Abstract
Heparin-binding epidermal growth factor (HB-EGF), a member of the epidermal growth factor (EGF) family, is implicated in a variety of biological processes, including reproduction. Previous studies describe increased levels of HB-EGF in the human endometrium during the midsecretory stage of the menstrual cycle, suggesting a function for HB-EGF in implantation of the human blastocyst. Here we have investigated the expression and function of the soluble and transmembrane forms of HB-EGF in the human endometrium. We show that the expression of the transmembrane form of HB-EGF in the human endometrium is modulated according to the stage of the menstrual cycle. We present data demonstrating that both the soluble and transmembrane forms of HB-EGF induce DNA synthesis in human endometrial stromal cells. Furthermore, TNFα has a cooperative effect on HB-EGF, EGF, TGFα, and betacellulin-induced DNA synthesis in stromal cells, suggesting roles for the EGF family and TNFα in regeneration and maturation of human endometrium. Induction of DNA synthesis by HB-EGF and its modulation by TNFα in endometrial stromal cells are mediated by the EGF receptor and not the HB-EGF receptor ErbB4. Our data suggest key functions for HB-EGF, TNFα, and the EGF receptor in endometrial maturation, via autocrine/paracrine and juxtacrine pathways, in preparation for embryo implantation.
Abbreviations: BTC, Betacellulin; CHO, Chinese hamster ovary; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; HB-EGF, heparin-binding epidermal growth factor; HRP, horseradish peroxidase; PMSF, phenylmethylsulfonylfluoride; sol-, soluble form; tm-, transmembrane form
Heparin-binding epidermal growth factor (HB-EGF) is a member of the epidermal growth factor (EGF) family and has been implicated in a variety of biological processes, such as wound healing, tumor growth, smooth muscle cell hyperplasia, angiogenesis, and reproduction (1–3). It is synthesized by many cell types (4–8) as a 208-amino acid transmembrane precursor (tm-HB-EGF) containing EGF, heparin-binding, transmembrane, and cytoplasmic domains. The extracellular domain can be released as a 12- to 22-kDa soluble form of HB-EGF (sol-HB-EGF), probably as a result of the action of metalloproteinases (9–11), although a substantial amount of transmembrane precursor can remain uncleaved on the cell surface (12). Both tm-HB-EGF and sol-HB-EGF are biologically active (12). The biological functions of both sol- and tm-HB-EGF are mediated by the EGF receptor (EGFR; HER1) and ErbB4 (HER4) (1, 13, 14). Activation of these types of receptors is believed to occur as a consequence of ligand-induced receptor homo-or heterodimerization (14).
The spatio-temporal expression of HB-EGF in rodents suggests a function in uterine receptivity, embryonic well-being, and implantation of the blastocyst (15, 16). In functional studies, cells expressing tm-HB-EGF adhered to mouse blastocysts (17), and sol-HB-EGF was found to stimulate proliferation, hatching, outgrowth of trophoblast, and tyrosine phosphorylation of EGFR in mouse blastocysts (16). It has also been suggested that HB-EGF binds to ErbB4 expressed on the trophectoderm of mouse blastocysts (18). Studies in the baboon revealed that HB-EGF protein accumulation increases in the endometrium on d 5 and 10 postovulation (19). These data from animal studies are consistent with reports addressing the human endometrium, in which HB-EGF mRNA is detected throughout the menstrual cycle and its levels increased before the implantation window (d 19–21 of the menstrual cycle) (8, 20, 21). Furthermore, the expression of both receptors for HB-EGF, EGFR and ErbB4, in the human endometrium also undergoes cyclic changes (22–24). It has also been suggested that HB-EGF has a function for sol-HB-EGF in promoting the development of in vitro fertilized human embryos to the blastocyst stage and subsequent hatching (25).
The endometrial stroma undergoes extensive expansion in preparation for embryo implantation, which is subsequently shed during the menstrual stage of the cycle if the embryo fails to implant. Several reports suggest that TNFα has a major role in regulation of the onset of menstruation. Expression of TNFα mRNA has been reported to increase during the secretory stage of the cycle (26, 27). Also, endometrial biopsies obtained from the menstrual stage of the cycle have been found to secrete higher levels of soluble TNFα than biopsies taken at any other stage (28). In addition, TNFα has been reported to inhibit proliferation and to induce apoptosis in endometrial epithelial cells (29). However, little is known about possible functions in the endometrial stroma.
Here we have investigated the potential function of HB-EGF and other EGFR ligands EGF, TGFα, betacellulin (BTC), and TNFα, in the regeneration of the human endometrial stroma. We describe the localization of the membrane-anchored, as opposed to the soluble, form of HB-EGF, present evidence for the modulation of DNA synthesis in endometrial stromal cells by sol-HB-EGF and tm-HB-EGF, and demonstrate that TNFα cooperates with HB-EGF, EGF, TGFα, and BTC to increase the proliferative capacity of stromal cells.
Materials and Methods
Tissue samples
Tissue samples were collected in accordance with the requirements of the Central Oxford Research Ethics committee. Endometrial samples were obtained from patients, aged 20–49 yr, undergoing hysterectomy for benign indications, who had regular 26- to 33-d menstrual cycles, and who had received no hormonal medication in the preceding 3 months. The cycle stage of the endometrium was assessed according to Noyes et al. (30) criteria.
Tissue staining
Endometrial tissue samples were obtained from 12 women (7 tissues from the proliferative and 5 from the secretory stages of menstrual cycle). Sections (7 μm) from paraffin-embedded tissue samples were prepared as described previously (31). Tissue sections were incubated in 10 μg/ml goat antihuman HB-EGF antibodies (recognizing the intracellular domain of the HB-EGF-precursor, detecting tm-HB-EGF; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 h at room temperature. Control staining was performed with the same antibodies preincubated with the corresponding antigenic peptides. Sections were washed in three changes of PBS, then incubated in horseradish peroxidase (HRP)-conjugated antigoat IgG for 1 h at room temperature and washed as described above. Bound antibodies were detected with HRP substrate diaminobenzidine tetrachloride (Sigma, Poole, UK).
