Abstract
Objective
Auditory verbal hallucinations (AVHs) likely result from disorders, as yet unspecified, of the neural mechanisms of language. Here we examine the functional neuroanatomy of single-word reading in patients with and without a history of AVH.
Method
Eighteen medicated schizophrenia patients (8 with AVH and 10 without AVH) and 12 healthy control subjects were scanned with PET 15O-water technique under 2 conditions: reading aloud English nouns and passively looking at English nouns without reading them.
Results
The contrast between the 2 conditions shows higher activation in Wernicke's area during the reading condition in the patient group and a reversed laterality index for the supplementary motor area in the AVH group.
Conclusions
These findings provide indications about the possible mechanisms of AVH. We suggest that the abnormal laterality of the supplementary motor area activity accounts for the failure to attribute speech generated by one's own brain to one's self and that the activation of Wernicke's area accounts for the perceptual nature (hearing) of the patient's experience.
Medical subject headings: schizophrenia; auditory verbal hallucinations (AVHs); PET; lexical processing; attribution bias; corollary discharge, SMA
Abstract
Objectif
Les hallucinations auditivo-verbales (HAV) sont dues vraisemblablement à des désordres, non encore précisés, des mécanismes neuronaux du langage. Nous étudions ici la neuroanatomie fonctionnelle de la lecture d'un seul mot chez des patients ayant ou non des antécédents d'HAV.
Méthode
Dix-huit patients schizophrènes sous médication (8 avec des HAV et 10 sans HAV) et 12 sujets témoins en santé ont été soumis à une TEP 150 avec injection d'eau sous deux contextes : lire à voix haute des noms en anglais et regarder passivement des noms en anglais sans les lire.
Résultats
L'écart entre les deux contextes révèle une activation plus élevée dans l'aire de Wernicke pendant la lecture dans le groupe sans HAV et un indice de latéralité inversée pour l'activité supplémentaire dans le cortex moteur chez le groupe des patients.
Conclusions
Ces résultats fournissent des indications sur les mécanismes possibles des HAV. Nous croyons que la latéralité anormale de l'activité supplémentaire dans le cortex moteur est responsable de l'absence d'attribution du langage généré dans le cerveau à la personne elle-même et que l'activation de l'aire de Wernicke explique la nature perceptuelle (auditive) de l'expérience du patient.
Introduction
Auditory verbal hallucinations (AVHs) likely result from a disorder of language neural mechanisms.1–4 Neuropsychological theories suggest that patients who suffer from AVH experience their own inner speech (thinking in words) as someone else speaking. To explain the nonself attribution of inner speech, different mechanisms are suggested.
Frith3 suggests that nonself attribution results from a deficit in a system of corollary discharge networks associated with “willed actions” that allow these actions to be labelled as one's own. The early research of oculomotoricity5 shows evidence of a corollary discharge from the motor to the visual perceptive areas when the eye moves voluntarily, but not when movement is imposed on the eye. The primary function of this discharge is to allow the cortical visual areas to adjust the perceptual experience according to the movement. As a result, we perceive still images, although the images of the world move across the retina. However, because the corollary discharge occurs only during voluntary eye movements, it could convey information that the movement is self-generated.
Frith6 and Frith and Done7 showed that patients with schizophrenia are less likely to correct errors committed during voluntary movements; they considered this to be an indication that the self-labelling system of “willed actions” was impaired. The authors theorized that these monitoring deficits extend to self-generated inner speech. Consequently, inner speech could occur without the self-label and be experienced as AVHs.
Recently, electrophysiological studies showed direct evidence of deficits of the corollary discharge during speech in patients with schizophrenia. In these studies, healthy control subjects, but not schizophrenia patients, exhibited a reduction in the amplitude of event-related potential (ERP) component N1 while speaking aloud, relative to listening,8 and during directed inner speech.9 This reduction was attributed to a physiological dampening of the auditory cortex during self-generated inner and external speech that, apparently, does not occur in patients with schizophrenia. Therefore, schizophrenia patients could have different perceptual experiences of their own speech and may perceive their own speech as louder or clearer. However, whether this perceptual abnormality is sufficient to attribute one's own speech to others is a question open to debate.
In another theory,2 Hoffman suggests that AVHs result from an altered preconscious planning of discourse that produces involuntary inner speech. These “verbal images” are experienced as hallucinations and are attributed to someone else because they are “unintended.” Hoffman10 agrees that unintended simple action (such as nervous tick) is usually considered self-generated but suggests that an unintended complex action (such as inner speech) should be attributed to a nonself agent. However, we are not aware of empirical data supporting this argument. In summary, it is widely accepted that AVHs result from a disorder of language. However, the nature of this disorder and the mechanisms attributing one's own inner speech to another are subject to debate.
In a recent study of the phenomenological space of AVHs,11 it was found that AVHs occur differentially at multiple levels of linguistic complexity, including single words, sentences and conversations. This is a probable indication that a dysfunction of neural mechanisms of language at the lexical, sentencial and discourse levels may give rise to AVH with corresponding levels of linguistic complexity. Therefore, examining language processes at each of these levels could help clarify the language disorder underlying AVH.
