Abstract
In previous functional neuroimaging studies, left anterior temporal and temporal–parietal areas responded more strongly to sentences than to randomly ordered lists of words. The smaller response for word lists could be explained by either (1) less activation of syntactic processes due to the absence of syntactic structure in the random word lists or (2) less activation of semantic processes resulting from failure to combine the content words into a global meaning. To test these two explanations, we conducted a functional magnetic resonance imaging study in which word order and combinatorial word meaning were independently manipulated during auditory comprehension. Subjects heard six different stimuli: normal sentences, semantically incongruent sentences in which content words were randomly replaced with other content words, pseudoword sentences, and versions of these three sentence types in which word order was randomized to remove syntactic structure. Effects of syntactic structure (greater activation to sentences than to word lists) were observed in the left anterior superior temporal sulcus and left angular gyrus. Semantic effects (greater activation to semantically congruent stimuli than either incongruent or pseudoword stimuli) were seen in widespread, bilateral temporal lobe areas and the angular gyrus. Of the two regions that responded to syntactic structure, the angular gyrus showed a greater response to semantic structure, suggesting that reduced activation for word lists in this area is related to a disruption in semantic processing. The anterior temporal lobe, on the other hand, was relatively insensitive to manipulations of semantic structure, suggesting that syntactic information plays a greater role in driving activation in this area.
INTRODUCTION
Sentence comprehension requires the analysis of structural relationships between words. A distinction can be made between syntactic structure, which is determined by the grammatical identity and the order of words, and semantic structure, which is based on relationships between individual word meanings. To fully understand a sentence, a listener must make use of both sources of information. Evidence from studies investigating the neural basis of sentence processing suggests that these two components may be processed by partly distinct brain systems. The traditional view from neuropsychology is that parts of the frontal lobe, such as the left inferior frontal gyrus (IFG), take part in syntactic analysis (Berndt & Caramazza, 1981; Caramazza & Zurif, 1976), whereas the left posterior superior temporal lobe plays a dominant role in semantic analysis (Hickok & Poeppel, 2000; Geschwind, 1970). More recent data suggest that the actual picture may be more complex, as lesions outside of the left IFG have been found to produce syntactic processing deficits (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Grodzinsky, 2000), and many cortical regions throughout the temporal lobe have been associated with semantic processing (Binder & Price, 2001; Martin, 2001; Grabowski & Damasio, 2000).
A first step in syntactic parsing involves analyzing relationships between individual words based on information such as lexical category and word order. In this process, called phrase-level or constituent parsing, basic grammatical elements of the sentence—noun and verb phrases and their components, auxiliaries, modals, prepositions, and so forth—are first represented. Later stages of syntactic analysis are thought to involve processes related to linking together displaced syntactic elements (Grodzinsky, 2000), repairing and reanalyzing misparsed fragments (Kaan & Swaab, 2003), and representing syntactic information in working memory (Caplan & Waters, 1999). Semantic processing, on the other hand, involves determining an overall semantic representation of the sentence by fitting the meanings of individual words into the framework of the sentence (i.e., Who did what to whom, and what are the implications of such?). Event-related potential (ERP) experiments provide some evidence for the existence of separate neural systems for syntactic and semantic analysis. For example, several ERP responses have been found in association with different kinds of sentence manipulations, including components such as the left anterior negativity (ELAN, LAN) thought to be involved in early parsing (Friederici, Hahne, & Mecklinger, 1996; Münte, Heinze, & Mangun, 1993), a component (P600) considered to be involved in syntactic reanalysis (Hagoort & Brown, 2000), and a component (N400) sensitive to semantic expectancy (Kutas & Hillyard, 1980).
Neuroimaging experiments concerning the brain areas involved in sentence processing have mainly focused on later stages of syntactic analysis. For example, experimenters have manipulated the relative syntactic complexity of sentences. Compared to simple sentences, syntactically complex sentences are thought to make greater demands on resources such as working memory and transformational analysis of misplaced syntactic fragments (Wartenburger et al., 2004). Given that the number of words was controlled in these studies, however, the relative amount of low-level constituent parsing should be fairly equivalent across conditions. Thus, these experiments, which have mainly found activation differences in the left IFG (Keller, Carpenter, & Just, 2001; Caplan, Alpert, & Waters, 1998; Inui et al., 1998; Just, Carpenter, Keller, Eddy, & Thulborn, 1996) and left posterior temporal and inferior parietal areas (Keller et al., 2001; Just et al., 1996), may not reflect activation of the entire syntactic processing system. Other studies have attempted to isolate components of sentence processing by using violations in syntactic (Friederici, Rüschemeyer, Hahne, & Fiebach, 2003; Meyer, Friederici, & von Cramon, 2000; Ni et al., 2000) or semantic (Friederici et al., 2003; Newman, Just, Keller, Roth, & Carpenter, 2003; Luke, Liu, Wai, Wan, & Tan, 2002; Kuperberg et al., 2000; Ni et al., 2000; Kang, Constable, Gore, & Avrutin, 1999) structure. However, violations may induce additional cognitive processes, such as error detection and syntactic reparsing, which, given the poor temporal sensitivity of fMRI, cannot be distinguished from initial syntactic parsing processes.
An alternative approach to investigating sentence processing, one intended to highlight early stages of parsing, is to compare listening to or reading sentences with a linguistic control lacking coherent sentence structure. Such control stimuli are typically created by selecting random lists of words, resulting in stimuli that are equal in length and lexical content to the sentences but lack syntactic structure. This approach differs from the previously mentioned studies in that it is designed to activate all postlexical components of sentence processing instead of focusing on later components. When such a contrast is performed, greater activation is seen for sentences compared to word lists in the lateral aspect of the anterior temporal lobe (ATL) (Humphries, Swinney, Love, & Hickok, 2005; Vandenberghe, Nobre, & Price, 2002; Friederici, Meyer, & von Cramon, 2000; Stowe et al., 1999; Bottini et al., 1994; Mazoyer, Tzourio, Frak, & Syrota, 1993) and in the angular gyrus (AG) (Humphries et al., 2005; Vandenberghe et al., 2002; Bottini et al., 1994). Differences in the left IFG have generally not been reported. One difficulty in interpreting these results is that sentences are likely to engage systems related to both syntax and semantics to a greater extent than random word lists. That is, the individual content words of a meaningful sentence can be combined to produce a larger, global semantic interpretation describing plausible events with real-world implications, whereas this is often not possible when content words are selected at random. Therefore, it is difficult to determine if the activations seen in this contrast represent syntactic processing, semantic processing, or both.
Vandenberghe et al. (2002) addressed this issue by including, in addition to sentences and word lists, conditions designed to manipulate combinatorial semantic processing. In their study, the content words of both sentences and word lists were either semantically related or selected at random. This created sentence and word list conditions in which the degree of overall meaning was systematically varied. The results showed the expected ATL and AG activation for sentences over lists. A subset of the activated ATL voxels also showed a main effect of the semantic manipulation, with greater activation for the “semantically random” conditions. The authors concluded that this region of the ATL is involved in integrating semantic information during sentence processing. Greater activation in the semantically random conditions was thought to result from a greater effort exerted in attempting to integrate such stimuli into a semantic whole. A surprising result was the lack of posterior temporal lobe activation as seen in other studies of sentence-level semantic processing (Friederici et al., 2003; Newman et al., 2003; Kuperberg et al., 2000; Ni et al., 2000). One potential problem with the design of this experiment is that the subjects performed a repetition monitoring task that did not necessarily require them to integrate semantic information.
