protocols approved by the Institutional Animal Care Committees of the University of California.
Neuropathic surgeries. Spinal nerve ligation was performed as described by Kim and Chung (1992). Briefly, the left L5/L6 lumbar spinal nerves were exposed in anesthetized male Harlan rats (100-150 gm) and ligated tightly with 6.0 silk sutures between the DRGs and the conjunction to form the sciatic nerve. In some animals, ipsilateral dorsal rhizotomy was performed during the same operation. Briefly, an incision was made at the L1 and L2 dorsal aspect, and a left hemilaminectomy was performed. After the dura was opened, the dorsal roots of L5/L6 DRG were exposed and cut to remove ∼2 mm in length (Luo et al., 2001). The anatomical connections of the rhizotomized dorsal roots were confirmed after tissue collections. Sham ligation and rhizotomy were performed in the same ways except that spinal nerves and dorsal roots were not ligated or transected, respectively.
Intrathecal antisense oligonucleotide treatment. Antisense and mismatch oligonucleotides (21 nucleotides each) with phosphothioate modification on three nucleotides each at the 5′ and 3′ ends were synthesized and purified with High Purity Salt Free method by MWG Biotech (High Point, NC). The antisense oligonucleotide sequence against a region containing the start codon ATG on the rat brain α2δ-1 gene (GenBank accession number M86621) was AGCCATCTTCGCGATCGAAG, and the mismatch control sequence was CGATACCTCGCTGGCTAAAG. These oligonucleotides were precipitated and washed in 75% ethanol solutions and dissolved in sterile saline before injecting into allodynic rats through intrathecal catheters (Yaksh and Rudy, 1976) in a total volume of 10 μl twice daily for 4 d, starting 3 weeks after the nerve ligation. The same amount of sterile saline was injected into some allodynic rats as control. Sterile saline (10 μl) was used to flash the catheter after each injection. Behavioral testing was performed before the first injection and daily before the first daily injections each day for 4 d as described below.
Behavioral testing. Tactile allodynia was tested as described by Chaplan et al. (1994). Briefly, rats were allowed to acclimate for at least 15 min in a clear plastic cage with a wire mesh bottom and were then tested for the 50% paw withdrawal threshold (PWT) to von Frey filaments (Stoelting, Wood Dale, IL) using a modified up-down method of Dixon (1980). A calibrated filament with a 2.0 gm buckling weight was applied to the plantar surface of the left hindpaw with a force causing the filament to bend. Paw lifting within 5 sec indicated a positive response and led to the use of the next weaker filament, whereas absence of a paw lifting after 5 sec led to the use of the next filament with increasing weight. This paradigm continued until six measurements starting from the one before the first positive response were made, or until four consecutive positive (assigned a score of 0.25 gm) or five consecutive negative (assigned a score of 15 gm) responses had occurred. The 50% paw withdrawal threshold was calculated from the resulting scores as described previously (Luo et al., 2001).
Western blot. Frozen spinal cord and DRG samples were pulverized and extracted in 50 mm Tris buffer, pH 8.0, containing 0.5% Triton, 150 mm NaCl, 1 mm EDTA, and protease inhibitors. The cell extracts were applied to NuPAGE Tris-acetate gels under reducing conditions (0.05 m DTT) for electrophoresis and then transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH) electrophoretically. The membranes were then incubated with α2 monoclonal antibodies in PBS with 0.1% Tween 20 for 1 hr at room temperature or overnight at 4°C after nonspecific binding sites were blocked with 5% low-fat milk in PBS. Secondary antibodies labeled with horseradish peroxidase were used to detect the antibody-protein complexes by incubating with the membrane at room temperature for 1 hr followed by washing, addition of chemiluminescent reagents, and x-ray radiography. In our experiments, the positive bands detected by the primary antibody reflect the α2 subunit only because the δ peptide separates from the α2 subunit under reducing conditions (Jay et al., 1991). The band densities were quantified by densitometry within the linear range of the film sensitivity curve.
