Abstract
Specific immunoglobulin (IgY) from egg yolk against Aeromonas hydrophila was produced by immunization of White Leghorn hens with formalin-killed whole cells of A. hydrophila. ELISA test using A. hydrophila as the coating antigen revealed that the specific antibody titer started to increase in the egg yolk at the 13th day post-immunization (P/N=2.18), reached the peak at the 56th day (P/N=13.82), and remained at high level until day 133 (P/N=7.03). The antibody was purified by saturated ammonium sulphate with a recovery rate of 63.5%. The specific IgY inhibited the growth of A. hydrophila at a concentration of 1.0 mg/ml during the 18 h incubation. Pre-treatment of polyploid gibel carps Carassius auratus Gibelio with specific IgY had a protection rate of 60% (6/10) against challenge with A. hydrophila, while none of the fishes in the control groups receiving sterile phosphate buffered saline (PBS) or non-specific IgY survived the challenge. Treatment of fishes with the specific IgY 4 h after the challenge also had lower mortality (70%, 7/10), a 30% reduction against the control PBS or non-specific IgY groups (10/10). These results indicate that specific IgY antibodies could be obtained easily from hens immunized with an inactivated A. hydrophila and could provide a novel alternative approach to control of diseases in fishes caused by this organism.
Keywords: Aeromonas hydrophila, Immunoglobulins, Hen egg yolk, Polyploid gibel carps, Carassius auratus Gibelio
INTRODUCTION
Aeromonas spp. are autochthonous inhabitants of aquatic, sewage and soil environments (Janda and Abbott, 1998; Chowdhury et al., 1990) and consist of two major groups, the psychrophilic group and the mesophilic group. Aeromonas hydrophila is one of the most important representative species in the mesophilic group (Gonzalez et al., 2001). The bacterium is of scientific and economic interest because of its pathogenicity to human and fishes (Elwitigala et al., 2005; Austin et al., 1998). A. hydrophila infection in fishes occurs from time to time in Asian countries including China, Philippines, Thailand and India (Chen and Lu, 1991). Freshwater aquatic species infected by A. hydrophila could exhibit different symptoms such as hemorrhagic enteritis, festering neck disease or furuncles in turtles, red body disease or septicemia in shrimps, and festering gill or hemorrhagic enteritis in fish species such as Hypopthalmichthys molitrix, Ctenopharyngodon idella, Aristichthys nobilis, Carassius auratus Gibelio. Antibiotics are often used in closed and open waters for therapy of furunculosis of farmed aquatic species (Kampfer et al., 1999). Vaccination is an important strategy in the control of this disease caused by A. hydropila among farmed fish (Asha et al., 2004). However, vaccination varies considerably in efficacy, and epizootic events occur frequently in fish farms. Therefore, it is important to search for effective methods as alternative to antibiotics for treatment of A. hydrophila infections in the intensively farmed aquatic species.
Laying hens transfer large amounts of immunoglobulin from serum to egg yolk of their eggs, where it serves as a means of passively protecting the developing chicks (Kariyawasam et al., 2004; Rosenberger et al., 1985). An average egg may contain 100~150 mg of yolk immunoglobulins (IgY), and substantial amounts of specific antibodies may be collected and purified from the eggs of immunized hens (Akita and Nakai, 1993). The availability of large amounts of relatively inexpensive IgY from egg yolks makes it feasible to use these antibodies for passive immunization by oral administration or injection (Carlander at al., 2000). The efficacy of this approach has been shown in human and veterinary medicine for rotavirus diarrhea in humans (Ebina, 1996; Kuroki et al., 1997; Sarker et al., 2001), Escherichia coli infections in pigs (Yokoyama et al., 1992; Ikemori et al., 1992; Marquardt et al., 1999; Hennig-Pauka et al., 2003), and Streptococcus mutans-induced dental caries (Krüger et al., 2004; Smith et al., 2001). In aquatic species, IgY against Edwardsiella tarda was administered orally to passively immunize Japanese eels (Mine and Kovacs-Nolan, 2002). These studies demonstrated that IgY could serve as an effective means against bacterial and viral infections (van Nguyen et al., 2006). Nevertheless, there has been no report so far on the use of IgY in the prevention and treatment of A. hydrophila infections. In this paper, we report the antibody response of laying hens to immunization with inactivated whole cells of A. hydrophila and the efficacy of the specific IgY preparation on control of fish diseases caused by A. hydrophila infection in Carassius auratus Gibelio.