In the current experiment, we examine these issues further using a design based partially on the experiment by Vandenberghe et al. (2002). This includes the four conditions used in that study: normal sentences, sentences in which the content words have been replaced by random content words, and “scrambled” versions of these two stimuli in which the word order is randomized (Table 1). Here we refer to the normal sentences and their randomly reordered versions as semantically “congruent” because the content words can be thematically linked to produce more complex meanings. Our design differs from that of Vandenberghe et al., however, in several respects. Whereas Vandenberghe et al. used PET imaging with stimuli presented in blocks, we chose an event-related fMRI design. One important advantage of an unblocked design is that subjects are unable to anticipate the type of stimulus that will appear next and so are less likely to systematically change their level of attention and effort across conditions. In addition, we included two conditions in which content words were replaced by pseudowords. The pseudoword conditions are designed to disrupt not only the sentence-level semantic information in the stimuli but also access to word-level semantics, thereby providing a more pure manipulation of syntactic structure. One problem in interpreting the results from the Vandenberghe et al. study was that the task used during scanning did not specifically require sentence comprehension. In contrast, we had participants perform a behavioral task during scanning that was designed to engage both semantic and syntactic processing. In this task, which has been used in other studies of sentence comprehension (Kuperberg et al., 2000; Ni et al., 2000), subjects rated the “meaningfulness” of each of the stimuli on a 4-point scale. We expected activation in known semantic and syntactic networks involving the frontal, temporal, and parietal lobes; however, our hypotheses mainly concern differences in temporal and parietal lobe regions. We expected ATL and AG activation in the syntactic contrast (sentences > lists), as seen in other studies. With the addition of a sentence comprehension task, we also expected the semantic manipulation to produce activation in temporal lobe regions known to be involved in semantic processing. We predicted that semantically congruent stimuli would activate lateral and inferior temporal lobe areas relative to both the semantically random and pseudoword stimuli. On the basis of previous studies of single word lexical access (Price et al., 1996; Small et al., 1996; Howard et al., 1992), we also expected the semantically random word stimuli to activate portions of the middle temporal gyrus relative to the pseudoword stimuli.
Table 1.
Example Stimuli
| Semantically congruent sentence | the man on a vacation lost a bag and a wallet |
| Semantically congruent word list | on vacation lost then a and bag wallet man then a |
| Semantically random sentence | the freeway on a pie watched a house and a window |
| Semantically random word list | a ball the a the spilled librarian in sign through fire |
| Pseudoword sentence | the solims on a sonting grilloted a yome and a sovir |
| Pseudoword word list | rooned the sif into lilf the and the foig aurene to |
METHODS
Subjects
Twenty-one right-handed, native English-speaking subjects (7 men, 14 women; ages 23–48 years) participated. Subjects gave informed consent under a protocol approved by the Institutional Review Board of the Medical College of Wisconsin.
Materials
The experiment used a factorial design with two independent variables. The first variable, syntactic structure, had two levels: sentences and word lists. The second variable, semantic structure (i.e., meaning), had three levels: congruent, random, and pseudoword. This gave a total of six conditions (see Table 1 for examples). The conditions were created in the following ways. For the semantically congruent sentences, 40 novel sentences were created using several constraints. All sentences described concrete events and were in active voice. Sentences were composed of either one independent clause, two independent clauses, or an independent and a dependent clause. Each sentence contained five content words, that is, nouns, verbs, and adjectives. The verbs in the sentences were all in the past tense and included transitive, bitransitive, and intransitive verbs. The nouns were all singular and concrete. The number of words (content and function) for each sentence varied between 9 and 13 (mean = 10.8).
The remaining five conditions were generated from the semantically congruent sentences using a computer program (see Table 1 for examples). The semantically random sentences were created by replacing all of the content words in each original sentence with randomly selected content words. The new content words were chosen by randomly sampling from the entire list of content words used in all of the original sentences, with the constraint that content words of a particular grammatical category replaced words of the same category. For example, nouns were replaced by nouns, adjectives by adjectives, intransitive verbs by intransitive verbs, and so on. Thus, the new sentences were grammatically correct, but the overall meaning of the sentence was disrupted. In addition, because the random sentences were the same length and included the same set of content words as the semantically congruent sentences, they were well controlled for nuisance factors such as stimulus length, word frequency, and word imageability.
The pseudoword sentences (i.e., “jabberwocky sentences”) were created by replacing the content words in the original sentence with randomly generated pseudowords. The pseudowords were generated using a Markov chaining program based on bigram frequencies from the CELEX lexical database (Baayen, Piepenbrock, & Gulikers, 1995). For each pseudoword that replaced a verb, the morphological affix “-ed” was added to the end of the word to signify its grammatical role as a past tense verb. Pseudowords were matched to the content words used in the other conditions in terms of syllable and phoneme length.
Finally, three sets of word lists (congruent, random, and pseudoword) were generated from each of the three sentence conditions in the following way. First, all of the function words were replaced by randomly chosen functions words using the same random sampling procedure that was used for the content words. Second, the order of resulting words was randomized.
All words and pseudowords used in the study were natural speech utterances spoken by a single male speaker and digitized to a computer. During recording, the words were read individually from a list in random order and without sentence prosody. Each individual word was normalized by the total energy in the recording. The stimuli used in the experiment were then created by piecing together the individual words or pseudowords to form the sentences and lists. The spacing between individual items was varied so that all stimuli were 6.1 sec in length. This process was used to generate stimuli without any intelligible sentence-level prosody (Humphries et al., 2005).
Procedure
The stimuli were generated from computer files and presented using the E-Prime software package (Psychology Software Tools, Pittsburgh, PA). Sounds were presented using wave-guide headphones (Avotec Corp., Stuart, FL). The headphones consisted of ear inserts surrounded by foam padding designed to reduce the sound level of the scanner noise. The stimuli were played at a volume loud enough that subjects could easily perceive individual words in each stimulus. A four-key response box was used to record subjects' responses.
Examples of each of the six types of stimuli were presented to subjects before scanning. During scanning, subjects performed a rating task in which they judged the “meaningfulness” of each stimulus on a scale from 4 (most meaningful) to 1 (least meaningful). Subjects were instructed to make their response after the termination of each stimulus.
The experiment used an event-related design. There were eight separate imaging runs. Each run contained 30 trials. Each trial consisted of 6.1 sec of stimulus followed by either 3.9, 5.9, 7.9, or 9.9 sec of rest. The length of the rest period was varied to increase statistical power (Birn, Cox, & Bandettini, 2002). The length of each run was 396 sec. There were 40 trials for each of the six conditions over the entire experiment. The conditions were pseudorandomly ordered such that the probability of any condition being followed by another particular condition was equal over all conditions.
Imaging was performed on a GE scanner operating at 1.5 T. Each subject underwent a series of functional runs followed by a high-resolution anatomical scan. For the functional images, 18 axial slices were collected continuously every 2 sec. The images were collected using an echo-planar imaging pulse sequence (FOV = 240 mm, matrix = 64 × 64, voxel size = 3.75 × 3.75 mm, thickness = 4 mm, TE = 40 msec, flip angle = 90°). Care was taken, for each subject, to choose slice positions that covered the entire temporal lobe, inferior parietal lobe, and inferior frontal lobe. The anatomical scan was collected using a spoiled gradient recalled echo sequence (FOV = 240 mm, matrix = 256 × 256, voxel size = 0.9375 × 0.9375 mm, thickness = 1.2 mm, flip angle = 40° in the sagitta plane.
To correct for head motion artifact, the functional images of each subject were aligned to the first volume in the series using a 3-D rigid body, six parameter model in the AIR 5.0 program (Woods, Grafton, Holmes, Cherry, & Mazziotta, 1998). The functional volumes were then coregistered to the corresponding anatomical image. Finally, the images were normalized to the MNI atlas using a fifth-order polynomial model in the AIR 5.0 program.