In this study, we focus on the neural correlates of single-word reading in patients with and without hallucinations. We hypothesize that the neural correlates of reading aloud should differentiate the patients who have hallucinations from those who do not.
Method
We studied 18 male patients meeting the Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV),40 criteria for schizophrenia. Eight patients had a history of experiencing AVH, and 10 never experienced AVH (NAVH subgroup). The subjects were recruited from the outpatient clinic at the Minneapolis Veterans Affairs Medical Center (VAMC). Diagnosis was made by the Comprehensive Assessment of Symptoms and History.12 Patients were medicated but were symptomatic. In the AVH subgroup, 4 patients were taking conventional antipsychotic drugs and 4 were taking atypical antipsychotic medications. In the NAVH subgroup, 7 patients were taking conventional and 2 were taking atypical antipsychotic medications; 1 was taking both. We estimated the chlorpromazine equivalent doses of medications according to the methods of Woods13 and Van Kammen and Marder.14 Measures of verbal intelligence were obtained from the information survey subscale of the Wechsler Adult Intelligence Scale-Revised (WAIS-R),15 which has high correlations with verbal intelligence. We obtained measures of general psychopathology with the Brief Psychiatric Rating Scale (BPRS)16 and the Positive and Negative Symptoms Scale (PANSS)17 for all patients. The duration of illness was derived from a record review by a research assistant who was blind to the planned comparison (AVH, NAVH). Unfortunately, the clinical assessment data on 7 patients were lost during a change in office space. To ensure that the AVH and NAVH subgroups were matched on clinical variables, in addition to AVH, we further reviewed the patients' records and obtained information about lifetime incidence of thought disorder, delusions and affective blunting or guardedness.
The AVH and NAVH groups were compared with respect to age, level of education, verbal intelligence, duration of illness, BPRS and PANSS scores, and chlorpromazine equivalent doses of medication. All these variables, except the chlorpromazine equivalent doses, were normally distributed. Thus we used parametric and the Mann–Whitney nonparametric t tests, respectively. No significant difference between the 2 groups was found on any of the above measures at a significance level of p < 0.05. The 2 groups of patients were also compared with respect to the incidence of formal thought disorder (3 AVH and 6 NAVH), delusions (6 AVH and 7 NAVH) and affective blunting or guardedness (6 AVH and 6 NAVH). Chi-square and Fisher's exact tests did not reveal significant differences between the 2 groups on any of these variables at a p < 0.05 significance level. Therefore, only the propensity for verbal hallucinations distinguished the AVH from the NAVH subgroups of patients. Further, to assess the impact of the missing clinical data on positron emission tomography (PET) results, we analyzed the PET data on all patients and on those with available clinical data separately. Consistent results were found in the 2 sets of analyses.
We also studied 12 healthy male control subjects. They were recruited by advertisement at the university and at Veterans Affairs hospitals. The subjects were screened for mental illness by the DSMIII-R Computerized Screener18 and had neither a history of mental illness nor a family history of schizophrenia in first-degree relatives.
All subjects gave written informed consent. The protocol was approved by the institutional review boards at the VAMC and the University of Minnesota and the Radioactive Drug Research Committee at the VAMC. We determined control subjects' ability to make informed consent on the basis of their understanding the voluntary nature of their participation, the risks associated with venous access and radiation exposure and the possible discomfort during the PET procedure. The control group did not differ significantly from the patient group with respect to age, level of education or verbal intelligence. All subjects were native English speakers and were right-handed. Handedness was assessed by the Edinburgh Handedness Inventory.19 Table 1 summarizes the characteristics of the subjects.
Table 1
Task paradigm
Stimuli were common concrete nouns presented in lower-case letters above the fixation mark. A fixation crosshair was always present in the centre of the video monitor. The words subtended approximately 3° vertically and 5° horizontally. The stimuli appeared on a video monitor for 2.75 seconds with a 250-millisecond interstimulus interval. The task conditions presented here were part of a larger protocol also involving other language tasks that will be presented in another report. In the reading condition (Read), subjects maintained fixation and read loudly and clearly the words displayed. In the control condition (Look), subjects were instructed to maintain fixation at the crosshair but to not read (aloud or silently) the displayed words. We anticipated that the Read condition would activate neural resources for sensory visual, orthographic, semantic and phonological processing and motor planning20,21 and that the Look condition would activate only sensory neural resources. Subjects might have read the words in the Look condition, in violation with the instruction not to do so. In this case, given that both reading aloud and silently activates largely the same neural correlates,21 the difference would be minimized between the 2 conditions, with respect to orthographic, semantic and phonological word processing but not motor planning.
Both groups read the words aloud successfully. Word lists were counterbalanced across conditions, and task order was counterbalanced across subjects. No stimulus was repeated during a scan session. Stimuli were programmed in MEL v2.0 (Psychology Software Tools, Pittsburgh, Pa.).