Isolation and immunohistochemical staining of human endometrial stromal cells
Endometrial tissues were processed for stromal cell culture as described previously (32). The purity of the isolated cells was assessed by staining with Thy-1 and vimentin, and in this study cells were either used at consecutive passages between passages 1–6 or thawed from early passage cultures. The percentages of Thy-1- and vimentin-positive cells were approximately 84% and 92%, respectively, and remained consistent with passage. Cultures of endometrial stromal cells seeded onto coverslips were stained by the use of immunofluorescent techniques as described previously (33). Specific antigens were detected by incubation in 4 μg/ml goat anti-HB-EGF (Santa Cruz Biotechnology, Inc.), 15 μg/ml anti-ErbB4 (clone HFR1) (34), or 5 μg/ml mouse anti-EGFR (BD PharMingen, San Diego, CA), followed by 15 μg/ml of either rabbit antigoat fluorescein isothiocyanate (FITC)-conjugated IgG (Sigma) or donkey antimouse Texas Red-conjugated IgG (Jackson ImmuoResearch Laboratories, Inc., West Grove, PA) antibodies. Goat and mouse IgG were used in negative controls as appropriate. Coverslips were mounted in Vectashield medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). The staining was observed using a Leitz DMRBE microscope (Leica Corp., Wetzlar, Germany) and Openlab imaging software (Improvision, Coventry, UK).
Generation of Chinese hamster ovary (CHO)/HB-EGF cells and membrane preparations
CHO cells expressing tm-HB-EGF were generated by transfection with a cDNA encoding the full-length HB-EGF precursor cloned into pEE14 (CHO/HB-EGF) using standard calcium phosphate precipitation procedures. The control cell line (CHO/vector) was transfected with pEE14. One-step amplification with 25 μm methionine sulfoximine (Sigma) was performed as described previously (35). Expression of tm-HB-EGF was assessed by Western blot analysis of crude membrane preparations derived from CHO/HB-EGF and CHO/vector. For this purpose, confluent CHO cells in 75-cm2 flasks were washed with PBS, scraped, and resuspended in 5 ml homogenization buffer [25 mm Tris (pH 7.5), 0.25 m sucrose, 1 mm EDTA, 1 mm dithiothreitol, 2 mm phenylmethylsulfonylfluoride (PMSF), 10 mg/ml leupeptin, and 1 mg/ml pep-statin] and passed through a glass cell homogenizer. The cell homogenate was centrifuged at 5,000 × g for 10 min, followed by centrifugation at 100,000 × g for 2 h. The pellet was washed with homogenization buffer and resuspended in RIPA (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate in PBS) supplemented with 2 mm PMSF, 10 mg/ml leupeptin, and 1 mg/ml pepstatin. Proteins were separated by 12% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Roche, Mannheim, Germany). Western blotting was performed with 2 μg/ml goat antihuman HB-EGF (Santa Cruz Biotechnology, Inc.), followed by 60 ng/ml rabbit antigoat IgG, and bound antibodies were detected with the use of the Super Signal West Pico Chemiluminescent Substrate (Pierce Chemical Co., Rockford, IL) according to the manufacturer’s instructions.
Cell preparations for testing the juxtacrine activity of tm-HB-EGF were prepared from CHO/HB-EGF or CHO/vector cells plated into 9-cm petri dishes in Glasgow MEM (First Link Ltd., Birmingham, UK) containing 10% dialyzed fetal calf serum (FCS), penicillin, streptomycin, and 25 μm methionine sulfoximine (Sigma). Confluent cultures were washed twice in 2 mm NaCl in PBS and fixed with 3% paraformaldehyde in PBS for 5 min. The fixed cells were washed twice in DMEM containing 10% FCS, scraped from the plate, and resuspended in serum-free DMEM. Fixed cells (105) were added to each monolayer of stromal cells in 96-well plates (plated at 104 cells/well) that had been previously serum-starved for 18 h. The cocultures were incubated in serum-free medium for 24 h, and thymidine incorporation assays were performed as described below.
DNA synthesis assays
Stromal cells were seeded into 96-well plates (104 cells/well) in DMEM supplemented with 10% FCS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. After 24 h the medium was changed to serum-free DMEM, and the cells were incubated for a further 18 h. Cells were then stimulated with combinations of recombinant HB-EGF, EGF, TGFα, BTC, and TNFα (all from R&D Systems, Abingdon, UK), and PD153035, a specific inhibitor of the tyrosine kinase activity of EGFR (Calbiochem, La Jolla, CA), in serum-free DMEM for 24 h. Juxtacrine activity was measured using a modification of a method described previously (36). Fixed CHO/HB-EGF or CHO/vector cell membrane preparations, prepared as described above, were added to each monolayer of stromal cells in 96-well plates (plated at 104 cells/well) and serum-starved as described above. [3H]Thymidine (1 μCi; Amersham Pharmacia Biotech, Little Chalfont, UK) was added to each well for the last 4 h of incubation. Cells were washed three times in PBS and harvested, and the amount of incorporated [3H]thymidine was determined using a β-plate counter (Wallac, Inc., Turku, Finland).
Immunoprecipitation and Western blotting
Confluent stromal cell cultures in 9-cm petri dishes were starved in serum-free medium for 18 h and then stimulated with 10 ng/ml HB-EGF or overlaid with fixed CHO/HB-EGF (as described above) in serum-free DMEM for 3 min at 37 C. Petri dishes were placed on ice and washed twice with ice-cold PBS supplemented with 2 mm orthovanadate. Cells were lysed for 10 min in RIPA supplemented with 2 mm orthovanadate, 2 mm sodium fluoride, 2 mm sodium pyrophosphate, 2 mm PMSF, 10 μg/ml leupeptin, and 1 μg/ml pepstatin in PBS. The cell lysates were clarified by microcentrifugation at 5000 × g for 15 min at 4 C. Immunoprecipitation was performed with 5 μg of either anti-EGFR mouse monoclonal (BD PharMingen) or anti-ErbB4 rabbit polyclonal (Santa Cruz Biotechnology, Inc.) antibodies for 4 h at 4 C. The immune complexes were collected by binding to protein A/G-Sepharose (Santa Cruz Biotechnology, Inc.) using standard protocols. Proteins were separated by 7.5% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Roche). Phosphotyrosines were detected by incubation in 1 μg/ml antibody clone 4G10 (Upstate Biotechnology, Inc., Lake Placid, NY). EGFR and ErbB4 were detected in parallel blots incubated with 2 μg/ml rabbit anti-EGFR (Santa Cruz Biotechnology, Inc.) or rabbit anti-ErbB4 (Santa Cruz Biotechnology, Inc.). Bound antibodies were detected by incubation in either 50 ng/ml sheep antimouse IgG (Amersham Pharmacia Biotech) or 250 ng/ml rabbit antigoat IgG (DAKO Corp., Copenhagen, Denmark), both conjugated with HRP, with the use of the methods described above.