Statistical analyses. Data were reported as means ± SE (SEM). Unpaired or paired Student's t tests were performed where significance was indicated by two-tailed p values <0.05.
Results
Peripheral nerve injury induced upregulation of the α2δ-1 subunit in dorsal spinal cord that correlated with neuropathic tactile allodynia
Our previous experiments have shown that spinal nerve ligation-induced injury in rats causes upregulation of the α2δ-1 subunit in DRG neurons that correlates with neuropathic allodynia development and maintenance, suggesting an important role of DRG α2δ-1 subunit expression in neuropathic pain (Luo et al., 2001; Valder et al., 2003). To investigate whether nerve injury induces α2δ-1 subunit expression in the spinal cord that also correlates with neuropathic pain development, we examined the α2δ-1 subunit levels in rat spinal cord and hindpaw withdrawal thresholds to mechanical stimulation at different time points after L5/L6 spinal nerve ligation. Spinal nerve ligation-induced injury caused upregulation of the α2δ-1 subunit in dorsal (Fig. 1A,B), but not ventral (data not shown), spinal cord. The increase was most dramatic at time points when the nerve-injured rats exhibited the most severe tactile allodynia. When the nerve-ligated animals were recovering from the allodynia state, elevated α2δ-1 subunit started to diminish, and by 17 weeks after nerve injury, both dorsal horn α2δ-1 subunit levels and the tactile allodynia were returned to the control levels (Fig. 1B). In addition, the peak increase of α2δ-1 subunit in spinal dorsal horn (5 weeks after injury) lacked behind that in DRG, which reached the maximal level 1-2 weeks after injury (Luo et al., 2001). These data suggest that injury-induced changes in dorsal horn α2δ-1 subunit expression may derive from changes in the DRG α2δ-1 subunit and contribute to injury-induced spinal neuroplasticity mediating neuropathic pain development and maintenance.
Figure 1.
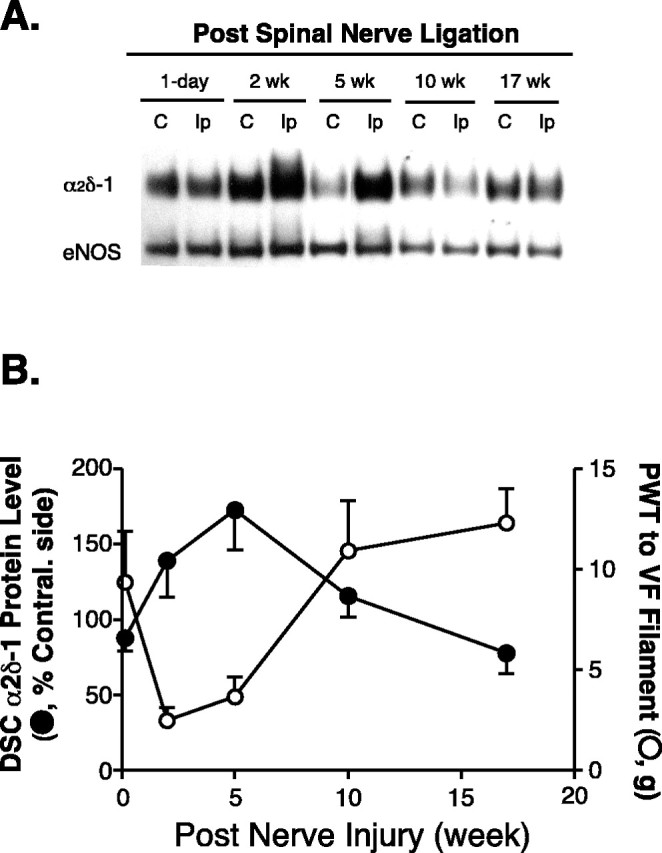
Injury-induced upregulation of spinal dorsal horn α2δ-1 subunit correlated with the development and maintenance of neuropathic allodynia. Unilateral L5/L6 spinal nerve ligation surgeries were performed, PWTs to von Frey filament stimulation were measured, and tissue samples were collected for Western blot analysis at indicated time points after nerve injury. A, Representative Western blots (from n = 4) showing time-dependent upregulation of the α2δ-1 subunit in spinal dorsal horn ipsilateral to spinal nerve ligation injury. The same blots were striped and reblotted with anti-eNOS antibodies for normalization of loading. B, Summarized injury-induced α2δ-1 subunit upregulation in dorsal spinal cord (DSC; as shown in A) and its reciprocal relationship with the development of tactile allodynia indicated as reduced PWTs to von Frey (VF) filament stimulation. Band density ratios of α2δ-1 subunit over eNOS were taken before signals from the ipsilateral (injury) side were compared with those from the contralateral (noninjury) side, which were taken as 100%. Normal PWT in sham-operated and naive animals are between 10 and 15 gm (Luo et al., 2001; Valder et al., 2003). Mean ± SEM from four rats in each group. C, Contralateral side; Ip, ipsilateral side.