Scanning and image processing
Scans were performed with a Siemens ECAT 953B PET camera (Siemens, Knoxville, Tenn.) in 2D mode (septae in place). A bolus of 40 mCi 15O-water was injected intravenously about 15 seconds after beginning the task. Integrated regional tissue activity was measured over 60 seconds after the arrival of the radiotracer to the head. A transmission scan corrected for attenuation. The images were reconstructed with filtered back projection with a Hanning filter (0.4 cycles/pixel). Images were visually inspected for movement artifacts, and parameters of shift and rotation between scans were obtained with an automated coregistration process.22 These parameters indicated that 8 subjects moved between scans, sometimes beyond a centimetre. Movement correction from the initial attenuation scan was carried out by a procedure similar to that described by Andersson and colleagues.23 Normalization for global activity, coregistration between scans within each subject and linear warping to Talairach space24 were carried out by a software package provided by Dr. Satoshi Minoshima.22,25,26 Image smoothing (final image resolution of 12- mm FWHM) and statistical analysis were performed with SPM99 (Welcome Department of Cognitive Neurology, London, GB; threshold Z = 3.0).
Pixel-wise analysis
All statistical comparisons, both within and between groups, were performed with random effects modelling. Within-group comparisons of the Read and Look conditions were performed with a paired t test on mean condition images for each subject. To examine differences between groups, a 2-way analysis of variance (ANOVA) task (Read v. Look) by group (control v. patient) was approximated with 3 separate comparisons. We assessed the main effect of the task, using a paired t test between the 2 experimental conditions across all subjects. The main effect of the group was examined with a 2-sample t test performed on individual subject images, generated by averaging across conditions. The group-task interaction was assessed with a 2-sample t test on individual subtraction images for each subject.
Because clinical data were available on only 11 patients, the pixel-wise analyses were repeated including only these patients. This was undertaken in an attempt to examine the sensitivity of these analyses.
Region of interest (ROI) analysis
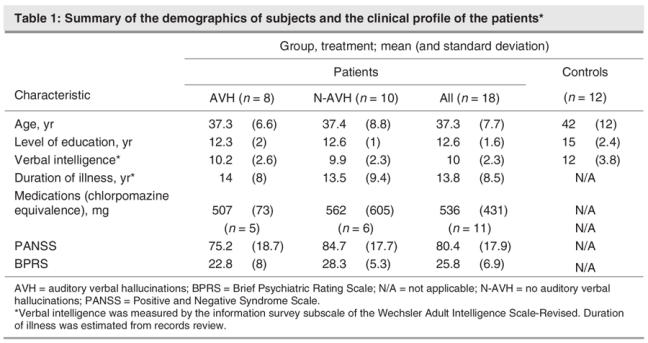
To investigate differences in lexical processing between the 2 patient subgroups (AVH and NAVH), given the limited number of patients and the multiple comparison problem, we restricted our analyses to ROI on areas classically implicated in language processing: the “Wernicke's area,” “Broca's area” and supplementary motor area (SMA)/pre-SMA. Given the variability across subjects and the uncertainty of the exact location of these areas, the ROIs were created from clusters of activity surpassing a voxel-level threshold of p < 0.05 (corrected for multiple comparisons) in the main effect of the task performed across all subjects (Fig. 1). The selected volumes included a cluster near the left posterior superior temporal gyrus (putative Wernicke's Area 684 mm3), a cluster in the left premotor frontal cortex (putative Broca's Area, 808 mm3) and a symmetric cluster near the left and right SMA (900 mm3 per hemisphere). Mean activity within the ROIs were analyzed for between-group differences across the control, AVH and NAVH groups. Kruskal–Wallis nonparametric ANOVA and Mann–Whitney U tests examined the relative contribution of AVH and NAVH subgroups to the observed abnormal activity in these areas.
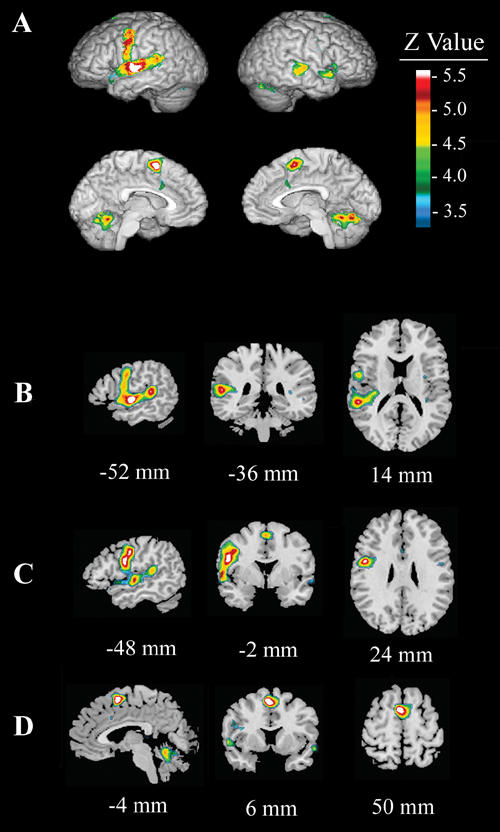
Fig. 1: Main effect of condition (Read – Look) across all subjects (patients and healthy control subjects). The figure shows (A) activation in the left motor and premotor cortices, left anterior and posterior superior temporal gyrus, bilateral supplementary motor area (SMA) and cerebellum. This image was used to draw 3 region of interests: (B) Wernicke's area (arrow; left posterior superior temporal gyrus), (C) Broca's area (anterior and inferior frontal focus), and (D) left and right SMA regions.