MATERIALS AND METHODS
Immunization of hens
The A. hydrophila strain AS 1.927 was originally from China General Microbiological Culture Collection Center. The bacterium was grown in tryptic soy broth (Difco, Detroit, MI) at 35 °C for 24 h in a flask with shaking. After three washes with sterile 0.1 mol/L phosphate-buffered saline (pH 7.2), the number of cells was adjusted to 2×109 cfu/ml with sterile PBS. The culture was treated with 0.4% formalin for 24 h and tested for viability. This antigen suspension was mixed and emulsified with equal volume of Freund’s complete adjuvant (FCA) or Freund’s incomplete adjuvant (FIA) (Sigma, St. Louis, MO). A group of 12 White Leghorn hens, 25 weeks old, were kept for immunization and egg production in the Central Animal Facility at Zhejiang University. Each hen received 1 ml of emulsified antigen in FCA intramuscularly at four sites (0.25 ml per site). Booster injections were given intramuscularly at 2, 4, and 6 weeks after the first injection with the emulsified antigen containing FIA. Eggs were collected from the time of first vaccination and then once a week during the experimental period (till day 133).
Purification of IgY
The crude antibody from yolk was extracted by the water-soluble fraction as described by Akita and Nakai (1993) with modification. Egg yolk was separated from the white, and the yolk preparation was diluted at 1 to 9 ratio with distilled water at pH 5.3. The mixtures were kept overnight at 4 °C. After centrifugation at 10000×g at 4 °C for 30 min, the water-soluble fraction (WSF) was carefully collected and further purified by 33% (v/v) saturated ammonium sulphate solution and ultrafiltration (UF) using a UF membrane (Millipore Corp., Bedford, Mass). Purified IgY was freeze-dried. The recovery rate of immunoreactive IgY was calculated using the formula OD 492 nm (A)/OD 492 nm (B)×100, where A is OD 492 nm in the crude extract (WSF) or (NH4)2SO4-purified product at a given dilution and B, OD 492 nm in the egg yolk pooled at the same dilution.
Antibody titration
Enzyme linked immunosorbent assay (ELISA) was used to check the titer of the specific antibody. Each well of the 96-well polystyrene plates (NUNC, Denmark) was coated overnight at 4 °C with 100 μl of A. hydrophila whole cell suspension (109/well) in 0.05 mol/L carbonate buffer (pH 9.6). The wells were washed with phosphate-buffered saline (PBS)-Tween (0.05% Tween 20 in PBS at pH 7.2, PBST), and then blocked by incubation for 1 h at 37 °C with 150 μl of 1.0% (w/v) bovine serum albumin (BSA, Sigma) in carbonated buffer. After three washings with PBS-Tween, appropriately diluted IgY preparations were added to the wells and the plates were incubated for 1 h at 37 °C. One hundred microlitres of HRP conjugated rabbit anti-chicken IgG (BioChain Institute, Inc., Hayward, USA) at 500-fold dilution with PBST with 0.05 BSA was added to the wells after another three washings. The plates were incubated at 37 °C for 1 h and washed with PBS-Tween for 3 times. The substrate solution (20 ml PBS+8 mg o-phenylene-diamine+100 μl H2O2) was added. After 20 min of incubation at 37 °C, 50 μl of 2 mol/L H2SO4 was added to stop the reaction. The color reaction was read on an ELISA plate reader (Model Elx800, Bio-Tek, USA) at 492 nm. The antibody titer was expressed as P/N values, where P represents the OD 492 nm of IgY from immunized hens at a given dilution and N, the OD 492 nm of IgY from non-immunized hens at the same dilution.
SDS-PAGE electrophoresis
Purification of the antibody was visualized by SDS-PAGE at 6% gel concentration according to the method of Laemmli (1970) on a Mini-PROTEAN II Cell (Bio-Rad Laboratories, Hercules, Calif). The protein concentration using the micro-BCA protein assay (Pierce, IL, USA) was adjusted to 1 mg/ml and mixed with the sample buffer (pH 6.8, 62.6 mmol/L Tris-HCl, 25% (v/v) glycine, 2% (w/v) SDS). The samples were heated for 3 min at 100 °C. Fifteen microlitres of the samples were loaded into corresponding wells for electrophoresis. The gel was stained with a mixture of Coomassie brilliant blue R-250 (Sigma) in 10% acetic acid and 30% methanol. The protein bands from the samples were compared with reference IgY from Jackson Immuno Research Laboratories.