The subsequent analysis was performed using Matlab (Mathworks Inc., Natick, MA). A gaussian spatial filter (FWHM = 6 mm) was applied to each image. A mask was applied so that voxels outside the brain were excluded from the analysis. The data were then temporally filtered to remove low-frequency noise using a high-pass filter (13-order Butterworth filter; 0.01 Hz). A regression analysis was applied to each voxel time course in each individual subject. Three regressors were used for each condition to model the signal at time lags 5, 6, and 7 (10, 12, and 14 sec after stimulus onset). These lags were chosen because they fell within the range of peak activities of brain regions outside of the primary auditory cortex for all six conditions. After the regression coefficients were calculated, voxelwise contrasts were performed to quantify differences between the conditions for each subject. A random effects analysis was then used to test the significance of the contrast maps across subjects (Friston, Holmes, & Worsley, 1999). Finally, the resulting statistical maps were thresholded using a cluster-based thresholding procedure. First, the statistical images were thresholded at an uncorrected p value of .01, contiguous clusters were identified, and the clusters were then thresholded based on spatial extent (Worsley et al., 2002). The resulting clusters are significant with a corrected p value of less than .05. Cluster centers, based on center of mass, and individual local maxima are reported in coordinates based on the MNI brain. Local peak maxima of clusters were found using a criterion that excluded peaks that were closer than 24 mm. Bar graphs of activation level above baseline for individual clusters were derived by calculating the mean regression coefficient for each condition across voxels in the cluster and across subjects.
RESULTS
Behavioral
Behavioral responses from 19 subjects are shown in Figure 1. Behavioral data from two other subjects were not collected due to equipment malfunction. Ratings differed significantly across conditions, F(5,108) = 193.29, p < .0001. Of the six conditions, the congruent sentences were rated the most meaningful, and the pseudoword lists were rated the least meaningful. We were interested in whether subjects perceived differences in meaningfulness between each of the six conditions, so paired t tests were conducted for all condition pairs. All conditions were significantly different except for the semantically congruent word lists and the semantically random sentences [congruent sentences vs. congruent lists: t(18) = 15.0, p < .0001; congruent sentences vs. random sentences: t(18) = 19.5, p < .0001; congruent sentences vs. random lists: t(18) = 20.4, p < .0001; congruent sentences vs. pseudoword sentences: t(18) = 38.0, p < .0001; congruent sentences vs. pseudoword lists: t(18) = 76.5, p < .0001; congruent lists vs. random sentences: t(18) = .287, p = .78; congruent lists vs. random lists: t(18) = 9.42, p < .0001; congruent lists vs. pseudoword sentences: t(18) = 8.25, p < .0001; congruent lists vs. pseudoword lists: t(18) = 14.0, p < .0001; random sentences vs. random lists: t(18) = 6.34, p < .0001; random sentences vs. pseudoword sentences:t(18) = 14.7, p < .0001; random sentences vs. pseudoword lists: t(18) = 23.6, p < .0001; random lists vs. pseudoword sentences: t(18) = 4.53, p < .001; random lists vs. pseudoword lists: t(18) = 9.33, p < .0001; pseudoword sentences vs. pseudoword lists: t(18) = 5.14, p < .0001].
Figure 1.
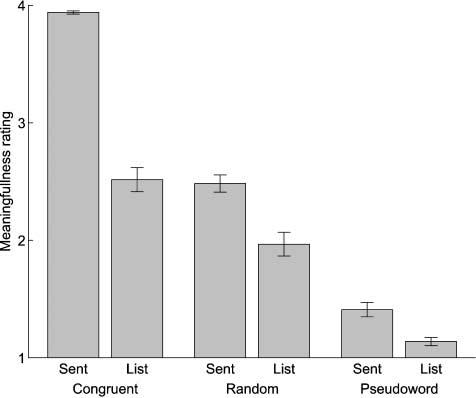
Behavioral results for stimulus meaningfulness judgments. Numbers are the mean response across subjects per condition and range from 1 (least meaningful) to 4 (most meaningful). Error bars in this and subsequent figures represent standard error of the mean across subjects.
Imaging
When contrasted with rest, each of the six conditions showed activation in expected auditory, language, and executive processing areas in the temporal and frontal lobes, including bilateral anterior, middle and posterior superior temporal gyrus (STG), bilateral superior temporal sulcus (STS), and bilateral IFG (Figure 2).
Figure 2.
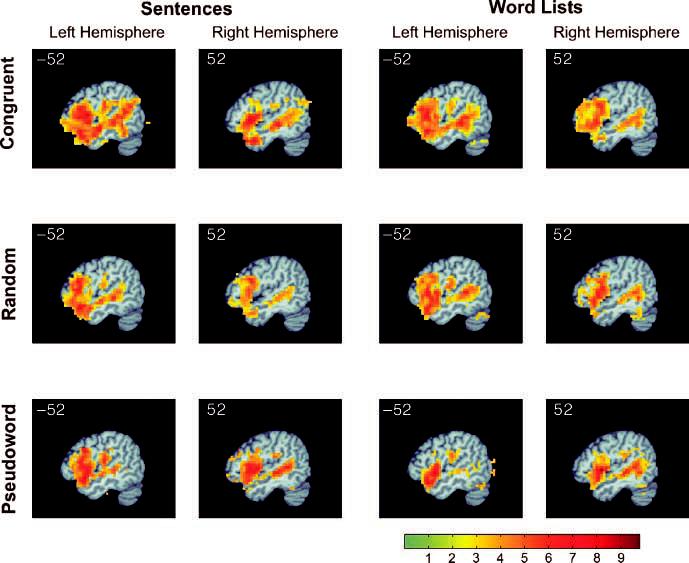
Functional activation map for each condition against resting baseline. The sagittal slice position in this and subsequent figures is shown in the upper left corner. The right hemisphere sagittal slice is at position 52 (MNI coordinates). The color scale represents t statistics from a random-effects analysis.
In the following analyses, our aim was to test specific hypotheses about temporal and parietal lobe processing, therefore, we confined our analyses to voxels located in the temporal and parietal lobes. To examine the differences between activation for sentences and word lists, a contrast of the main effect of syntactic structure was calculated, collapsed across all three semantic levels (congruent, random, and pseudoword). A significant cluster of activation (center = [−49, 2, −25]) was seen for sentences over word lists in the left anterior STS (Figure 3). Coordinates of peak activation for this and the remaining contrasts are presented in Table 2.
Figure 3.
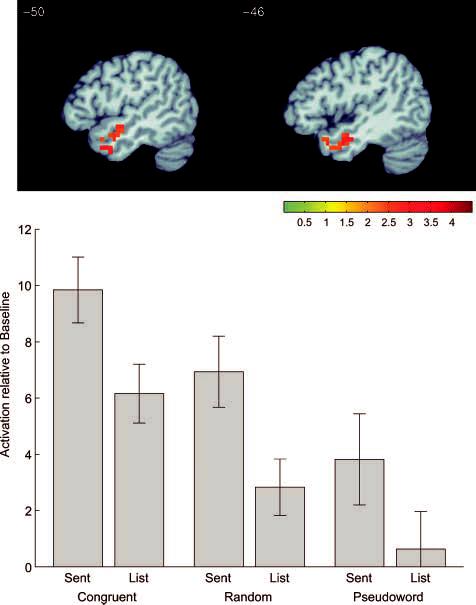
Functional activation map of the main effect of syntactic structure. Displayed voxels had greater activation levels for sentences over word lists collapsed across semantic conditions (congruent, random, pseudoword). The graph shows mean BOLD signal (in arbitrary units relative to the resting interstimulus interval) for each of the six conditions, averaged over the activated voxels and the subjects.
Table 2.