Flow cytometry
Cultures of stromal cells were trypsinized, fixed in 0.25% paraformaldehyde and 2% glucose in PBS for 10 min at 4 C, and permeabilized with 0.1% saponin and 0.1% BSA in PBS for 20 min at 4 C. Labeling was performed with 5 μg/ml of either mouse anti-EGFR monoclonal antibodies (BD PharMingen, San Diego, CA) or mouse IgG (Sigma) in 2% glucose and 5% normal human serum in PBS for 30 min at 4 C. Cells were washed in 2% glucose and 0.1% BSA in PBS and incubated with 15 μg/ml goat antimouse FITC-conjugated antibodies (Sigma) for 30 min on ice. The cells were washed in 2% glucose and 5% normal human serum in PBS and resuspended in PBS containing 2% FCS and 0.1% NaN3. Samples were analyzed on a Coulter EPICS Elite flow cytometer that was calibrated before each experiment with Flow Set Beads (Beckman Coulter, Inc., High Wycombe, UK). FITC fluorescence was detected at 525 nm. Ten thousand events were collected, and the results were expressed as the mean channel brightness.
Statistical analysis
Data were analyzed using ANOVA (StatView 5.01 software, SAS Institute, Inc., Cary, NC), and P value less than 0.05 indicated significant differences between datasets.
Results
Expression of tm-HB-EGF in the human endometrium is modulated during the menstrual cycle
Previous data from this and other laboratories have reported expression of HB-EGF mRNA and sol-HB-EGF protein in the endometrium (8, 21). Here we have analyzed in detail the expression of the tm-HB-EGF-precursor (hereafter referred to as tm-HB-EGF) in the human endometrium. Tissue sections derived from different stages of the cycle were stained with antibodies recognizing the C-terminal end of tm-HB-EGF (Fig. 1). Proliferative stage endometrial tissues exhibited staining for HB-EGF in both stroma and glandular epithelium. Staining was most intense in the basalis, decreasing in intensity through the functionalis, and was not detectable in the area adjacent to the lumenal part of the endometrium (Fig. 1, A and C). Staining for tm-HB-EGF in early to midsecretory stage tissues was intense throughout the stroma and epithelium of both the basalis and functionalis layers of the endometrium (Fig. 1B). High magnification of secretory stage tissues revealed intense, membrane-associated staining for tm-HB-EGF (Fig. 1C). There was a punctate pattern of staining around the basal and apical surfaces of glandular epithelium and some stromal cells. Staining with anti-HB-EGF preincubated with control peptide showed no reactivity with tm-HB-EGF (Fig. 1D).
Fig. 1.
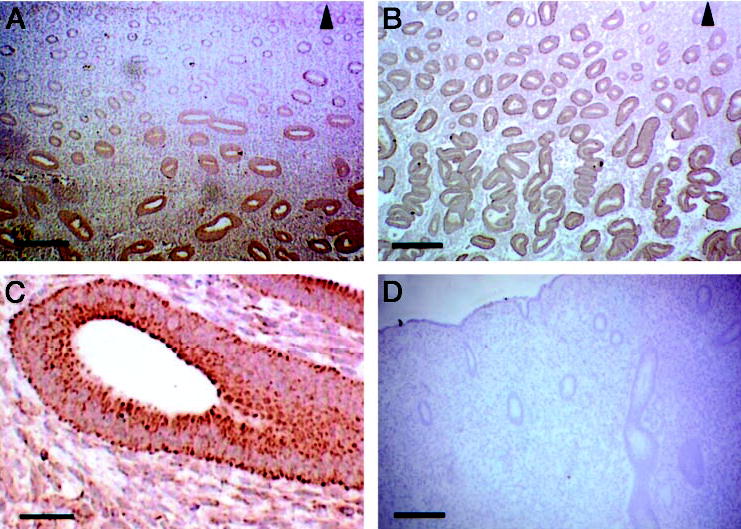
Expression of tm-HB-EGF in the human endometrium. Tissue sections derived from proliferative (A and C) and secretory (B) endometrium were stained with anti tm-HB-EGF antibodies. Control staining was performed with antibodies preincubated with the appropriate control peptide (D). The region adjacent to the lumenal surface is marked by an arrow. Scale bars, 50 μm (in A–C) and 125 μm (D).
tm-HB-EGF, EGFR, and ErbB4 are expressed in cultures of endometrial stromal cells
The HB-EGF receptors EGFR and ErbB4 previously have been shown to be expressed in human endometrial tissues (23, 34). As the functions of sol- and tm-HB-EGF were to be determined in cultures of endometrial stromal cells, the expression of EGFR, ErbB4, and tm-HB-EGF by these cells in culture was assessed (Fig. 2). Stromal cells stained positively with antibodies to the HB-EGF precursor, displaying a punctate pattern of staining for tm-HB-EGF (Fig. 2A). Control cultures treated with goat IgG were negative (Fig. 2B). The receptors EGFR (Fig. 2C) and ErbB4 (Fig. 2D) were detected in stromal cells, and both exhibited a punctate pattern of staining. Although the punctate tm-HB-EGF staining was reminiscent of that seen in vivo, the significance of this pattern of staining for the ligand and the receptors is not clear. Control cultures treated with mouse IgG were negative (Fig. 2E).
Fig. 2.