Inhibition of A. hydrophila growth in vitro
The specific antibody from A. hydrophila vaccinated hen eggs and the non-specific control from those of non-immunized hens were purified with 33% (v/v) saturated ammonium sulphate solution, followed by ultrafiltration. The specific and control antibodies were adjusted to the level of 2 mg/ml and serial 2-fold dilutions were made. The antibody solutions were then added into corresponding wells of a 96-well polystyrene plate containing 108 cfu/ml A. hydrophila in 100 μl of 2× sterilized tryptic soy broth. Inhibition of bacterial growth was continuously recorded as changes of OD 560 nm at the 30-min interval for 18 h at 35 °C on the SPECTRAMAX M2 spectrophotometer (Molecular Devices Corporation, California, USA) preset to shake the plate for 5 s before each measurement.
Fish experiments
All fish experiments were conducted according to the guidelines for the care and use of animals in research and teaching of Zhejiang University. Polyploid gibel carps Carassius auratus Gibelio were clinically healthy and free of any external abnormalities. The fishes were maintained at (24±2) °C in stock tanks supplied with flowing water and fed a diet of a commercial fish food. All experiments were done after fishes were acclimatized for one week.
For prevention experiments, three groups, each with 10 fishes ((27±1) g), were randomly assigned to the experimental tanks. Each fish was injected by intraperitoneal route either with 0.25 ml of 30 mg/ml specific IgY preparation (s-IgY group), 0.25 ml of 30 mg/ml non-specific IgY (n-IgY group) or sterile PBS (control). The fishes were kept for 4 h in the tanks and then challenged with 0.25 ml of AS 1.927 (1×109 cfu/ml) via intraperitoneal route. Distress or death of the fishes was monitored at frequent intervals until day 8 post-challenge. For therapeutic experiments, the groups were assigned as above. Fishes in each group were challenged first with 0.25 ml with 1×109 cfu/ml of AS 1.927. After 4 h in the tanks, the fishes were injected intraperitoneally with 0.25 ml of 30 mg/ml specific IgY, non-specific IgY or sterile PBS as above. They were monitored for distress or death at frequent intervals until the 8th day post-challenge.
At the end of each experiment, fishes that died during the experiment were necropsized for examination of the presence of A. hydrophila in the kidney. Kidney samples from each fish were placed into sterile Whirl-Pak bags and weighed. A volume of sterile saline (1:10 ratio, w/v) was added before processing with a stomacher (Lab-Blender 80) for 1 min. The suspensions were further diluted, and 100 μl each of the dilutions was spotted onto tryptic soy agar plates to screen A. hydrophila. After incubation at 35 °C for 24 h, colonies on the plates were tested for cytochrome c oxidase reaction (positive for A. hydrophila) and counted.
RESULTS AND DISCUSSION
Specific antibody production
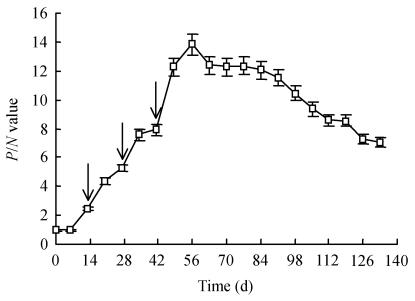
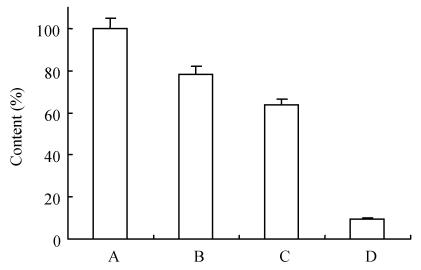
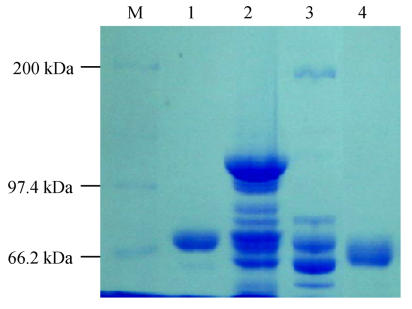
It is well-known that a specific antibody against a particular pathogen can be obtained in large quantities from yolk of eggs laid by hyper-immunized hens. In avian medicine, immunization of hens is often used to transfer maternal antibodies to chicks for preventive purposes. In the food or feed industry, the egg yolk could be utilized directly either for nutritional supplements or for therapeutic purposes because of its high IgY content (Larsson and Carlander, 2003; Camenisch et al., 1999; Tini et al., 2002). Therefore, the approach for use of yolk IgY in aquatic medicine seems to be plausible. ELISA test showed that the specific antibody started to increase in the egg yolk at day 13 from the 1st immunization (P/N=2.18), reached the peak at day 56 (P/N=13.82), and kept at high level until day 133 (P/N=7.03) (Fig.1). This is similar to the findings by Lee et al.