Coordinates of Peak Activation
| Contrast | x | y | z | Hemisphere | Region |
|---|---|---|---|---|---|
| Sentences > word lists (congruent, random, pseudoword) | −54 | 6 | −36 | L | Middle temporal gyrus |
| Congruent > random | −58 | −46 | 4 | L | Posterior middle temporal gyrus |
| −58 | −19 | −12 | L | Middle temporal gyrus | |
| −54 | −53 | 32 | L | Supramarginal gyrus/angular gyrus | |
| −47 | −1 | −32 | L | Inferior temporal gyrus | |
| 65 | −57 | 8 | R | Posterior middle temporal gyrus | |
| 51 | −38 | −4 | R | Posterior superior temporal sulcus | |
| 54 | −61 | 32 | R | Angular gyrus | |
| 51 | −16 | 28 | R | Inferior temporal gyrus | |
| Congruent > pseudoword | −51 | −50 | 12 | L | Posterior superior temporal gyrus |
| −47 | −27 | −12 | L | Middle temporal gyrus | |
| −58 | −54 | −12 | L | Inferior temporal gyrus | |
| 43 | −80 | 32 | R | Angular gyrus | |
| 43 | −50 | 8 | R | Posterior superior temporal sulcus | |
| 51 | 6 | −32 | R | Anterior middle temporal gyrus | |
| Random > pseudoword | −54 | −39 | −4 | L | Middle temporal gyrus |
| −51 | 21 | −24 | L | Anterior superior temporal gyrus | |
| Interaction (congruent [sentences > word lists] > random [sentences > word lists]) | −58 | −31 | 12 | L | Posterior superior temporal gyrus |
| −47 | 6 | −12 | L | Anterior superior temporal gyrus | |
| −39 | −76 | −12 | L | Fusiform/middle occipital gyri | |
| 51 | −20 | 8 | R | Posterior superior temporal/angular gyri | |
| 43 | 10 | −16 | R | Anterior superior temporal gyrus | |
| 51 | −9 | −28 | R | Middle temporal gyrus | |
| 9 | −95 | 16 | R | Middle occipital gyrus | |
| Interaction (congruent [sentences > word lists] > [pseudoword (sentences > word lists]) | −54 | −57 | 16 | L | Posterior superior temporal/angular gyri |
| −66 | −24 | 8 | L | Middle superior temporal gyrus | |
| −54 | 10 | −8 | L | Anterior superior temporal gyrus | |
| 54 | −9 | −28 | R | Middle temporal gyrus | |
| 66 | −31 | −8 | R | Middle temporal gyrus | |
| Sentences > Word lists (Congruent, Random) | −54 | −57 | 24 | L | Angular gyrus |
| −47 | 14 | −28 | L | Anterior superior temporal sulcus | |
| −47 | −12 | −36 | L | Inferior temporal gyrus | |
| 51 | 6 | −32 | R | Anterior middle temporal gyrus |
L = left; R = right.
The next set of contrasts tested for differences between the semantic levels, collapsed across syntactic structure. The main contrast of interest was between semantically congruent and semantically random word stimuli, as this comparison should show areas that respond more to stimuli with coherent semantic structure while controlling for word-level semantic input. The resulting map included a large expanse of activation in the bilateral anterior, middle, and posterior STS and middle temporal gyrus (MTG) as well as bilateral ITG and bilateral (left > right) AG (Figure 4A). Additional contrasts were calculated between the congruent and pseudoword conditions and between the random and pseudoword conditions. In these contrasts, the congruent and random stimuli, which both contain meaningful content words, are compared against the pseudoword stimuli, which lack meaningful content words. Thus, these comparisons should identify additional areas involved in processing meaning at the level of individual words. The contrast between congruent and pseudoword conditions showed a pattern of temporal-parietal activation very similar to the contrast between congruent and random stimuli (Figure 4B), including the anterior, middle, and posterior STS and MTG, left ITG, and bilateral AG. The contrast between random and pseudoword stimuli (Figure 4C), on the other hand, showed a spatially more confined region of activation in the temporal lobe, involving primarily the left anterior, middle, and posterior STS.
Figure 4.
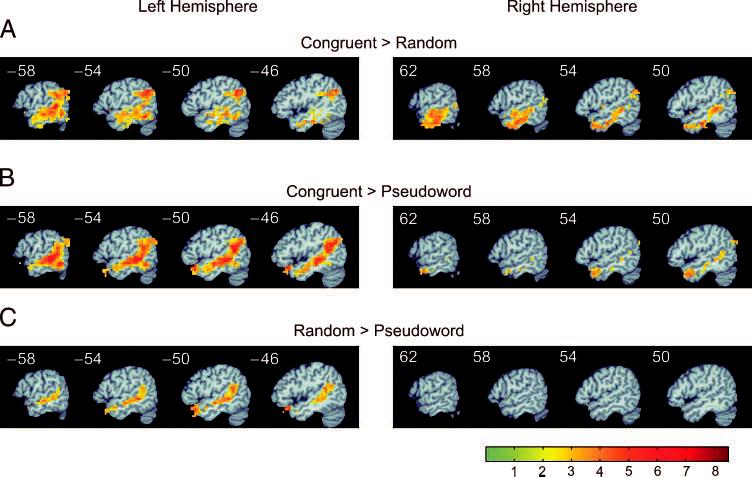
Temporal and parietal lobe functional activation maps of the main effects of semantic structure. (A) Left and right hemisphere activation for congruent over random (sentences and lists). (B) Activation for congruent over pseudoword (sentences and lists). (C) Activation for random over pseudoword (sentences and lists).
In order to examine possible dependencies between the syntax and semantics conditions, interactions were calculated (Figure 5). Most of the temporal lobe regions that had shown effects of the semantic factor (MTG, ITG) did not show significant interactions with the syntactic manipulation. Interactions between syntax and the congruent and random semantic levels were observed in the left and right STG, and in small regions of the right MTG (Figure 5A). Interactions between syntax and the congruent and pseudoword semantic levels were observed in the STG bilaterally, right MTG, and left AG (Figure 5B). No significant interactions were found between syntax and semantics with the random and pseudoword stimuli. Given this evidence for significant interactions between the syntactic and semantic factors, a number of more specific contrasts were carried out to examine effects of syntactic and semantic structure for the word conditions alone, as well as effects of semantic structure separately for sentences and word lists.
Figure 5.
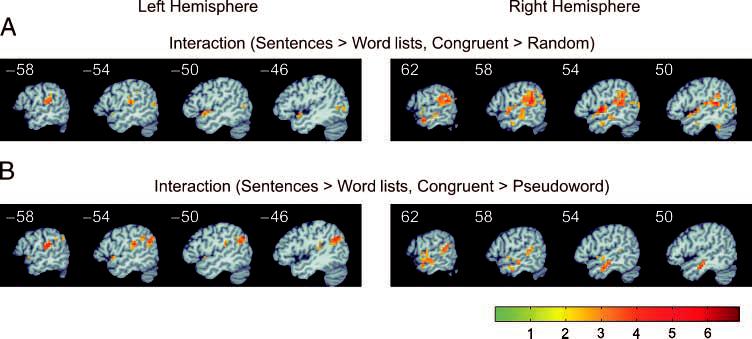
Temporal and parietal lobe functional activation map of the interaction between syntactic structure and semantics. (A) Left and right hemisphere activation for the interaction between sentences over lists and congruent over random. (B) Left and right hemisphere activation for the interaction between sentences over lists and congruent over pseudoword.