Results
Read–Look comparisons
In healthy control subjects, the reading condition minus the looking condition (Fig. 2, Table 2) activated the left SMA (BA 6), left and right superior temporal gyrus (STG, BA 22), Broca's area (BA 44), the left and right precentral gyri (BA 4) and the cerebellum. Reading, compared with looking, deactivated the right superior occipital gyrus (BA 19), right inferior temporal gyrus (BA 19/37), right middle frontal gyrus (BA 9), left occipital gyrus (BA 19), right superior frontal gyrus (BA 10) and right middle frontal gyrus (BA 8).
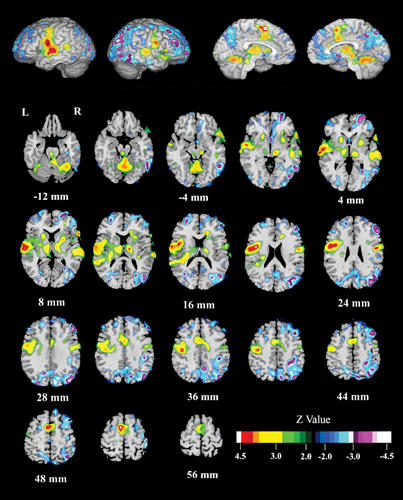
Fig. 2: Effect of condition (Read – Look) for the healthy control subjects. In healthy control subjects, reading relative to looking activated the left supplementary motor area (SMA), bilateral superior temporal gyrus (STG), Broca's area, bilateral precentral gyri and the cerebellum. Deactivation is noted in the right superior occipital, right inferior temporal, right middle frontal, left occipital, right superior frontal, and the right middle frontal gyri.
Table 2
In patients with schizophrenia, the reading condition (Fig. 3, Table 2) activated the left and right STG (BA 21, 22); left posterior STG (Wernicke's area; BA 41); left precentral gyrus (BA 6); bilateral, but predominantly right-sided, SMA (BA 6); cerebellum; and right inferior frontal gyrus (BA 47). Reading deactivated the left and right precuneus (BA 7), right fusiform gyrus (BA 36), right inferior occipital gyrus (BA 18), right medial frontal gyrus (BA 10), the right occipital gyrus (BA 19) and the left middle frontal gyrus (BA 8).
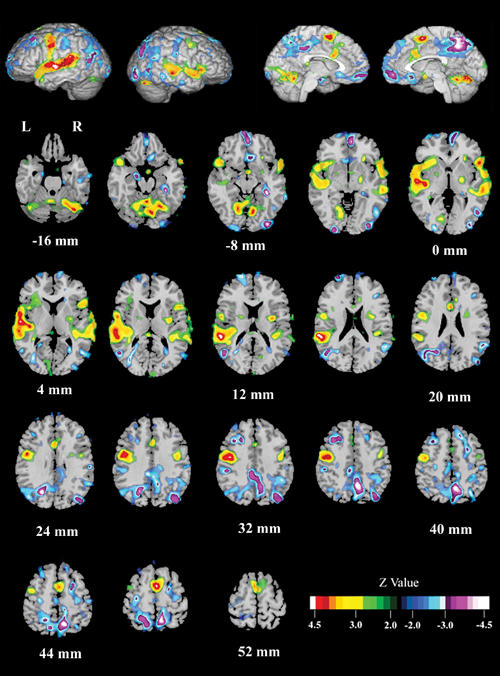
Fig. 3: Effect of condition (Read – Look) for all schizophrenia patients combined (AVH and NAVH).Schizophrenia patients reading single words activated bilateral anterior superior temporal gyrus (STG); left posterior STG (Wernicke's area); left precentral gyrus; bilateral, but predominantly right, SMA; cerebellum; and the right inferior frontal gyrus. Reading relative to looking deactivated the left orbital gyrus, the right fusiform gyrus, the right precuneus, the right inferior occipital gyrus, the right median frontal gyrus, the right orbital gyrus, and the left middle frontal gyrus.
In the AVH subgroup, an increase in bloodflow is noted in the left and right STG, Wernicke's and Broca's areas, the left precentral gyrus, and the cerebellum. Bloodflow decrease is noted in the right medial frontal gyrus, right precuneus, left middle frontal gyrus, right inferior occipital gyrus left middle temporal gyrus and left caudate nucleus.
In the NAVH subgroup, an increase in bloodflow was observed in the left and right STG, Wernicke's area, the left SMA, left precentral gyrus, left caudate, right inferior frontal gyrus and cerebellum. Decrease in bloodflow was observed in the right and left precuneus and the right superior occipital gyrus. Table 3 summarizes these findings and Figure 4 shows a comparison between AVH, NAVH and the control group.