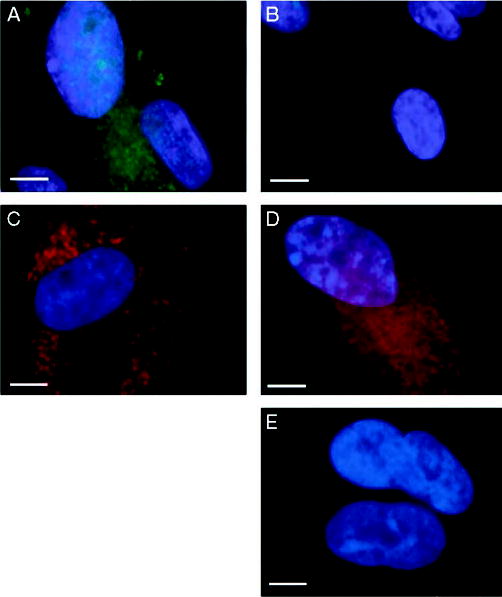
Cultured endometrial stromal cells express both HB-EGF receptors, EGFR and ErbB4, and tm-HB-EGF. Stromal cells were grown on coverslips and stained for tm-HB-EGF (A), EGFR (C), and ErbB4 (D). Control staining was performed with either goat or mouse IgG (B and E, respectively). Scale bars, 10 μm.
sol- and tm-HB-EGF promote DNA synthesis in endometrial stromal cells
The effects of sol- and tm-HB-EGF on DNA synthesis in human endometrial stromal cells were analyzed (Fig. 3) First we assessed the response to HB-EGF of stromal cells at passages 1–6 in serum-free medium (Fig. 3A). Our analyses confirmed that the cells retain the capacity to respond to HB-EGF to a similar extent at the passages we used in this study, and there was no significant difference in HB-EGF responsiveness between passages.
Fig. 3.
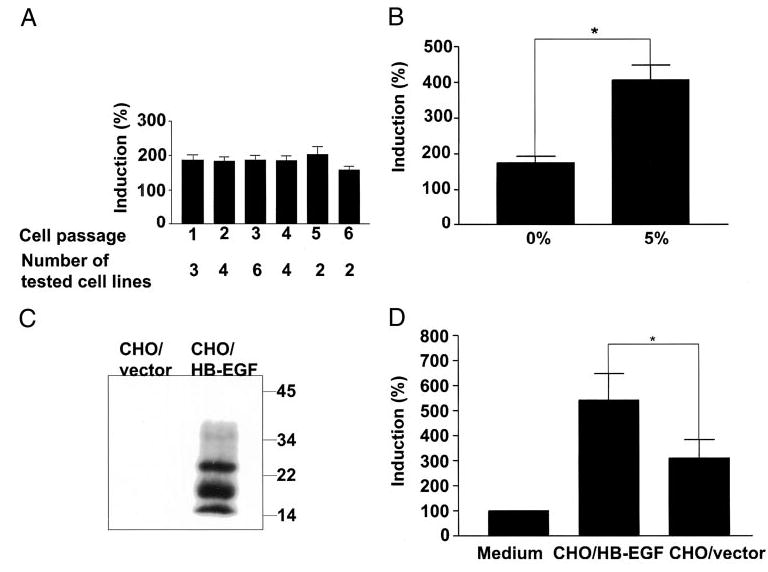
Sol- and HB-EGF-precursor stimulate DNA synthesis in human endometrial stromal cells. Stromal cell lines (n = 15), each derived from a different human endometrium, were cultured with or without 10 ng/ml sol-HB-EGF in serum-free medium, and DNA synthesis assays were performed at passages 1–6 (A). Stromal cells were cultured with or without 10 ng/ml sol-HB-EGF in serum-free medium or in medium supplemented with 5% FCS (n = 7; B). Crude membrane preparations from CHO/vector and CHO/HB-EGF were analyzed by Western blot with antibodies to HB-EGF precursor (C). Stromal cells cultures overlaid with medium, fixed CHO/HB-EGF, or fixed CHO/vector and cultured in serum-free medium (n = 6; D). DNA synthesis was assessed by measurement of the incorporation of [3H]thymidine into the cells. Incorporation of [3H]thymidine into cells in the absence of HB-EGF was taken as 100% (control). Each bar represents the mean ± sem of at least four independent experiments with each individual cell line. *, Significant difference (P < 0.05) compared with the control.
In serum-free medium, sol-HB-EGF induced up to a 2-fold increase in DNA synthesis compared with cells cultured in the absence of sol-HB-EGF. However, in the presence of 5% charcoal-stripped serum, stromal cells responded to sol-HB-EGF with a 3- to 5-fold increase in DNA synthesis compared with untreated controls (Fig. 3B), indicating that additional factors in serum may act cooperatively with HB-EGF in promoting DNA synthesis.
To analyze the juxtacrine activity of HB-EGF we generated CHO expressing the tm-HB-EGF, CHO/HB-EGF, and a control cell line transfected with the vector, CHO/vector. In crude membrane preparations from CHO/HB-EGF cells tm-HB-EGF was detected as several species ranging from 15–35 kDa (Fig. 3C), correlating with the previously reported pattern of protein species (37). Juxtacrine activity of HB-EGF was tested by coincubating CHO/HB-EGF and CHO/vector cells with stromal cells (Fig. 3D). In serum-free medium the CHO/HB-EGF preparation induced a 2-fold increase in [3H]thymidine incorporation compared with the CHO/vector. Cell preparations from CHO/vector also induced DNA synthesis in stromal cells 3- to 6-fold more than in cells cultured in medium alone, indicating that additional membrane-associated factors also contributed to the induction of DNA synthesis in stromal cells via a juxtacrine pathway.
HB-EGF-induces phosphorylation of EGFR, but not ErbB4
Previously, HB-EGF has been shown to induce phosphorylation of EGFR (9) and ErbB4 (14) in transfected cell lines overexpressing these receptors. We therefore examined receptor phosphorylation in endometrial stromal cells exposed to sol- and tm-HB-EGF to determine which of the receptors, EGFR or ErbB4, mediates the activity of HB-EGF in these cells (Fig. 4). Our data demonstrated that EGFR, but not ErbB4, was phosphorylated in response to sol-HB-EGF (Fig. 4A). Phosphorylation of EGFR in response to HB-EGF was consistently detected in stromal cells of different passages (data not shown). Phosphorylation of ErbB4 was not manifested when cells were induced with sol-HB-EGF, even in medium containing 10% serum (results not shown). Stimulation of cells with CHO/HB-EGF cell preparations also resulted in phosphorylation of EGFR, but not that of ErbB4 (Fig. 4B). The presence of both EGFR and ErbB4 in the stromal cells was demonstrated in Western blots of the cell lysates (Fig. 4C). A major band of approximately 150 kDa corresponding to the full-length EGFR was detected. The major band in the ErbB4 blot was approximately 100 kDa, and there were also minor bands of higher molecular weight, but lower than the predicted full-length ErbB4 species (>140 kDa).