Endogenous α2δ-1 subunit is likely expressed in spinal dorsal horn presynaptically and postsynaptically, and injury-induced presynaptic α2δ-1 expression may contribute to genesis and maintenance of neuropathic allodynia
The basal level of α2δ-1 subunit in spinal dorsal horn could be expressed at the presynaptic terminals and/or postsynaptic dorsal horn neurons and other cell types, thus contributing differentially to presynaptic and postsynaptic neurotransmission. Similarly, nerve injury can increase presynaptic α2δ-1 subunit expression through central transport of increased α2δ-1 subunit from DRG cell bodies and/or induce trans-synaptic expression of α2δ-1 subunit in postsynaptic dorsal horn neurons (Coull et al., 2003). To distinguish these possibilities, we examined nerve injury-induced dorsal horn α2δ-1 subunit expression and its relationships to DRG α2δ-1 subunit regulation and tactile allodynia in nerve-ligated rats with or without dorsal rhizotomy. Western blot analyses indicated that nerve injury induced upregulation of the α2δ-1 subunit in DRG and spinal dorsal horn 1 week after spinal nerve injury (Fig. 2). The increased dorsal horn α2δ-1 subunit mimicked the slower migration rate of the DRG α2δ-1 subunit (Fig. 2A) (Luo et al., 2001), suggesting that it may come from injured DRG neurons. Dorsal rhizotomy, which posted minimal effects on DRG α2δ-1 subunit expression, blocked the injury-induced dorsal horn α2δ-1 subunit upregulation to a level even lower than that of the endogenous α2δ-1 subunit seen in the contralateral side (Fig. 2). Importantly, dorsal rhizotomy also blocked spinal nerve ligation-induced tactile allodynia (Fig. 3). These data suggest that under normal conditions, the α2δ-1 subunit is likely expressed in both presynaptic and postsynaptic terminals in the dorsal horn, and the presynaptic α2δ-1 subunit is mainly transported from DRG neurons through their central axons. Nerve injury induces upregulation of the presynaptic dorsal horn α2δ-1 subunit that is originated from DRG.
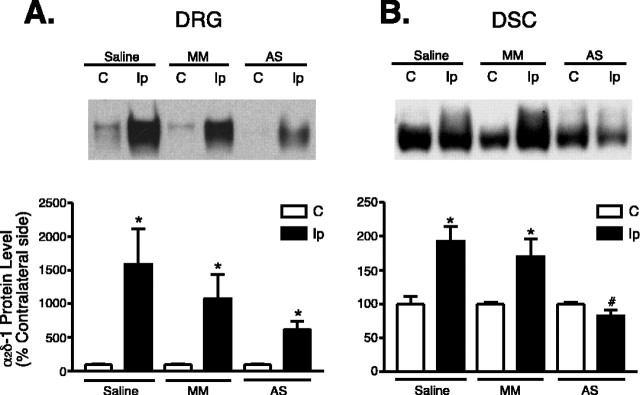
Figure 2.