Table 3
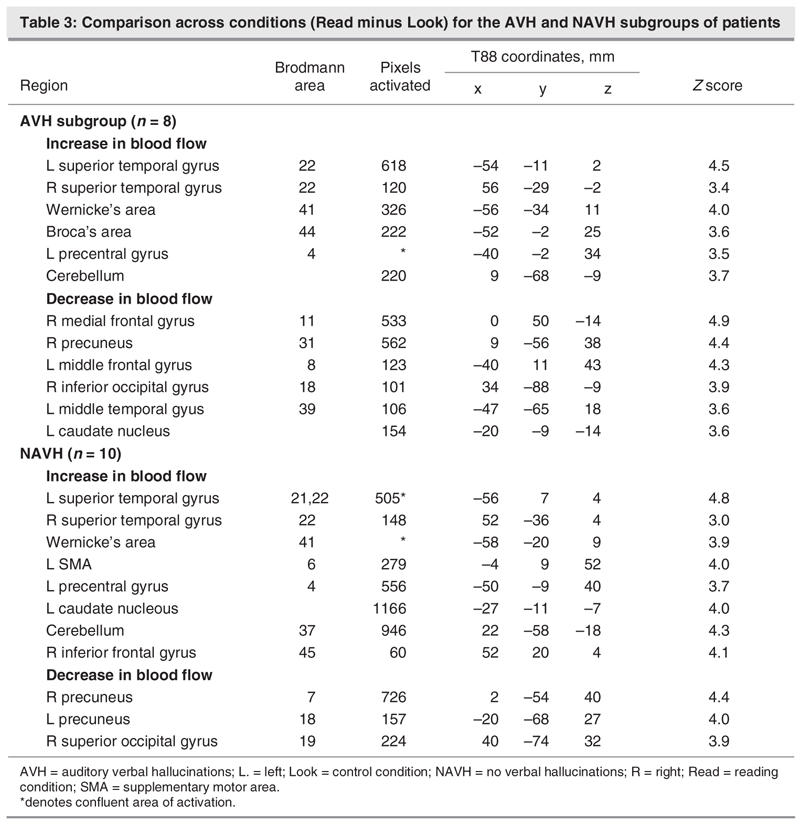
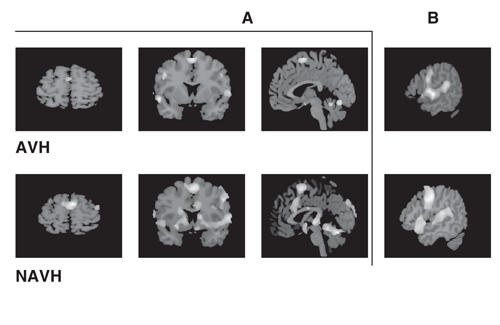
Fig. 4: Effect of condition (Read – Look) for the auditory verbal hallucination (AVH) group and the group without AVH (NAVH) separately. Bilateral activation of the supplementary motor area (SMA) in the AVH subgroup and predominantly left SMA activation in the NAVH subgroup (A). Activation of the Wernicke's area can be seen in both subgroups (B).
Main effect of the group
To study differences in rCBF between the patients and the healthy control group (not related to the task), we examined the main effect of the group (patients v. control subjects), using the sum of the Read and the Look images.
In the patients, higher activity was found in the right striatum (25,9,0), left temporal-parietal junction (BA 21[–47,–47,9]), and right midcingulate gyrus (BA 31 [7,–25,40]) (not shown in the figures). Relative decreases in activation arose in the anterior cingulate gyrus (BA 24 ([22,14,36]) and BA 32 ([–2,50,4]; not shown in the figures).
Patient–control subject comparison
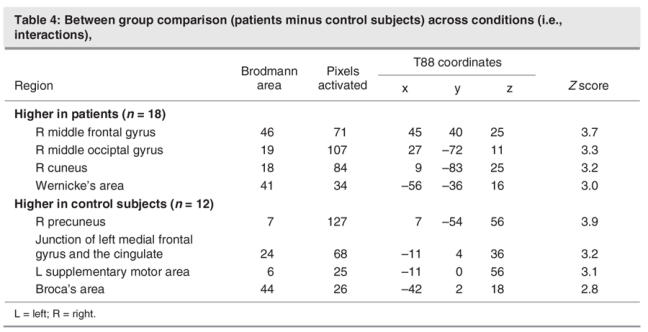
Figure 5 shows the interaction of task (Read minus Look) by group (schizophrenia patients minus healthy control subjects). Read minus Look scores were higher in patients in the right middle frontal and occipital gyri (BA 46 and BA 19), right cuneus (BA 18) and Wernicke's area (BA 41). Conversely, the activation of reading relative to looking was higher in healthy control subjects in the right precuneus (BA 7), an area at the junction between the left medial frontal gyrus and the cingulate (BA 24), and the left SMA (BA 6). In Broca's area (BA 44), the activation of reading relative to looking was higher in healthy control subjects at a trend level. Table 4 summarizes the pixel-wise analysis findings. In light of the within-group findings across tasks presented above, we concluded that reading elicits higher activity in the Wernicke's area in patients and higher activity in the left SMA and Broca's area in healthy control subjects.