Fig. 4.
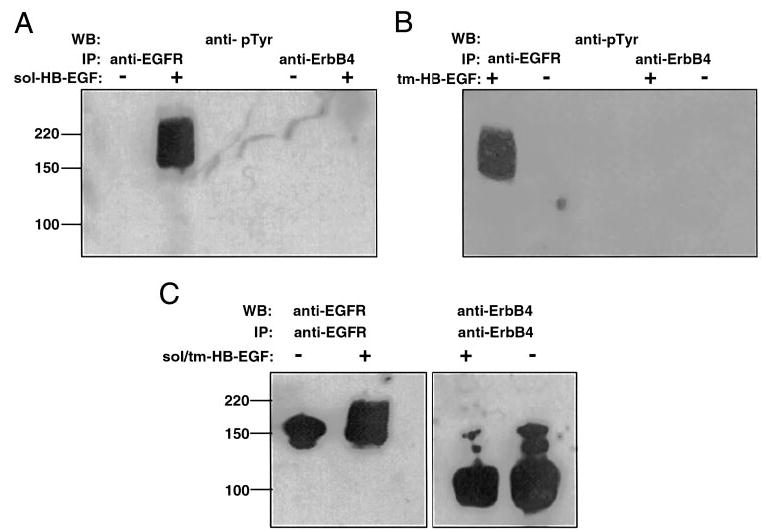
HB-EGF induces phosphorylation of EGFR, but not ErbB4. Serum-starved endometrial stromal cells were stimulated with either sol-HB-EGF (A) or HB-EGF-precursor (B). Control cells were treated with medium alone. Whole cell lysates were prepared and immunoprecipitated (IP) with either anti-EGFR or anti-ErbB4. Samples were separated by 7.5% SDS-PAGE and subjected to Western blotting (WB) with antiphosphotyrosine antibodies (A and B), anti-EGFR, or anti-ErbB4 antibodies (C). Molecular weight markers are indicated.
EGFR mediates DNA synthesis in endometrial stromal cells
Having determined that EGFR is phosphorylated in response to HB-EGF, we investigated the role of EGFR in mediating the downstream functional effects of HB-EGF by the use of PD153035, which selectively inhibits the protein tyrosine kinase activity of EGFR (Fig. 5). Our data showed that PD153035 suppressed sol-HB-EGF-induced DNA synthesis in stromal cells to levels comparable to those in the medium control.
Fig. 5.
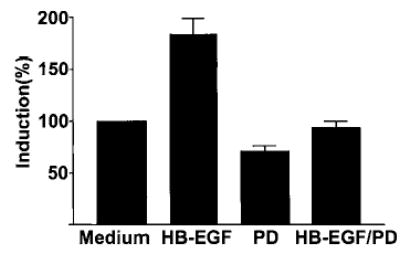
Induction of DNA synthesis in endometrial stromal cells by HB-EGF is mediated by EGFR. DNA synthesis in endometrial stromal cells induced by sol-HB-EGF (10 ng/ml) is suppressed by PD153035 (2 μm; HB-EGF/PD). Each bar represents the mean ± sem of four replicates in each of three independent experiments.
TNFα acts cooperatively with HB-EGF and the EGFR ligands EGF, TGFα, and BTC to induce DNA synthesis in endometrial stromal cells
The increase in HB-EGF-induced DNA synthesis we observed in the presence of serum (see Fig. 3B) suggested that additional factors present in serum have an additive effect on HB-EGF activity. We examined the possibility that TNFα can modulate HB-EGF function and that of other EGF family ligands that bind EGFR (EGF, TGFα, and BTC) in endometrial stromal cells (Fig. 6). TNFα did not significantly induce DNA synthesis in stromal cells, whereas EGF, TGFα, and BTC induced up to a 2-fold increase in each case. However, simultaneous stimulation of stromal cells with TNFα and HB-EGF, EGF, TGFα, or BTC in serum-free medium resulted in a 3.5- to 4-fold increase in DNA synthesis compared with the controls, more than the sum of the increase induced by each ligand on its own (Fig. 6).
Fig. 6.
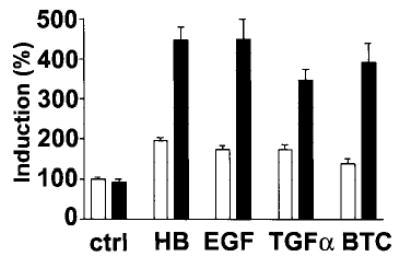
Cooperativity of TNFα and HB-EGF and EGFR ligands, EGF, TGFα, and BTC, in the induction of DNA synthesis in endometrial stromal cells. Stromal cells were stimulated with 10 ng/ml HB-EGF (HB), EGF, TGFα, and BTC alone (□) or in the presence of 10 ng/ml TNFα (▪). Control cells (ctrl) were cultured with (▪) or without (□) 10 ng/ml TNFα. Incorporation of [3H]thymidine in cells in the absence of the exogenous factors was taken as 100% (ctrl; □). Each bar represents the mean ± sem of four replicates in each of four independent experiments with different cell lines.
TNFα increases the levels of EGFR on endometrial stromal cells
We examined one of the possible mechanisms by which the modulation of HB-EGF activity by TNFα could be facilitated by assessing the expression of EGFR in endometrial stromal cells in response to TNFα by the use of quantitative flow cytometry (Fig. 7). TNFα induced increased levels of EGFR that were detected on the membranes of the endometrial stromal cells relative to the controls labeled with IgG.
Fig. 7.