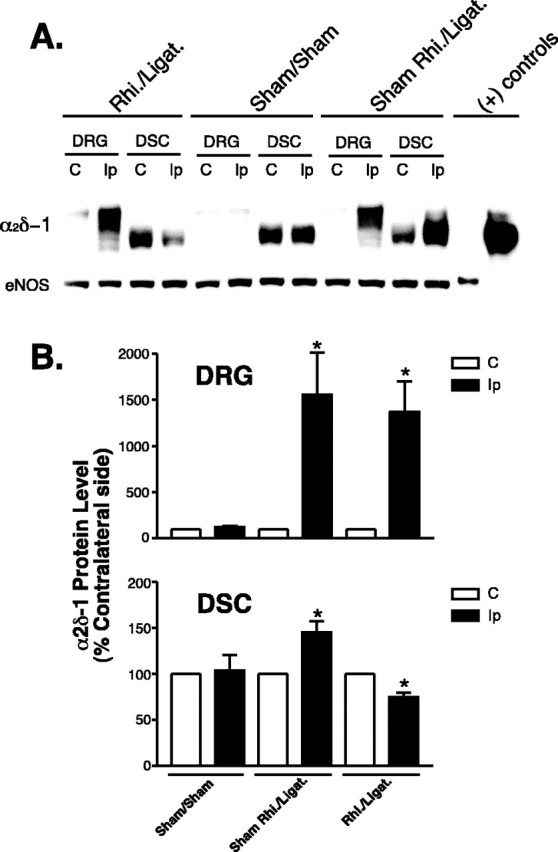
Effects of rhizotomy onα2δ-1 subunit expression in dorsal spinal cord and DRG of sham and nerve-injured rats. Rhizotomy (Rhi.) and spinal nerve ligation (Ligat.) surgeries were performed at the same time, and tissue samples were collected for Western blot analyses 1 week after the surgeries. A shows a representative Western blot (from n = 5) indicating α2δ-1 protein levels in DRG and dorsal spinal cord (DSC) of neuropathic rats with or without dorsal rhizotomy. The same blots were stripped and reblotted with antibodies against eNOS for determination of equal loading. B shows summarized α2δ-1 protein levels in DRG and dorsal spinal cord detected with Western blots shown in A. Data shown are means ± SEM of percentage changes compared with that in contralateral sides (chosen as 100%) from at least five independent animals in each group. The asterisk indicates significant changes compared with the control values (p < 0.05) as determined by unpaired two-tailed Student's t test. C, Contralateral side; Ip, ipsilateral side; (+), positive.
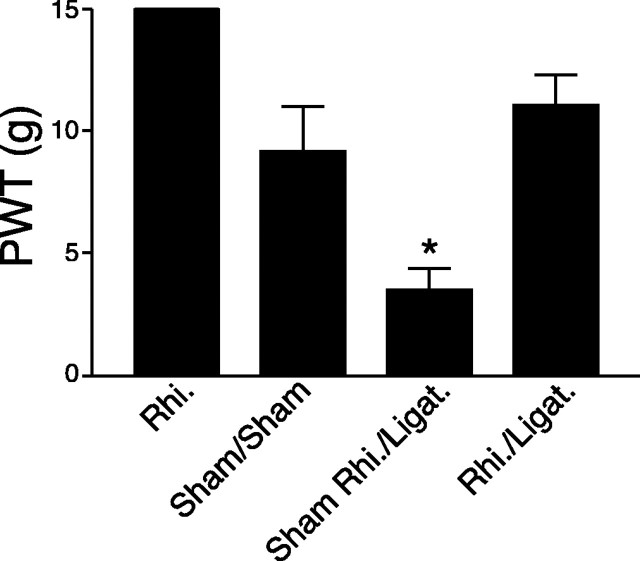
Figure 3.