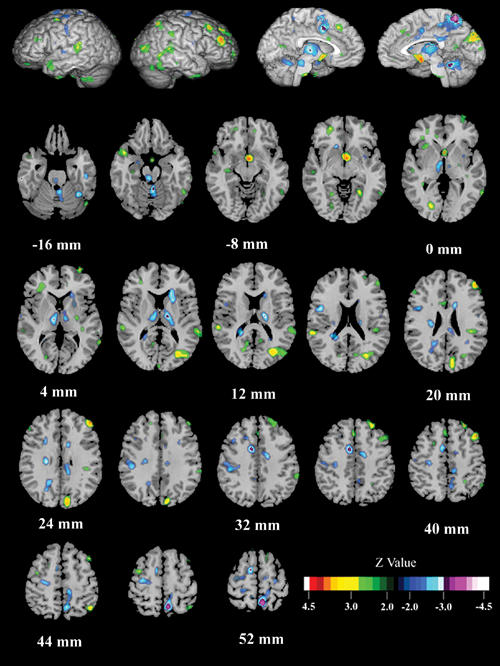
Fig. 5: Between-group (schizophrenia vs. control subjects) and across-task (Read – Look) comparison (i.e., interaction). Patients, relative to control subjects, had higher activation during reading in the right middle frontal and occipital gyri, the right cuneus, and Wernicke's area (left, posterior, superior temporal gyrus). Decreased activation, relative to the control contrast, was found in patients in the right precuneus, an area at the junction between the left medial frontal gyrus and the cingulate, and the left supplementary motor area. A trend toward lower activation was found in Broca's area in patients.
Table 4
ROI analyses
In the ROI analysis, the distributions of the variables (normalized regional cerebral bloodflow [rCBF]) in each area and in each group were tested for normality. Since almost all the distributions were nonnormal (p < 0.05, Lilliefors test), we performed the Kruskal–Wallis nonparametric analysis of variance to test for differences between the 3 groups (AVH, NAVH and healthy control subjects), followed by specific pairwise group comparisons (Mann–Whitney with Bonferroni correction for multiple comparisons). The 3 groups were significantly different in the change of rCBF in Wernicke's area (Fig. 6A; Read minus Look difference scores; p = 0.03, Kruskal–Wallis). Pairwise group comparisons revealed that the NAVH group showed a significantly larger increase in rCBF than did the control group (p = 0.01, Mann–Whitney, Bonferroni corrected). The change in rCBF in Broca's area was not significantly different between groups (p = 0.40, Kruskal–Wallis; Fig. 6B).

Fig. 6: Region of interest analyses. Activation of Wernicke's area (note black regions of interest [ROIs] in the cortex) in the subgroup without auditory verbal hallucination (AVH) was significantly different from that of healthy control subjects and the AVH subgroup (A). The AVH subgroup had higher baseline activity (nonsignificant) in Wernicke's area than both the NAVH and the healthy control groups. This could be related to an ongoing experience of verbal hallucinations. Activation of Broca's area (note black ROIs in the cortext) was not different across the 3 groups (B). Both the healthy control subjects and the NAVH groups showed higher activity in the left SMA than in the right SMA (note black ROI and white ROI at midline) (C). The inverse is true in the AVH group.
Because bilateral activation of the SMA was noted in the patient group, the laterality index was calculated with a formula similar to that in Binder and others27: [(ARead – ALook)L – (ARead – ALook)R] / [(ARead – ALook)L + (ARead – ALook)] %, where ARead and ALook refer to the normalized rCBF during the Read and Look conditions, respectively, and L and R refer to the left and right SMA, respectively. Kruskal–Wallis nonparametric ANOVA revealed a significant between-group effect (p = 0.007). Pairwise comparisons showed a significantly lower laterality index in the AVH group relative to the healthy control subjects (p = 0.02, Mann–Whitney, Bonferroni corrected) and NAVH groups (p = 0.03, Mann–Whitney, Bonferroni corrected). The laterality index in the NAVH group was not significantly different from the healthy control group (p = 0.49, Mann–Whitney, uncorrected; Fig. 6C).
Discussion
The comparison (Read – Look) in this study activated brain regions such as Broca's area, Wernicke's area, and the SMA. These areas are known to be involved in phonetic and semantic processing and motor planning.28,29 Therefore, this contrast reflects, as anticipated, the neural correlates of these aspects of processing associated with the action of reading single words.
Both reading aloud and reading silently activates largely the same neural correlates.21 Therefore, if subjects silently read the stimuli during the Look condition, this would only minimize the difference (Read – Look). However, as both patients and control subjects exhibited the activation of language-specific areas, it is reasonable to conclude that all subjects largely performed the Look condition correctly.