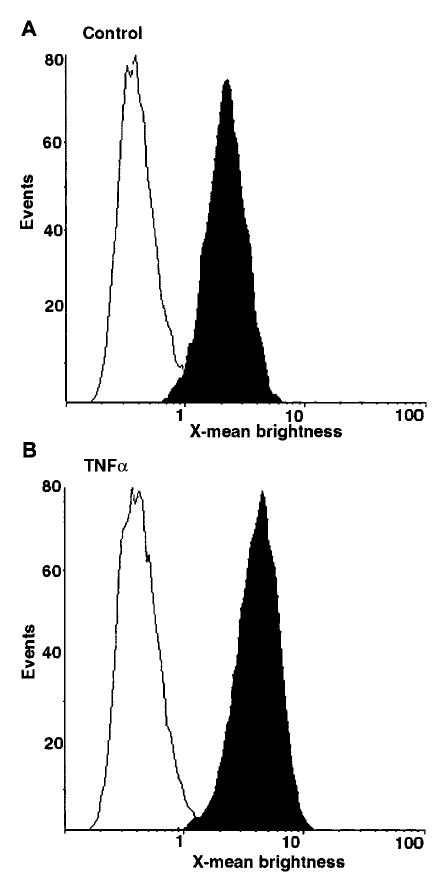
TNFα increases levels of EGFR in endometrial stromal cells. Flow cytometry analysis of stromal cells incubated in the absence (A) or presence (B) of 10 ng/ml TNFα, labeled with mouse anti-EGFR antibodies (▪) or mouse IgG (□). The graphs are representative of data obtained from 11 independent stromal cell lines.
Discussion
There is persuasive evidence suggesting that HB-EGF and other EGFR ligands have important functions in reproduction. The results presented here suggest a function for sol- and tm-HB-EGF and the EGFR ligands EGF, TGFα, and BTC in the proliferation of human endometrial stromal cells. Our experiments demonstrate that 1) tm-HB-EGF expression in the human endometrium is spatially modulated in a cycle stage-dependent fashion; 2) sol- and tm-HB-EGF modulate the proliferative capacity of stromal cells; 3) the proliferative capacity of both soland tm-HB-EGF is mediated via EGFR and not ErbB4; and 4) TNFα can act cooperatively with sol-HB-EGF, EGF, TGFα, and BTC to induce DNA synthesis in stromal cells.
The expression of HB-EGF mRNA and sol-HB-EGF protein in the human endometrium has been shown previously to increase before the implantation window (8, 21). Although there are conflicting data on the expression of EGFR, Srinivasan et al. (24) showed that ErbB4 is expressed at high levels in the endometrial stroma and that it accumulates in the glands during the secretory stage of the cycle. Here we have demonstrated that during the proliferative stage of the menstrual cycle tm-HB-EGF is expressed in the glands and stroma of the basalis layer of the endometrium. With the progression of the cycle to the secretory stage, the level of tm-HB-EGF increases, and it can be detected on the basal membrane of glands and on stromal cell membranes in both the basalis and functionalis layers, suggesting a function for tm-HB-EGF in endometrial maturation before blastocyst implantation. Here we used human endometrial stromal cell lines as a model for investigating possible functions for HB-EGF function. We have demonstrated that these cultured stromal cells retain the expression profile of a number of markers and their responsiveness to a number of growth-inducing factors, the collective data for which are not within the scope of the present report. Pertinent to this study are our data showing that the cultured endometrial stromal cells we describe retain their expression of tm-HB-EGF and its receptors, EGFR and ErbB4, and their responsiveness to HB-EGF with passaging, thus validating the use of these cell lines as a suitable model system for investigating the function of endometrial HB-EGF.
The preparation of the endometrium for implantation of the embryo involves a dramatic increase in endometrial mass that is regulated by steroid hormones. However, it is clear that the effects of steroid hormones on endometrial cells are mediated in part by a number of growth factors (38, 39). A previous report demonstrated that EGF and TGFα can stimulate the proliferation of endometrial stromal cells in culture, whereas TNFα has no effect (40) and is thus in agreement with our data, while others have reported that EGF has no effect on stromal cell proliferation (41). Also, it has been shown that HB-EGF levels are differentially regulated by estradiol and progesterone in rat uterine epithelium and stroma (42) and in the baboon (19) in vivo, indicating that it is a good candidate for a molecular mediator of steroid action in the endometrium. Here we show that human stromal cells increase DNA synthesis in response to sol-HB-EGF, indicating that sol-HB-EGF may contribute to endometrial maturation by promoting the proliferation of endometrial stromal cells.
We tested the potential mitogenic activity of tm-HB-EGF in the endometrium by the use of CHO cell lines overex-pressing tm-HB-EGF on the cell surface. These experiments demonstrate that CHO/HB-EGF cells can promote DNA synthesis and suggest a function for tm-HB-EGF as a juxtacrine growth factor in the human endometrium. Interestingly, the control cell line, CHO/vector, also had a significant effect on DNA synthesis, suggesting that additional membrane-bound factors are involved in juxtacrine activity. We therefore propose that HB-EGF can act as a mitogenic factor for stromal cells in the human endometrium and that the mitogenic activity of HB-EGF can be mediated or stimulated by additional soluble or membrane-associated regulators. Further investigations will elucidate the importance of the association of tm-HB-EGF with such activity-modulating factors, for example CD9 (43) and α3β1 (44), in the context of the endometrial stromal cell membrane.
The mechanism of HB-EGF action was analyzed by examining the effect of HB-EGF on the tyrosine phosphorylation status of HB-EGF receptors, EGFR and ErbB4, both of which are expressed in human endometrium (24, 34, 45). We demonstrate that the addition of HB-EGF to stromal cells in culture results in tyrosine phosphorylation of EGFR, but ErbB4 phosphorylation was not detected, suggesting that HB-EGF-induced proliferation in these cells is mediated via EGFR. This was corroborated by the observation that PD153035, a specific inhibitor of EGFR tyrosine kinase (46), suppresses HB-EGF-induced DNA synthesis in stromal cells. These results are in agreement with previous studies that suggest a role for EGFR, but not ErbB4, in mediating a proliferative response in transformed cell lines (14). Previous reports indicate that ErbB4 activation is coupled to a differentiation, rather than proliferation, pathway (34, 47). Therefore, we cannot rule out the possibility that activation of endometrial ErbB4 may be associated with differentiation or other cellular processes in the endometrial stroma.