Effects of rhizotomy on spinal nerve ligation induced tactile allodynia in rats. Rhizotomy (Rhi.) and spinal nerve ligation (Ligat.) surgeries were performed at the same time. PWTs to von Frey filament stimulation were measured in the injured paws 1 week after surgery and reported as means ± SEM from five independent animals in each group. In rhizotomized animals, the PWT to similar stimulation is 15 gm, similar to that in sham-operated and naive rats (between 10 and 15 gm) (Luo et al., 2001). The asterisk indicates significant changes (p < 0.05) compared with the sham control values determined by unpaired two-tailed Student's t test.
Intrathecal antisense oligonucleotide treatment diminished and blocked injury-induced α2δ-1 subunit in DRG and dorsal spinal cord, respectively, and reversed tactile allodynia
To investigate whether elevated α2δ-1 subunit in dorsal spinal cord and DRG plays a causal role in nerve injury-induced tactile allodynia, we treated the nerve-injured animals with antisense oligonucleotides complementary to a region in the α2δ-1 gene that contains the start codon ATG. As indicated in Figure 4, intrathecal treatment with antisense oligonucleotides at a dose of 30 μg per rat, twice daily for 4 d, but not with saline or mismatch oligonucleotide controls, diminished endogenous (contralateral side) as well as nerve injury-induced (ipsilateral side) α2δ-1 subunit expression in DRG, and blocked injury-induced α2δ-1 subunit upregulation in the spinal dorsal horn. In addition, the same antisense treatment also diminished injury-induced tactile allodynia in a dose-dependent manner (Fig. 5). However, the reversal of neuropathic allodynia after antisense oligonucleotide treatment was not complete, and we observed significant variations in the treatment outcome as indicated by the size of the error bars in Figure 5.
Figure 4.
Effects of intrathecal treatments with α2δ-1 antisense oligonucleotides on α2δ-1 subunit protein levels in dorsal spinal cord and DRG of nerve-injured rats. Spinal nerve ligation surgeries were performed 2 weeks before intrathecal catheterization. Thirty micrograms per rat of antisense (AS) or mismatch (MM) oligonucleotides in 10 μl of sterile saline, or 10 μl of sterile saline alone, followed by a 10 μl sterile saline flush were administered twice daily for 4 d through the intrathecal catheter at least 1 week after intrathecal catheterization. Western blot analyses were performed to detect the α2δ-1 protein levels in DRG (A) and dorsal spinal cord (DSC; B). The same plots were stripped and reblotted with antibodies against either eNOS or GAPDH for loading controls. Band density ratios of the α2δ-1 subunit over the eNOS or GAPDH controls were taken before signals from the ipsilateral (injury) side were compared with those from the contralateral (noninjury) side, which were taken as 100%. Summarized data were reported as means ± SEM from three to five independent animals examined in duplicate. The asterisk indicates significant changes (p < 0.05) compared with values from the contralateral side determined by paired two-tailed Student's t test. The # symbol indicates significant changes (p < 0.05) compared with values from saline-treated samples ipsilateral to the injury determined by unpaired two-tailed Student's t test. C, Contralateral side; Ip, ipsilateral side.
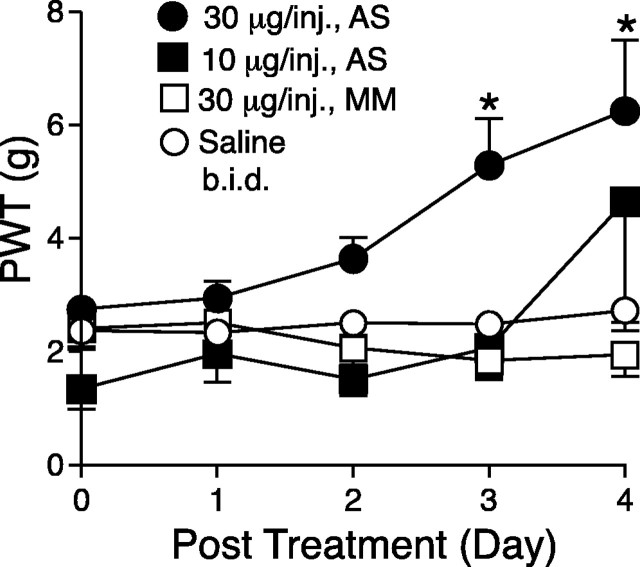
Figure 5.