This study reveals differences in the neural correlates for reading single words between the patient and control groups and between the AVH and the NAVH subgroups of patients. The patients differed from the control subjects in the activation of Wernicke's area, the right SMA, the right cuneus and the right inferior frontal gyrus (RIFG). The higher activation of Wernicke's area could indicate difficulty with semantic processing of single words. Further, patients activated the RIFG and the cuneus, which are usually associated with sentence-level semantic processing,30,31 during a task requiring only lexical processing. It is generally accepted that language calls on a neural network of distinct, nonoverlapping and interconnected processors specific to the lexical, sentencial and discourse32 components. Therefore, the above findings may reflect either a defective lexical processor and/or abnormal connectivity between language processors.
The AVH subgroup differed from the NAVH subgroup by the reversal of the laterality index of the activation of the SMA (right more than left) in the former and a higher activation of Wernicke's area in the latter subgroup. The 2 subgroups of patients have similar clinical profiles, differing only with respect to the presence of a propensity for verbal hallucinations in the AVH subgroup. Therefore, these findings provide clues about the possible mechanisms of AVH.
The higher activation of Wernicke's area during reading in the patient group is consistent with the theory that an abnormal corollary discharge in schizophrenia fails to dampen Wernicke's area during self-generated speech and, consequently, gives rise to AVH. However, when patients were divided according to the propensity to verbal hallucinations, higher activation in Wernicke's area was found in the NAVH group only. Thus failure of the corollary discharge is not by itself sufficient to explain the misattribution of one's own voice to another (as present in the subgroup without hallucinations). It could, nonetheless, result in a different perceptual experience of one's own voice.
The AVH subgroup activated Wernicke's area in the Read–Look comparison; this activation was lower than the NAVH subgroup, as reflected in the ROI analysis. This observation could be related to higher baseline activity due to the experience of AVH.33,34 Indeed, the baseline activity in Wernicke's area was higher in the AVH subgroup, compared with the NAVH subgroup and healthy control subjects, but it did not reach statistical significance.
What clearly differentiated the hallucinating from the nonhallucinating group was the laterality of the SMA activation. A reversal of the laterality index was shown in the AVH group, whereas the NAVH and control groups showed normal lateralization. Lesions in the SMA have been shown to give rise to alien limb syndrome35; thus the SMA is considered necessary for self-attribution of self-initiated actions (such as movements, speech or reading). Right-handed people could carry out actions with their left hand; however, this would be less effective, and occasional failure could arise. Similarly, patients with abnormal lateralization of the SMA activity could occasionally fail to attribute to themselves a self-generated action. In patients with hallucinations, the ineffective function of the SMA during external speech could extend to inner speech, given a common developmental origin36,37 and neural correlates38 of inner and external speech. Therefore, inner speech could be generated without self-attribution and be experienced as hallucinations. This finding is consistent with an imaging study39 showing a suboptimal level of function of the SMA. In this study, hypoactivation of the SMA is found in schizophrenia patients with hallucinations during tasks that entail generation and monitoring of inner speech.
By comparing the group with AVH with the group without, we were able to control for the medication effect. The effects of the antipsychotic medications on bloodflow are unclear; however, it is unlikely that the aberrant activity of the SMA in the AVH group and of Wernicke's area in the NAVH group are related to medication effects. Both groups were on equivalent doses and similar types of medications. Nonetheless, they differed from each other (as well as from healthy control subjects) in the activation of these brain areas. The small sample size of the AVH and NAVH subgroups should be taken into account when interpreting these findings.
The lateralization failure of the SMA activity in schizophrenia patients with hallucinations, combined with the failure of corollary discharge to inhibit Wernicke's area during the action of speaking, favours speech generation pathology as a mechanism for AVH3,4 and provides a more comprehensive explanation for the experience of verbal hallucinations. The first finding accounts for the failure to attribute self-generated speech to self (and therefore attribution to nonself); the second finding explains the perceptual nature (hearing) of the patient experience.
Acknowledgments
This work was supported in part by the Department of Veterans Affairs (MS, JTL, MK, JVP), NARSAD (JVP), Matt Kaul Schizophrenia Research Foundation (JVP), Minnesota Medical Foundation (MS, JVP), Martha and William Muska Fund at the Saint Paul Foundation (MS), and an NRSA F31 MH-12575 (MCH).
Footnotes
Contributors: Drs. Stephane, P. Pardo and J. Pardo designed the study. Drs. Uecker, P. Pardo and J. Pardo acquired the data; Drs. Stephane Hagen, Lee, P. Pardo, Kuskowski and J. Pardo analyzed it. Drs. Stephane, P. Pardo and J. Pardo wrote the article; they and Drs. Hagen, Lee, Uecker and Kuskowski critically reviewed it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. José V. Pardo, Cognitive Neuroimaging Unit (11P), Veterans Affairs Medical Center, One Veterans Drive, Minneapolis MN 55417; fax 612 725-2449; jvpardo@james.psych.umn.edu
References
- 1.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull 1978;4:636-40. [DOI] [PubMed]
- 2.Hoffman RE. Verbal hallucinations and language production processes in schizophrenia. Behav Brain Sci 1986;9:503-48.