The first analysis focused on activation for the congruent and random stimuli not including the pseudoword conditions (Figure 6). This allowed a more direct comparison of the current results to those of the Vandenberghe et al. (2002) study, which used only congruent and random word conditions. Although this analysis was based only on the congruent and random word conditions, activation levels depicted for individual clusters also give results for the two pseudoword conditions for comparison. A syntactic contrast was performed between sentences and word lists that included only the semantically congruent and random word conditions. As in the full main effect of syntactic structure, a cluster was found in the anterior STS (center = [−46, −3, −25]). Additional clusters were seen in the left AG (center = [−52, −56, 27]) and right anterior MTG (center = [52, 1, −32]). When this activation map is plotted against the semantic contrast of congruent and random, overlap can be seen in the left AG and in a region of the left anterior STS (Figure 6A). In the AG, the cluster identified in the syntactic contrast was completely overlapped by the semantic activation. A plot of the mean activation for all six conditions for this AG region of overlap shows that the activation in this area is dominated by the semantically congruent sentences, which show much higher activity than the congruent word lists or random sentences (Figure 6B). An interaction between the syntactic and semantic factors is apparent in this region, in that the large effect of syntactic structure observed for the semantically congruent stimuli was not observed for the pseudoword stimuli. Overlap was also seen between the syntactic and semantic maps in the ATL; however, the majority of the syntactic activation in this region did not show overlap. Plots of the mean activation for those voxels in the syntactic cluster that overlap with the semantic activation and those that do not are shown in Figure 6D and E. The voxels in the overlap region show only weak responses to the random word stimuli and no response to the pseudowords, suggesting sensitivity to semantic structure (Figure 6D). In contrast, the nonoverlapping voxels (Figure 6E) show a strong response to both the random and pseudoword sentences, suggesting that they are less sensitive to semantic structure. Finally, a plot of the activation levels for those temporal lobe voxels that showed significant activation in the semantic contrast but not in the syntactic contrast is shown in Figure 6C. This extensive region of temporal cortex showed a strong preference for semantically congruent over random and pseudoword stimuli but no effect of syntactic structure.
Figure 6.
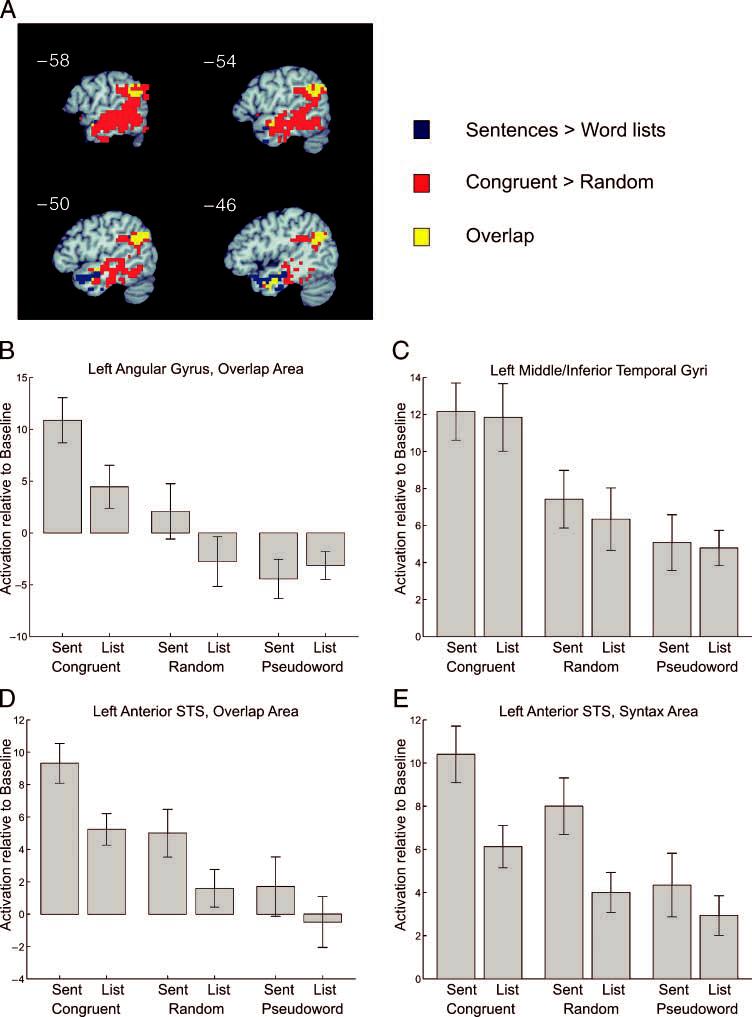
Temporal and parietal lobe effects of syntactic and semantic structure for semantically congruent and random word conditions. (A) Functional activation map of left-hemisphere areas showing an overlay of the effect of syntactic structure (sentences > word lists) in blue, the effect of semantic structure (congruent > random) in red, and overlap in yellow. (B) Plot of the mean activation levels for the voxels showing overlap between syntax and semantics (yellow) in the left angular gyrus. (C) Plot of mean activation levels for voxels showing activation for semantics (red) in the left middle temporal and inferior temporal gyri. (D) Plot of mean activation levels for voxels showing activation for both semantic and syntactic structure (yellow) in the left anterior temporal lobe. (E) Plot of mean activation levels for voxels showing activation only for syntactic structure (blue) in the left anterior temporal lobe.
A final analysis examined the effect of semantic structure (congruent > random) separately for sentences and word lists. A composite map of the two contrasts is shown in Figure 7. Voxels showing a significant difference between congruent and random word lists were located primarily in the middle part of the STS and MTG. In contrast, voxels showing a difference between congruent and random sentences were located in more inferior temporal lobe regions (ITS and ITG). A plot of the activation levels across all six conditions for those temporal lobe voxels that responded to the semantic effect only for word lists is shown in Figure 7B, whereas activation levels for the temporal lobe voxels showing a semantic effect only for sentences are shown in Figure 7C. For the voxels located in the MTG, which showed a greater semantic effect for word lists, high levels of activity were seen for all conditions, including the two pseudoword conditions, with slightly higher activation for congruent compared to random and random compared to pseudoword stimuli. In the areas of the temporal lobe that showed a greater semantic effect for sentences, activation was greater than baseline for only the two congruent stimuli. For these voxels, congruent sentences showed higher activation than congruent word lists.
Figure 7.
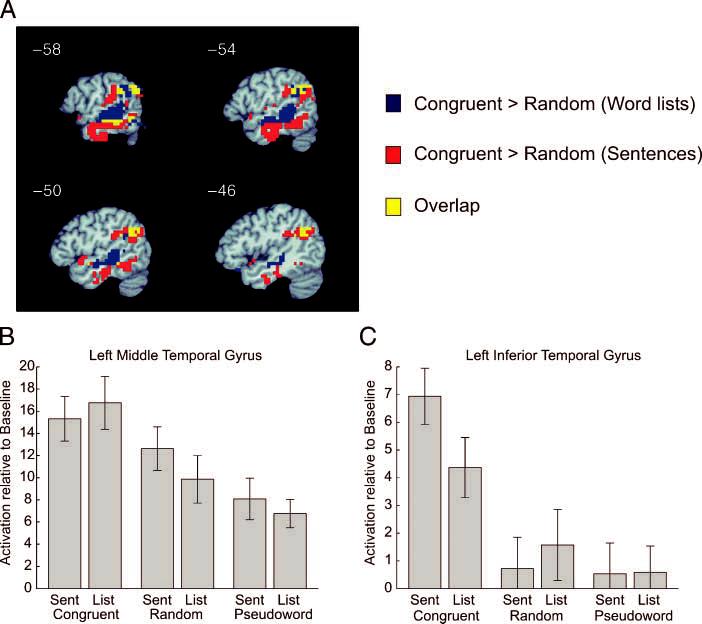
Effects of semantic structure, computed separately for sentences and word lists. (A) Functional activation map of left-hemisphere areas showing an overlay of the effect of semantics (congruent > random) for word lists in blue, the effect of semantics (congruent > random) for sentences in red, and overlap in yellow. (B) Plot of the mean activation levels for temporal lobe voxels showing a semantic effect for word lists (blue voxels) (C) Plot of mean activation levels for temporal lobe voxels showing a semantic effect for sentences (red voxels).