Effects of intrathecal treatments with α2δ-1 antisense oligonucleotides on tactile allodynia of nerve-injured rats. Spinal nerve ligation surgeries were performed 2 weeks before intrathecal catheterization. Antisense (AS) or mismatch (MM) oligonucleotides in 10 μl of sterile saline, or 10 μl of sterile saline alone, followed by a 10 μl sterile saline flush were administered twice daily for 4 d through the intrathecal catheter at least 1 week after intrathecal catheterization. PWTs in the injury side to von Frey filament stimulation were measured before the intrathecal treatment and daily before the first injection for 4 d and reported as means ± SEM from at least six animals in each group. The asterisk indicates significant changes (p < 0.05) compared with values from saline-treated animals determined by unpaired two-tailed Student's t test.
Discussion
Our data indicated that α2δ-1 subunit is upregulated in dorsal, but not ventral, spinal cord by peripheral nerve injury signals. Because dorsal spinal cord contains afferent central terminals and dorsal horn neurons as well as interneurons participating in sensory processing, our data suggest that injury-induced upregulation of the α2δ-1 subunit may contribute to spinal neuroplasticity, leading to abnormal sensations in nerve-injured animals. Three lines of independent evidences from our study support this hypothesis. First, upregulation of the spinal α2δ-1 subunit only occurs in the dorsal, but not ventral, horn and correlates temporally with injury-induced tactile allodynia. Second, both dorsal horn α2δ-1 subunit upregulation and tactile allodynia in nerve-ligated animals can be blocked by dorsal rhizotomy. Third, intrathecal antisense, but not mismatch, oligonucleotides can reverse at least partially the injury-induced α2δ-1 subunit upregulation and tactile allodynia.
It is known that calcium channels are expressed in both presynaptic terminals and postsynaptic dorsal horn neurons (Ahlijanian et al., 1990). Thus, we would expect both presynaptic and postsynaptic α2δ-1 expression. The postrhizotomy reduction, but not elimination, of α2δ-1 subunit expression in dorsal horn ipsilateral to the injury to a level lower than that in the contralateral dorsal spinal cord confirms the presynaptic and postsynaptic expression of the α2δ-1 subunit and suggests that, under a normal condition, the presynaptic α2δ-1 subunit derives from DRG α2δ-1 subunit that may undergo axonal transport to the central terminals.
The blockade of dorsal horn α2δ-1 subunit induction with rhizotomy indicates that the injury-induced spinal α2δ-1 subunit increase is mostly presynaptic and derived from increased α2δ-1 subunit expression in injured DRG. In addition to modulation of DRG neuron excitability, the injury-induced DRG α2δ-1 subunit can be transported to central terminals in the spinal dorsal horn where it modulates presynaptic neurotransmission. This presynaptic neuroplasticity may underlie neuropathic pain development and maintenance. Alternatively, rhizotomy could block injury-induced postsynaptic induction of the α2δ-1 subunit that may contribute to enhanced excitability of dorsal horn neurons leading to neuropathic pain. However, our data support a presynaptic mechanism based on the following observations. First, injury-induced maximal increase in spinal cord α2δ-1 subunit expression lacks behind that in DRG (Fig. 1) (Luo et al., 2001). Second, injury-induced spinal dorsal horn α2δ-1 subunit mimics the slow migration pattern of the DRG α2δ-1 subunit in Western blots (Fig. 2A), and rhizotomy blocks the induction and reverses tactile allodynia (Figs. 2, 3). Third, spinal nerve ligation and rhizotomy have opposite effects on spinal α2δ-1 subunit expression, suggesting that these injuries do not independently regulate spinal cord α2δ-1 subunit expression through a common injury-related mechanism, including that mediated by injury factors such as neurotrophic factors and cytokines generated at the injury sites. Rather, the outcome of rhizotomy on spinal dorsal horn α2δ-1 subunit expression is more consistent with blocking the effects of spinal nerve ligation on dorsal horn α2δ-1 subunit expression that is most likely derived from injured DRG central axons.