- 3.Frith CD, Done DJ. Toward a neuropsychology of schizophrenia. Br J Psychiatry 1988;153:437-43. [DOI] [PubMed]
- 4.Stephane M, Barton SN, Boutros NN. Auditory verbal hallucinations and dysfunction of the neural substrates of speech. Schizophr Res 2001;50:61-78. [DOI] [PubMed]
- 5.Von Helmholtz H. A treatise on physiological optics. New York: Dover; 1866.
- 6.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 1987;17:631-48. [DOI] [PubMed]
- 7.Frith CD, Done DJ. Experiences of alien control of schizophrenia reflect a disorder of central monitoring of action. Psychol Med 1989;19:359-64. [DOI] [PubMed]
- 8.Ford JM, Mathalon DH, Heinks T, et al. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry 2001;158:2069-71. [DOI] [PubMed]
- 9.Ford JM, Mathalon DH, Kalba S, et al. Cortical responsiveness during inner speech in schizophrenia: an event-related potential study. Am J Psychiatry 2001;158:1914-6. [DOI] [PubMed]
- 10.Hoffman RE. The Duphar Lecture: on the etiology of nonself attributes of schizophrenic ‚voices.' Psychopathology 1991;24:347-55. [DOI] [PubMed]
- 11.Stephane M, Thuras P, Nassrallah H, et al. The internal structure of the phenomenology of auditory verbal hallucinations. Schizophr Res 2003;61:185-93. [DOI] [PubMed]
- 12.Andreasen NC. Comprehensive assessment of symptoms and history (CASH). Iowa City: University of Iowa; 1986.
- 13.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663-7. [DOI] [PubMed]
- 14.Van Kammen D, Marder S. Dopamine receptor antagonists. In: Kaplan H, Sadock B, editors. Comprehensive text book of psychiatry. Baltimore: Williams & Wilkins; 1995. p. 1987-2022.
- 15.Wechsler D. WAIS-R Manual. New York: The Psychological Corporation; 1981.
- 16.Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS). Psychol Rep 1962;10:799-812.
- 17.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-7. [DOI] [PubMed]
- 18.Bucholz KK, Bomins LN, Shayka JJ, et al. Performance of two forms of a computer psychiatric screening interview: version I of the DISSI. Psychiatry Res 1991;25:117-29. [DOI] [PubMed]
- 19.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971;9:97-113. [DOI] [PubMed]
- 20.Posner MI, Abdullaev YG, McCandliss BD, et al. Anatomy, circuitry and plasticity of word reading. In: Everatt J, editor. Visual and attentional processes in reading and dyslexia. London: Routledge; 1995. p. 137-62.
- 21.Price CJ. Functional anatomy of reading. In: Frakowiak RSJ, Friston KJ, Frith CD, et al, editors. Human brain function. San Diego: Academic Press; 1997. p. 301-28.
- 22.Minoshima S, Berger KL, Lee KS, et al. An automated method for rotational correction and centering of three-dimensional functional brain images. J Nucl Med 1992;33:1579-85. [PubMed]
- 23.Andersson JL, Vagnhammar BE, Schneider H. Accurate attenuation correction despite movement during PET imaging. J Nucl Med 1995;36:670-8. [PubMed]
- 24.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme Verlag; 1988.
- 25.Minoshima S, Koeppe RA, Mintun MA, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med 1993;34:322-9. [PubMed]
- 26.Minoshima S, Koeppe RA, Frey KA, et al. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 1994;35:1528-37. [PubMed]
- 27.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996;46:978-84. [DOI] [PubMed]
- 28.Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A 1998;95:914-21. [DOI] [PMC free article] [PubMed]
- 29.Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat 2000;197 Pt 3:335-9. [DOI] [PMC free article] [PubMed]
- 30.MacSweeney M, Woll B, Campbell R, et al. Neural systems underlying British Sign Language and audio-visual English processing in native users. Brain 2002;125(Pt 7):1583-93. [DOI] [PubMed]
- 31.Balsamo LM, Xu B, Grandin CB, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol 2002;59:1168-74. [DOI] [PubMed]
- 32.Caplan D. Language structure, processing and disorders. Cambridge: The MIT Press; 1992.
- 33.Silbersweig DA, Stern E, Frith CD, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995;378:176-9. [DOI] [PubMed]
- 34.Stephane M, Hill T, Matthew E, et al. New phenomenon of abnormal auditory perception associated with emotional and head trauma: Pathological confirmation by SPECT scan. Brain Lang 2004;89:503-7. [DOI] [PubMed]
- 35.Goldberg G. Supplementary motor area structure and function: review and hypothesis. Behav Brain Sci 1985;8:567-615.
- 36.Piaget J. The language and thought of the child. New York: Meridian Books; 1955.
- 37.Vygotsky LS. Mind in society, the development of higher psychological processes. Cambridge: Harvard University Press; 1978.
- 38.MacKay DG. Constraints of theories of inner speech. In: Reisberg D, editor. Auditory imagery. New Jersey: Lawernce Erlbaum Associates; 1992. p. 121-42.
- 39.McGuire PK, Silbersweig DA, Wright I, et al. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet 1995;346:596-600. [DOI] [PubMed]
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: the Association; 1994.