The full-length ErbB4 protein is 180 kDa, yet the major band we detected in Western blots of cell lysates using anti-ErbB4 was approximately 100 kDa. Different molecular weight species of ErbB4 derived by differential glycosylation and/or proteolytic cleavage are seen in other cell types (48, 49). Taking into account the predicted molecular weight of the full-length ErbB4 (>144 kDa), it can be suggested that the vast majority of ErbB4 synthesized by endometrial stromal cells is cleaved and is not phosphorylated by HB-EGF. This introduces an additional mechanism by which HB-EGF function may be regulated in the human endometrium.
The onset of the menstrual stage of the cycle is thought to be regulated by many factors, including TNFα acting as an apoptosis-inducing factor that inhibits proliferation of epithelial cells and promotes endometrial shedding (28, 50, 51). TNFα is biologically active in both transmembrane and soluble forms (reviewed in Ref. 52). Previously it was shown to have a synergistic effect with fibroblast growth factor on the proliferation of endometrial stromal cells, whereas no stimulation of stromal cell proliferation was observed with TNFα alone (40). In other systems, TNFα has been shown to modulate the activity of EGFR and thus regulate the function of EGFR ligands (53–55). Here we observed that TNFα has an additive effect on the induction of DNA synthesis by sol-HB-EGF in endometrial stromal cells. Thus, both HB-EGF and TNFα may be involved in the process of endometrial regeneration and maturation. A cooperative effect of TNFα on other EGFR ligands, EGF, TGFα, and BTC expressed in human endometrium (24, 56), was also observed. TNFα has been shown to promote tyrosine phosphorylation and expression of EGFR in some cell lines (57, 58). We found that TNFα increases the levels of EGFR in endometrial stromal cells. Tyrosine phosphorylation of EGFR was not observed when stromal cells were stimulated with TNFα (data not shown). The TNFα-induced increase in EGFR levels could therefore account for the increase in the proliferative capacity of stromal cells in response to HB-EGF, EGF, TGFα, and BTC observed in the presence of TNFα.
In summary, we propose that in humans, sol- and tm-HB-EGF have a key function in endometrial regeneration and preparation of the endometrium for embryo implantation, and that TNFα is among the potential key factors regulating the activities of HB-EGF and other EGFR ligands. We suggest that endometrial HB-EGF acts as a mitogenic factor via juxtacrine as well as autocrine and paracrine pathways, and that TNFα acts cooperatively with HB-EGF to induce the proliferation of stromal cells in the endometrium. Finally, our data firmly support existing evidence that EGFR and ErbB4 may have distinct functions in the human endometrium.
Acknowledgments
We are grateful to Grant Nicholson for assistance with flow cytometry.
Footnotes
This work was supported by the Wellcome Trust and the Medical Research Council (United Kingdom).
References
- 1.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 2.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 3.Arkonac BM, Foster LC, Sibinga NE, Patterson C, Lai K, Tsai JC, Lee ME, Perrella MA, Haber E. Vascular endothelial growth factor induces heparin-binding epidermal growth factor-like growth factor in vascular endothelial cells [published erratum appears in J Biol Chem 1998 Apr 10;273(15):9352] J Biol Chem. 1998;273:4400–4405. doi: 10.1074/jbc.273.8.4400. [DOI] [PubMed] [Google Scholar]
- 4.Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz RA. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 1993;190:125–133. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy TG, Brown KD, Vaughan TJ. Expression of the genes for the epidermal growth factor receptor and its ligands in porcine oviduct and endometrium. Biol Reprod. 1994;50:751–756. doi: 10.1095/biolreprod50.4.751. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan TJ, Pascall JC, Brown KD. Tissue distribution of mRNA for heparin-binding epidermal growth factor. Biochem J. 1992;287:681–684. doi: 10.1042/bj2870681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshizumi M, Kourembanas S, Temizer DH, Cambria RP, Quertermous T, Lee ME. Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. J Biol Chem. 1992;267:9467–9469. [PubMed] [Google Scholar]
- 8.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–6212. [PubMed] [Google Scholar]
- 10.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 11.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-γ/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aviezer D, Yayon A. Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc Natl Acad Sci USA. 1994;91:12173–12177. doi: 10.1073/pnas.91.25.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Funk C, Glasser SR, Mulholland J. Progesterone regulation of heparin-binding epidermal growth factor-like growth factor gene expression during sensitization and decidualization in the rat uterus: effects of the anti-progestin, ZK 98.299. Endocrinology. 1994;135:1256–1263. doi: 10.1210/endo.135.3.8070371. [DOI] [PubMed] [Google Scholar]
- 16.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 17.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 18.Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 19.Leach RE, Khalifa R, Armant DR, Brudney A, Das SK, Dey SK, Fazleabas AT. Heparin-binding EGF-like growth factor modulation by antiprogestin and CG in the baboon (Papio anubis) J Clin Endocrinol Metab. 2001;86:4520–4528. doi: 10.1210/jcem.86.9.7835. [DOI] [PubMed] [Google Scholar]
- 20.Birdsall MA, Hopkisson JF, Grant KE, Barlow DH, Mardon HJ. Expression of heparin-binding epidermal growth factor messenger RNA in the human endometrium. Mol Hum Reprod. 1996;2:31–34. doi: 10.1093/molehr/2.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- 22.Niikura H, Sasano H, Kaga K, Sato S, Yajima A. Expression of epidermal growth factor family proteins and epidermal growth factor receptor in human endometrium. Hum Pathol. 1996;27:282–289. doi: 10.1016/s0046-8177(96)90070-2. [DOI] [PubMed] [Google Scholar]
- 23.Moller B, Rasmussen C, Lindbloom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reproduction. 2001;7:65–72. doi: 10.1093/molehr/7.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan R, Benton E, McCormick F, Thomas H, Gullick W. Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1α, neuregulin-1β, and betacellulin, in normal endometrium and endometrial cancer. Clin Cancer Res. 1999;5:2877–2883. [PubMed] [Google Scholar]