The involvement of dorsal horn α2δ-1 subunit in neuropathic pain is further supported by our finding that intrathecal antisense oligonucleotides diminish and block, respectively, injury-induced DRG and dorsal horn α2δ-1 subunit expression and reverse partial injury-induced tactile allodynia. The incomplete reversal of tactile allodynia after antisense treatments may be caused by many factors, including pharmacodynamics, pharmacokinetics, and distributions of injected oligonucleotides. The following may also be possible: (1) the antisense oligonucleotides may not be site specific to completely block injury-induced α2δ-1 subunit in presynaptic terminals, because the proteins are made in DRG, and intrathecal antisense treatment can only diminish, but not abolish, injury-induced DRG α2δ-1 subunit upregulation; (2) changes in α2δ-1 subunit expression may mediate one of many pathways involved in neuropathic pain; and (3) the α2δ-1 subunit may be a cofactor or comediator, but not a determinant, in neuropathic allodynia. These possibilities may not be mutually exclusive.
The causal role of α2δ-1 subunit in tactile allodynia is not clear at the present time. Findings from biochemical and biophysical studies have indicated that this subunit is critical in functional assembly and expression of the voltage-gated calcium channels (Brust et al., 1993; Gurnett et al., 1996, 1997; Kang et al., 2002). Recent data from electron cryomicroscopy study on the L-type calcium channels have indicated that the extracellular α2 subunit protrudes from the membrane in close proximity to the channel forming α1 subunit (Wolf et al., 2003), supporting an important role of this subunit to calcium channel functions. Thus, it is possible that this subunit is a rate-limiting subunit in calcium channel assembly, and overexpression of this subunit may lead to enhanced expression of functional calcium channels. Increased dorsal horn presynaptic calcium channels can lead to enhanced and/or prolonged presynaptic terminal excitability, resulting in elevated excitatory neurotransmitter release after non-noxious stimulation, which evokes a pain response (tactile allodynia). This hypothesis is supported by the findings that calcium channels are expressed at the presynaptic terminals and involved in excitatory neurotransmitter release (Matthews and Dickenson, 2001; Kochegarov, 2003), nerve injury modulates dorsal horn release of excitatory neurotransmitters (Skilling et al., 1992; Noguchi et al., 1995; Malcangio et al., 2000), and gabapentin, an antihyperalgesic drug that binds to the α2δ-1 subunit, inhibits excitatory neurotransmitter release from presynaptic terminals only in hyperalgesic, but not normal, rat spinal cord and caudal trigeminal nucleus slices (Patel et al., 2000; Maneuf et al., 2001). Colocalization of this subunit with other calcium channel-forming subunits in the presynaptic terminals and in vivo electrophysiological studies will provide direct evidences to prove or disprove this hypothesis.
Alternatively, increased α2δ-1 subunit may play a yet identified role, in addition to being the calcium channel structural subunit, in neuropathic pain processing. This is supported by our findings that nerve injury only induces α2δ-1, but not other, calcium channel subunit expression (Luo et al., 2001). In addition, the structure of DRG α2δ-1 subunit differs from that of α2δ-1 subunits from spinal cord, brain, and skeletal muscle (Luo, 2000), suggesting distinct functions for the DRG and presumably the presynaptic α2δ-1 subunit in sensory processing. Nevertheless, our data support a contributory role of spinal plasticity mediated by the calcium channel α2δ-1 subunit in neuropathic pain development and maintenance.
Footnotes
This study was supported in part by National Institutes of Health Grants DE13270, DE14545, and NS40135 (Z.D.L.). We thank A. Boroujerdi and Dr. K.-W. Li for assistance in some experiments.