DISCUSSION
The goal of this experiment was to explore the neural activation patterns associated with processing semantic and syntactic structure during sentence comprehension. Our results show two distinct patterns of activity modulation depending on whether word order (i.e., syntax) or combinatorial word meaning (i.e., semantics) was manipulated. Randomly ordered stimulus lists (word lists lacking syntactic structure) produced a reduced blood oxygen level dependent (BOLD) signal in the left anterior STS compared to syntactically well-formed sentences, regardless of whether the stimuli were composed of semantically congruent words, semantically random words, or pseudowords. This region thus appears to be sensitive primarily to the presence of syntactic structure in spoken sentences. Replacing content words in a sentence with randomly chosen content words or with pseudowords resulted in reduced activity in large regions of the temporal lobe (STS/MTG, ITG) bilaterally, and in the insula, frontal lobe, and the AG, regardless of whether the stimuli were syntactically well-formed or not. These regions thus appear to be sensitive to the degree of global semantic structure present in word strings. Brain areas that responded to both of these manipulations included the left AG and a small region of the left ATL immediately adjacent to the ATL region modulated by syntactic structure. Finally, the effect of semantic structure was different for sentences and word lists. Greater activation for congruent word lists over random word lists was observed in the middle aspect of the STS and MTG, whereas greater activation for congruent sentences over random sentences was seen in more inferior temporal lobe areas (ITS and ITG). The results indicate a network of temporal and parietal areas involved in semantic and syntactic aspects of auditory sentence comprehension.
Four of the conditions (congruent and random sentences and word lists) were similar to stimuli used by Vandenberghe et al. (2002). Similar results were found in the two studies for the contrast of sentences over word lists, showing clusters in the left ATL and left AG. Divergent results occurred, however, in the contrast between congruent and random stimuli. We found stronger activation for the semantically congruent stimuli throughout large regions of the temporal lobe. Vandenberghe et al., on the other hand, found a semantic effect only in a small set of voxels in the ATL, and this effect was in the opposite direction, with greater activation for the semantically random stimuli. These differences may be related to differences in the tasks performed during scanning. A repetition monitoring task was used in the Vandenberghe et al. study, in which participants responded if they saw a stimulus repeated. This task can be performed easily using orthographic or phonological analysis and thus does not require processing of combinatorial semantic information. In the current study, subjects rated the meaningfulness of each stimulus, a task that explicitly requires semantic and syntactic processing at both the word and sentence level. The differential demands on semantic processing in the two experiments could explain the different pattern of results. In what follows, we discuss the possible functional roles of individual brain areas in the temporal and parietal lobes based on the results of our experiment and previously published data.
Anterior Temporal Lobe
Activation in the ATL can be separated into two distinct regions based on response patterns. The first region, centered in the most anterior part of the STS, responded to the syntactic manipulation (sentences > lists) across all semantic conditions. A second group of voxels, located immediately posterior to the first, responded to both the syntactic manipulation and the semantic (congruent > random) manipulation.
Various accounts of the functional role of the ATL in sentence comprehension have been proposed. In one theory, the ATL is thought to be involved in sentence-level semantic integration (Stowe, Haverkort, & Zwarts, 2005; Noppeney & Price, 2004; Vandenberghe et al., 2002). This view is supported in part by studies of semantic dementia, a syndrome characterized by deficits in processing word meaning associated with degeneration in the anterior inferior temporal lobe (Hodges, Patterson, Oxbury, & Funnell, 1992). Additional evidence comes from neuroimaging experiments showing ATL activation during semantic manipulations such as semantic priming (Rossell, Price, & Nobre, 2003; Mummery, Shallice, & Price, 1999). According to this view, sentences produce greater activation in the ATL than word lists because scrambling the order of words in a sentence disrupts the ability of the ATL to integrate the semantic information. One prior result that this theory does not explain, however, is that pseudoword sentences, which presumably lack semantic information in the form of content words, can show levels of activity in the ATL similar to normal sentences (Friederici, Meyer, & von Kramon, 2000; Mazoyer et al., 1993). An alternative account of ATL function is that this region is involved in analyzing syntactic structure (Dronkers et al., 2004; Friederici & Kotz, 2003). Because the word lists used in these studies lacked coherent syntactic cues, such as word order or morphological markings, the sentence versus word list difference is attributed to a disruption of basic syntactic structure. More specifically, because the ATL does not show a differential response to sentences with simple versus complex syntactic structure (Keller et al., 2001; Just et al., 1996), it has been proposed that this area may be involved in an early stage of syntactic parsing related to constituent and phrase structure analysis (Friederici & Kotz, 2003; Meyer et al., 2000).
Constituent analysis refers to the rapid, highly efficient parsing processes that assign words their grammatical roles and construct phrase structure representations from these grammatical elements. At a minimum, this process requires simultaneous input from lexical systems, morphological decomposition mechanisms, and phonetic and prosodic systems that analyze word and phrase boundary cues (Clifton, Carlson, & Frazier, 2002; Cutler, Dahan, & van Donselaar, 1997; MacDonald et al., 1994; Shapiro, Zurif, & Grimshaw, 1987; Chomsky, 1957). The superior ATL, which includes the anterior STS, is most likely composed of polymodal and heteromodal cortex with strong connections to other regions of the temporal and frontal lobes. Because of these connections with various sensory, motor, and executive cortices, the ATL has been considered a “convergence zone” (Damasio & Damasio, 1994), and thus may be ideally suited for carrying out highly interactive processes like constituent parsing. Our results show a region of the left ATL that responds more to sentences than to randomly ordered lists and does not show a significant difference in response to semantically congruent and semantically random stimuli. This pattern suggests that this area is not simply processing the increased amount of semantic information present in a sentence but instead responds to the presence of syntactic structure. This region also showed a greater response to pseudoword sentences than to pseudoword lists, further suggesting that semantic information in the form of content words is not required to activate this area. Adjacent voxels were found that did respond to both syntactic and semantic manipulations, and voxels in slightly more posterior parts of the STS and MTG responded only to the semantic contrast. Thus, a possible functional division of the ATL may exist between the most anterior parts of the STS, which are sensitive primarily to syntactic structure, and more posterior parts of the ATL, which play a greater role in processing semantic information.
Angular Gyrus
The left AG responded robustly to both syntactic and semantic manipulations. Previous evidence strongly suggests a role for this region in semantic processing (Binder & Price, 2001). For example, increased activation has been observed in the AG for words over nonwords during visual lexical decision tasks (Binder, Westbury, Possing, McKiernan, & Medler, 2005; Ischebeck et al., 2004; Binder et al., 2003) and for concrete words over abstract words (Binder et al., 2005; Sabsevitz, Medler, Seidenberg, & Binder, 2005). Activation occurs in the left AG for single word semantic tasks over phonologic tasks (Scott, Leff, & Wise, 2003; Roskies, Fiez, Balota, Raichle, & Petersen, 2001; Binder, Frost, Hammeke, Bellgowan, et al., 1999; Mummery, Patterson, Hodges, & Price, 1998; Price, Moore, Humphreys, & Wise, 1997; Demonet et al., 1992). The AG also appears to be involved in semantic processing at the sentence level. Increased activation in the left AG is seen during reading of sentences compared with viewing consonant letter strings (Bavelier et al., 1997), hearing or reading sentences compared with lists of nonwords (Homae, Hashimoto, Nakajima, Miyashita, & Sakai, 2002), hearing sentences compared to lists of content words only (Humphries et al., 2005), and hearing or reading sentences compared to randomly ordered word lists (Humphries et al., 2005; Vandenberghe et al., 2002; Bottini et al., 1994). The left AG shows greater activation for sentences with semantic violations than for normal sentences (Friederici et al., 2003; Newman et al., 2003; Ni et al., 2000). During reading of syntactically complex and simple sentences, activation in the AG has been found to interact with the lexical frequency of words in the sentence, with low-frequency words producing greater activation in more complex sentences (Keller et al., 2001). Neuropsychological data also support the idea that the AG is involved in semantic processing. Lesions in the temporal–parietal junction including the AG can produce comprehension deficits in the form of transcortical sensory aphasia (Dronkers et al., 2004; Rapcszak & Rubens, 1994).