- 25.Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS, Chen HL, Hu XL, Tabibzadeh S. Tumor necrosis factor-α messenger ribonucleic acid and protein in human endometrium. Biol Reprod. 1992;47:141–147. doi: 10.1095/biolreprod47.1.141. [DOI] [PubMed] [Google Scholar]
- 27.Philippeaux MM, Piguet PF. Expression of tumor necrosis factor-α and its mRNA in the endometrial mucose during the menstrual cycle. Am J Pathol. 1993;143:480–486. [PMC free article] [PubMed] [Google Scholar]
- 28.Tabibzadeh S, Zupi E, Babakina A, Liu R, Marconi D, Romanini C. Site and menstrual cycle-dependent expression of proteins of the tumor necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNFα in human endometrium. Hum Reprod. 1995;10:277–286. doi: 10.1093/oxfordjournals.humrep.a135928. [DOI] [PubMed] [Google Scholar]
- 29.Tabibzadeh S, Kong QF, Satyaswaroop PG, Zupi E, Marconi D, Romanini C, Kapur S. Distinct regional and menstrual cycle dependent distribution of apoptosis in human endometrium. Potential regulatory role of T cells and TNFα. Endocr J. 1994;2:87–95. [Google Scholar]
- 30.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 31.Biddolph S, Jones M. Low temperature, heat-mediated antigen retrival (LTHMAR) on archival endometrial sections. Appl Immunohistochem Mol Morphol. 1999;7:289–293. [Google Scholar]
- 32.Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial stromal and glandular cells using immuno-magnetic microspheres. Hum Reprod. 1992;7:156–161. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 33.Altroff H, van der Walle C, Asselin J, Fairless R, Campbell ID, Mardon HJ. The eighth FIII domain of human fibronectin promotes integrin α5β1 binding via stabilization of the ninth FIII domain. J Biol Chem. 2001;276:38885–38892. doi: 10.1074/jbc.M105868200. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998;185:236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Simmons DC 1993 Cloning cell surface molecules by transient expression in mammalian cells. In: Hartley DA, ed. Cellular interaction in development: a practical approach. Oxford: IRL Press; 93–127
- 36.Takemura T, Kondo S, Homma T, Sakai M, Harris RC. The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem. 1997;272:31036–31042. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- 37.Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- 38.Cross JC, Werb Z, Fisher S. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 39.Kane MT, Morgan PM, Coonan C. Peptide growth factors and preimplantation development. Hum Reprod Update. 1997;3:137–157. doi: 10.1093/humupd/3.2.137. [DOI] [PubMed] [Google Scholar]
- 40.Hammond MG, Oh ST, Anners J, Surrey ES, Halme J. The effect of growth factors on the proliferation of human endometrial stromal cells in culture. Am J Obstet Gynecol. 1993;168:1131–1138. doi: 10.1016/0002-9378(93)90356-n. [DOI] [PubMed] [Google Scholar]
- 41.Haining RE, Cameron IT, van Papendorp C, Davenport AP, Prentice A, Thomas EJ, Smith SK. Epidermal growth factor in human endometrium: proliferative effects in culture and immunocytochemical localization in normal and endometriotic tissues. Hum Reprod. 1991;6:1200–1205. doi: 10.1093/oxfordjournals.humrep.a137512. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Funk C, Roy D, Glasser S, Mulholland J. Heparin-binding epidermal growth factor-like growth factor is differentially regulated by progesterone and estradiol in rat uterine epithelial and stromal cells. Endocrinology. 1994;134:1089–1094. doi: 10.1210/endo.134.3.8119147. [DOI] [PubMed] [Google Scholar]
- 43.Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell-cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berchuck A, Soisson AP, Olt GJ, Soper JT, Clarke-Pearson DL, Bast RCJ, McCarty KSJ. Epidermal growth factor receptor expression in normal and malignant endometrium. Am J Obstet Gynecol. 1989;161:1247–1252. doi: 10.1016/0002-9378(89)90676-5. [DOI] [PubMed] [Google Scholar]
- 46.Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. A specific inhibitor of epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 47.Schroeder JA, Lee DC. Dynamic expression and activation of ErbB receptors in the developing mouse mammary gland. Cell Growth Differ. 1998;9:451–464. [PubMed] [Google Scholar]
- 48.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–H2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 49.Ni C-Y, Murphy MP, Glode TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 50.Tabibzadeh S. Signals and molecular pathways involved in apoptosis, with special emphasis on human endometrium. Hum Reprod Update. 1995;1:303–323. doi: 10.1093/humupd/1.4.303. [DOI] [PubMed] [Google Scholar]
- 51.Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Network. 1996;7:93–124. [PubMed] [Google Scholar]
- 53.Perez M, Donato NJ. Activation of epidermal growth factor receptor tyrosine phosphorylation by tumor necrosis factor correlates with loss of cytotoxic activity. J Interferon Cytokine Res. 1996;16:307–314. doi: 10.1089/jir.1996.16.307. [DOI] [PubMed] [Google Scholar]
- 54.Donato NJ, Ince C, Rosenblum M, Gallick G. Early events in the anti-proliferative action of tumor necrosis factor are similar to the early events in epidermal growth factor stimulation. J Cell Biochem. 1989;41:139–157. doi: 10.1002/jcb.240410305. [DOI] [PubMed] [Google Scholar]
- 55.Bird TA, Saklatvala J. Down-modulation of epidermal growth factor receptor affinity in fibroblasts treated with interleukin 1 or tumor necrosis factor is associated with phosphorylation at a site other than threonine 654. J Biol Chem. 1990;265:235–240. [PubMed] [Google Scholar]
- 56.Imai T, Kurachi H, Adachi K, Adachi H, Yoshimoto Y, Homma H, Tadokoro C, Takeda S, Yamaguchi M, Sakata M, Sakoyama Y, Miyake A. Changes in epidermal growth factor receptor and the levels of its ligands during menstrual cycle in human endometrium. Biol Reprod. 1995;52:928–938. doi: 10.1095/biolreprod52.4.928. [DOI] [PubMed] [Google Scholar]
- 57.Adachi K, Belser P, Bender H, Li D, Rodeck U, Benveniste EN, Woo D, Schmiegel WH, Herlyn D. Enhancement of epidermal growth factor receptor expression on glioma cells by recombinant tumor necrosis factor α. Cancer Immunol Immunother. 1992;34:370–376. doi: 10.1007/BF01741746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalthoff H, Roeder C, Gieseking J, Humburg I, Schmiegel W. Inverse regulation of human ERBB2 and epidermal growth factor receptors by tumor necrosis factor α. Proc Natl Acad Sci USA. 1993;90:8972–8976. doi: 10.1073/pnas.90.19.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]