Correspondence should be addressed to Dr. Z. David Luo, Department of Anesthesiology, University of California, Irvine Medical Center, 101 The City Drive South, Orange, CA 92868. E-mail: zluo@uci.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248494-06$15.00/0
References
- Ahlijanian MK, Westenbroek RE, Catterall WA (1990) Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron 4: 819-832. [DOI] [PubMed] [Google Scholar]
- Brust PF, Simerson S, McCue AF, Deal CR, Schoonmaker S, Williams ME, Veliçelebi G, Johnson EC, Harpold MM, Ellis SB (1993) Human neuronal voltage-dependent calcium channels: studies on subunit structure and role in channel assembly. Neuropharmacology 32: 1089-1102. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55-63. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938-942. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Catterall WA (1990) Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem 265: 14738-14741. [PubMed] [Google Scholar]
- Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20: 441-462. [DOI] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM (1988) Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science 241: 1661-1664. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP (1996) Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron 16: 431-440. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP (1997) Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J Biol Chem 272: 18508-18512. [DOI] [PubMed] [Google Scholar]
- Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP (1991) Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem 266: 3287-3293. [PubMed] [Google Scholar]
- Kang MG, Felix R, Campbell KP (2002) Long-term regulation of voltage-gated Ca2+ channels by gabapentin. FEBS Lett 528: 177-182. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50: 355-363. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F (1999) Molecular diversity of the calcium channel α2δ subunit. J Neurosci 19: 684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochegarov AA (2003) Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium 33: 145-162. [DOI] [PubMed] [Google Scholar]
- Laird MA, Gidal BE (2000) Use of gabapentin in the treatment of neuropathic pain. Ann Pharmacother 34: 802-807. [DOI] [PubMed] [Google Scholar]
- Luo ZD (2000) Rat dorsal root ganglia express distinctive forms of the α2 calcium channel subunit. NeuroReport 11: 3449-3452. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL (2001) Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci 21: 1868-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR (2002) Injury type-specific calcium channel α2δ-1 subunit upregulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther 303: 1199-1205. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB (2000) Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci 12: 397-399. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Hughes J, McKnight AT (2001) Gabapentin inhibits the substance P-facilitated K+-evoked release of [3H] glutamate from rat caudal trigeminal nucleus slices. Pain 93: 191-196. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F (2001) Calcium channel α2δ subunits-structure and gabapentin binding. Mol Pharmacol 59: 1243-1248. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH (2001) Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain 92: 235-246. [DOI] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S (1991) Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350: 398-402. [DOI] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN (2001) Dorsal root ganglion neurons show increased expression of the calcium channel α2δ-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res 95: 1-8. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K (1995) Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci 15: 7633-7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MK, Gonzalez MI, Bramwell S, Pinnock RD, Lee K (2000) Gabapentin inhibits excitatory synaptic transmission in the hyperalgesic spinal cord. Br J Pharmacol 130: 1731-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR (2002) Molecular cloning and characterization of the human voltage-gated calcium channel α(2)δ-4 subunit. Mol Pharmacol 62: 485-496. [DOI] [PubMed] [Google Scholar]
- Skilling SR, Harkness DH, Larson AA (1992) Experimental peripheral neuropathy decreases the dose of substance P required to increase excitatory amino acid release in the CSF of the rat spinal cord. Neurosci Lett 139: 92-96. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L (1998) A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 29: 233-249. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD (2003) Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem 87: 560-573. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS (2002) Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience 114: 529-546. [DOI] [PubMed] [Google Scholar]
- Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM (1992) Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 8: 71-84. [DOI] [PubMed] [Google Scholar]
- Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N (2003) Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J Mol Biol 332: 171-182. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17: 1031-1036. [DOI] [PubMed] [Google Scholar]
- Yusaf SP, Goodman J, Pinnock RD, Dixon AK, Lee K (2001) Expression of voltage-gated calcium channel subunits in rat dorsal root ganglion neurons. Neurosci Lett 311: 137-141. [DOI] [PubMed] [Google Scholar]