Our results suggest that the left AG is particularly involved in sentence-level semantic processing. This region showed greater activation for semantically congruent stimuli than for random or pseudoword stimuli, with little difference between random and pseudoword conditions. Thus, single word semantics, which the random stimuli have in the form of content words and the pseudoword stimuli lack, did not play a large role in driving AG activation. Instead, the AG appears to be especially sensitive to stimuli that have combinatorial semantic structure, which arises when individual words fit together to form a more complex meaning (Gagné & Shoben, 1997; Smith & Osherson, 1984). For example, in the sentence, “the chef burned a hand on the hot oven,” the content words together describe a particular scene about a chef cooking in a kitchen. This is true even if the syntax of the sentence is disrupted; for example, in the semantically congruent word list, “chef for hand oven burned the a hot on,” the overall scene of the chef and the oven can be computed from the content words alone. Both sentences and word lists produced this combinatorial effect on left AG activation, suggesting that increased activation for semantically related words appears whether the words occur together in the context of a sentence or randomly within a list.
In addition to a semantic effect, the left AG also showed an effect of syntax for congruent and random stimuli. Sentences produced greater activation than word lists, suggesting that the left AG also uses syntactic structure. This pattern was not seen, however, in a contrast that included the two pseudoword conditions, indicating that the AG responds to syntactic structure only when lexical information is present in the input.
These patterns of activation strongly suggest that the AG integrates semantic and syntactic information to compute an overall sentence meaning. The fact that syntactic information is not strictly required to activate the AG (i.e., the AG also shows activation for semantically related word lists), but semantic information is required (i.e., pseudoword sentences do not activate the AG), suggests that the AG is engaged in primarily a semantic process. This process, which most likely involves integrating incoming pieces of semantic information, nevertheless appears to be modulated by syntactic information. This pattern can be contrasted with the anterior aspect of the ATL, which showed modulation primarily by syntactic information and much less sensitivity to semantic structure. Given the interactive nature of semantic and syntactic processing, it is likely that the processes carried out by the AG and ATL are also highly interactive, either through direct neural connections along the temporal lobe or through subcortical networks involving the thalamus or basal ganglia. This would explain the modulation of AG activity by syntactic information and the modulation of other ATL regions by semantic information.
Middle and Inferior Temporal Gyri
Complex effects of global semantic structure and single word meaning (congruent > random, congruent > pseudoword, random > pseudoword) were observed over large portions of the left STS, MTG, and ITG. These areas have all been strongly implicated in language comprehension (Dronkers et al., 2004; Binder, Frost, Hammeke, Cox, et al., 1997; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996; Demonet et al., 1992) and specifically in lexical–semantic processes (Martin, 2001; Damasio, Grabowski, Tranel, Hichwa, & Damasio, 1996). An interesting pattern was observed when the semantic structure effect (congruent > random) for word lists was compared with the semantic structure effect for sentences. Activation differences for the word lists were primarily seen in the middle part of the STS and MTG, whereas the same comparison for sentences showed activation in more inferior parts of the temporal lobe (Figure 7). The region showing a significant difference for word lists, in the middle STS and MTG, responded to all conditions above baseline, including the pseudoword conditions. The activation levels were greater for the more meaningful stimuli, with the highest levels of activity for the congruent word lists. The regions that showed semantic differences for sentences, on the other hand, had much greater activation for the congruent stimuli over both the random and pseudoword stimuli.
All of the temporal lobe areas seen in these contrasts show greater responses to stimuli with coherent semantic structure. Because the random stimuli contained the same words, randomized across trials, as the congruent stimuli, these activation differences cannot be explained by differences in lexical semantic information between the two conditions, but instead must be due to differences in the extent to which this information could be meaningfully combined. The two patterns of activation suggest somewhat different functional roles for midtemporal and more inferior temporal areas. The middle STS and MTG region responded to some extent to all six conditions and did not show large differences between sentences and word lists. We propose that this region plays a primary role in mapping from auditory word forms to lexical semantic representations. Mid-STS regions in the left hemisphere have recently been linked to phoneme recognition processes (Dehaene-Lambertz et al., 2005; Liebenthal, Binder, Spitzer, Possing, & Medler, 2005; Davis & Johnsrude, 2003; Binder et al., 2000; Scott, Blank, Rosen, & Wise, 2000). Activation in the STS and adjacent MTG may represent an intermediate processing stage in which combinations of phonemes activate semantic representations of single words. Pseudowords might activate this system somewhat due to the presence of familiar phoneme and syllable input, but are unable to produce strong activation of lexical semantic codes. Conversely, the presence of coherent semantic structure might induce somewhat stronger activation of such codes by facilitating the recognition of individual words. This interpretation is similar to earlier proposals that the left mid-STS and adjacent MTG represent neural correlates of the lexicon (Price et al., 1996; Small et al., 1996; Howard et al., 1992).
In contrast, more inferior parts of the temporal lobe (ITS and ITG) responded much more to stimuli with a coherent semantic structure, and showed very little activation for the random and pseudoword stimuli. In addition, these areas showed greater activation for congruent sentences than congruent word lists. These regions thus appear to be involved in representing more complex semantic information arising from the combination of individual word meanings, including such information as who did what to whom, where and when the event took place, the possible motivations of the actor in the sentence, and the possible implications of the event. This difference in activation pattern between midtemporal and surrounding regions is concordant with findings from a recent large-scale study of 64 left-hemisphere stroke patients, in which it was found that anterior, middle, and posterior temporal lobe lesions correlated with sentence comprehension deficits, whereas the patients with more isolated middle temporal lesions tended to also have greater deficits in single word comprehension (Dronkers et al., 2004).
Conclusion
In conclusion, our results suggest that syntactic and semantic processes engaged during sentence comprehension occur in distinct but overlapping parts of the temporal and parietal lobes. These regions make use of syntactic and semantic information in different ways. The middle part of the temporal lobe appears to function in initial access to semantic information at the word level, whereas more inferior, anterior, and posterior temporal and inferior parietal regions process combinatorial semantic information that arises when content words can be integrated to form a more complex meaning. Two areas, the ATL and the AG, appear to be specifically involved in sentence parsing. The ATL, which responded to syntactic structure regardless of the semantic structure present in the stimuli, appears to use syntactic information in building basic constituent structure. Semantic context could influence this process, and the fact that some voxels in the ATL sensitive to syntax showed overlap with semantic areas provides evidence for this interaction. The AG, on the other hand, responded only to sentences that contained recognizable content words. This suggests that the AG may be involved in combining discrete semantic information (i.e., content words) into a global meaning that is constrained by the syntactic structure of the sentence.
Acknowledgments
This work was supported by grants from the NINDS (RO1 NS33576) and NIH General Clinical Research Center (M01 RR00058).
Footnotes
The data reported in this experiment have been deposited with the fMRI Data Center (www.fmridc.org). The accession number is 2-2005-119DN.
REFERENCES
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX lexical database. University of Pennsylvania, Linguistic Data Consortium; Philadelphia: 1995. (Version 2.5) [CD-ROM] [Google Scholar]
- Bavelier D, Corina D, Jezzard P, Padmanabhan S, Clark VP, Karni A, Prinster A, Braun A, Lalwani A, Rauschecker JP, Turner R, Neville H. Sentence reading: A functional MRI study at 4 tesla. Journal of Cognitive Neuroscience. 1997;9:664–686. doi: 10.1162/jocn.1997.9.5.664. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Caramazza A. Syntactic aspects of aphasia. In: Sarno MT, editor. Acquired aphasia. Academic Press; New York: 1981. pp. 157–181. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Price CJ. Functional imaging of language. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of aphasia. MIT Press; Cambridge: 2001. pp. 187–251. [Google Scholar]
- Binder JR, Westbury CF, Possing ET, McKiernan KA, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: Choosing the optimal stimulus timing. Neuroimage. 2002;15:252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RSJ, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters GS. Effects of syntactic structure and prepositional number on patterns of regional cerebral blood flow. Journal of Cognitive Neuroscience. 1998;10:541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters GS. Verbal working memory and sentence comprehension. Behavioral and Brain Sciences. 1999;22:77–94. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in sentence comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Syntactic structures. Mouton; The Hague: 1957. [Google Scholar]
- Clifton C, Carlson K, Frazier L. Informative prosodic boundaries. Language and Speech. 2002;45:87–114. doi: 10.1177/00238309020450020101. [DOI] [PubMed] [Google Scholar]
- Cutler A, Dahan D, van Donselaar W. Prosody in the comprehension of spoken language: A literature review. Language and Speech. 1997;40:141–201. doi: 10.1177/002383099704000203. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H. Cortical systems for retrieval of concrete knowledge: The convergence zone framework. In: Koch C, editor. Large-scale neuronal theories of the brain. MIT Press; Cambridge: 1994. pp. 61–74. [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. Journal of Neuroscience. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Pallier C, Serniclaes W, Sprenger-Charolles L, Jobert A, Dehaene S. Neural correlates of switching from auditory to speech perception. Neuroimage. 2005;24:21–33. doi: 10.1016/j.neuroimage.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr., Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, Mecklinger A. Temporal structure of syntactic parsing: Early and late event-related brain potential effects. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1219–1248. doi: 10.1037//0278-7393.22.5.1219. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage. 2003;20:S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY. Auditory language comprehension: An event-related fMRI study on the processing of syntactic and lexical information. Brain and Language. 2000;74:289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Friederici A,D, Rüschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal gyrus and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Gagné CL, Shoben EJ. Influence of thematic relations on the comprehension of modifier–noun combinations. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:71–87. [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio AR. Investigating language with functional neuroimaging. In: Toga AW, Mazziotta JC, editors. Brain mapping: The systems. Academic Press; San Diego, CA: 2000. pp. 425–461. [Google Scholar]
- Grodzinsky Y. The neurology of syntax: Language use without Broca's area. Behavioral Brain Sciences. 2000;23:1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown CM. ERP effects of listening to speech compared to reading: The P600/SPS to syntactic violations in spoken sentences and rapid serial visual presentation. Neuropsychologia. 2000;38:1531–1549. doi: 10.1016/s0028-3932(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Homae F, Hashimoto R, Nakajima K, Miyashita Y, Sakai KL. From perception to sentence comprehension: The convergence of auditory and visual information of language in the left inferior frontal cortex. Neuroimage. 2002;16:883–900. doi: 10.1006/nimg.2002.1138. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons. Brain. 1992;115:1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Humphries C, Swinney D, Love T, Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Human Brain Mapping. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui T, Otsu Y, Tanaka S, Okada T, Nishizawa S, Konishi J. A functional MRI analysis of comprehension processes of Japanese sentences. NeuroReport. 1998;9:3325–3328. doi: 10.1097/00001756-199810050-00032. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Indefrey P, Usui N, Nose I, Hellwig F, Taira M. Reading in a regular orthography: An fMRI study investigating the role of visual familiarity. Journal of Cognitive Neuroscience. 2004;16:727–741. doi: 10.1162/089892904970708. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. Repair, revision, and complexity in syntactic analysis: An electrophysiological differentiation. Journal of Cognitive Neuroscience. 2003;15:98–110. doi: 10.1162/089892903321107855. [DOI] [PubMed] [Google Scholar]
- Kang AM, Constable RT, Gore JC, Avrutin S. An event-related fMRI study of implicit phrase-level syntactic and semantic processing. Neuroimage. 1999;10:555–561. doi: 10.1006/nimg.1999.0493. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: A fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SCR, David AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: An fMRI study. Journal of Cognitive Neuroscience. 2000;12:321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic anomaly. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonetic perception. Cerebral Cortex. 2005;15:1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- Luke KK, Liu HL, Wai YY, Wan YL, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Human Brain Mapping. 2002;16:133–145. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC, Pearlmutter NJ, Seidenberg MS. Lexical nature of syntactic ambiguity resolution. Psychological Review. 1994;101:676–703. doi: 10.1037/0033-295x.101.4.676. [DOI] [PubMed] [Google Scholar]
- Martin A. Functional imaging of semantic memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. MIT Press; Cambridge: 2001. pp. 153–186. [Google Scholar]
- Mazoyer B, Tzourio N, Frak V, Syrota A. The cortical representation of speech. Journal of Cognitive Neuroscience. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon YD. Neurocognition of auditory sentence comprehension: Event related fMRI reveals sensitivity to syntactic violations and task demands. Cognitive Brain Research. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: Divisible by what? Journal of Cognitive Neuroscience. 1998;10:766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: A functional imaging perspective. Neuroimage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Münte TF, Heinze HJ, Mangun GR. Dissociation of brain activity related to syntactic and semantic aspects of language. Journal of Cognitive Neuroscience. 1993;5:335–344. doi: 10.1162/jocn.1993.5.3.335. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA, Keller TA, Roth J, Carpenter PA. Differential effects of syntactic and semantic processing on the subregions of Broca's area. Cognitive Brain Research. 2003;16:297–307. doi: 10.1016/s0926-6410(02)00285-9. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fullbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. An fMRI study of syntactic adaptation. Journal of Cognitive Neuroscience. 2004;16:702–713. doi: 10.1162/089892904323057399. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RSJ, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Rubens AB. Localization of lesions in transcortical aphasia. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. Academic Press; San Diego, CA: 1994. pp. 297–329. [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank C, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Leff AP, Wise RJ. Going beyond the information given: A neural system supporting semantic interpretation. Neuroimage. 2003;19:870–876. doi: 10.1016/s1053-8119(03)00083-1. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Zurif E, Grimshaw J. Sentence processing and the mental representation of verbs. Cognition. 1987;27:219–246. doi: 10.1016/s0010-0277(87)80010-0. [DOI] [PubMed] [Google Scholar]
- Small SL, Noll DC, Perfetti CA, Hlustik P, Wellington R, Schneider W. Localizing the lexicon for reading aloud: Replication of a PET study using fMRI. NeuroReport. 1996;7:961–965. doi: 10.1097/00001756-199603220-00027. [DOI] [PubMed] [Google Scholar]
- Smith EE, Osherson DN. Conceptual combination with prototype concepts. Cognitive Science. 1984;8:337–361. [Google Scholar]
- Stowe LA, Haverkort M, Zwarts F. Rethinking the neurological basis of language. Lingua. 2005;115:997–1042. [Google Scholar]
- Stowe LA, Paans AMJ, Wijers AA, Zwarts F, Mulder G, Vaalburg W. Sentence comprehension and word repetition: A positronic emission tomography investigation. Psychophysiology. 1999;36:786–801. [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. Journal of Cognitive Neuroscience. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price CJ, Wise RJ, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Burchert F, Heinemann S, De Bleser R, Villringer A. Neural correlates of syntactic transformations. Human Brain Mapping. 2004;22:72–81. doi: 10.1002/hbm.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:141–154. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]