(2002) and Kariyawasam et al.(2004) who reported that the specific IgY from egg yolk could be detectable at the 14th day post-immunization. The antibody response in the yolk is delayed as compared to occurrence in the serum after immunization (Patterson et al., 1962). The recovery rate of IgY in the crude extract of the pooled egg yolks in the period of days 49~90 was 78.3%, and the rate was 63.5% upon further purification with 33% (NH4)2SO4 precipitation of the crude extract (Fig.2). SDS-PAGE analysis showed substantial decrease of irrelevant proteins upon extraction with water or purification with saturated ammonium sulphate solution (Fig.3). A 1 mg/ml solution of the purified IgY had a titer of 1:64 as estimated by ELISA using A. hydrophila as the coating antigen. These results indicate that IgY is highly specific to A. hydrophila. The motility shift of purified IgY seen as its lower MW (Fig.3, lane 4) than those of lanes 1, 2 and 3 may be due to structural changes as a result of treatment with saturated ammonium sulphate.
Fig. 1.
Changes of IgY titers over time following immunizations
The arrows (↓) indicate the booster injections. Each data point represents the average of three determinations of three eggs pooled and the error bars, the standard error of means
Fig. 2.
Recovery of immunoreactive IgY
A: Egg yolk pooled; B: Crude extract of IgY by water dilution; C: Purified IgY by 33% saturated ammonium sulfate; D: IgY from non-immunized hens
Fig. 3.
Coomassie blue stained SDS-PAGE gel of IgY extracted from egg yolk
M: Molecular weight marker; 1: Reference IgY; 2: Egg yolk in 10× dilution distilled water at pH 5.3; 3: Suspension of pellets from “2” after centrifugation; 4: Suspension from 33% saturated ammonium sulfate precipitates
In vitro inhibition of A. hydrophila growth
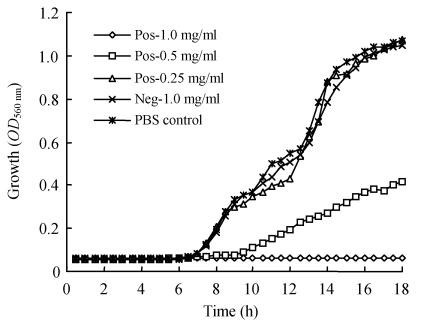
To determine the effect of the specific IgY against A. hydrophila, we compared its growth rate in the presence of specific IgY using the IgY from non-immunized hens as controls. Fig.4 indicates that the growth of A. hydrophila was completely inhibited at a concentration of 1.0 mg/ml specific IgY during the 18 h incubation. A concentration of 0.5 mg/ml was inhibitory to A. hydrophila as shown by a longer lag phase and less steep slope. However, the control IgY did not show any inhibitory effect at the level of 1 mg/ml. Sugita-Konishi et al.(1996) and Shin et al. (2002) reported that IgY obtained from hens immunized with a mixture of formalin-treated Pseudomonas aeruginosa and Helicobater pylori inhibited the growth of both pathogens. Production of Staphylococcus aureus enterotoxin A and adhesion of Salmonella typhimurium serovar Enteritidis to cultured human intestinal cells were also inhibited in the presence of corresponding IgY. The IgY antibodies inhibited the colonization of teeth by Streptococcus mutans, thus preventing plaque formation in humans (Hatta et al., 1997). However, there is no report so far on the use of IgY in the prevention or treatment of A. hydrophila infections in aquatic species.
Fig. 4.
Effect of specific IgY (Pos) on growth of A. hydrophila in vitro using non-specific IgY (Neg) and phosphate buffered saline (PBS) as controls
Passive protection of fishes
Table 1 indicates that 6 out of 10 fishes (60%) pretreated with A. hydrophila specific IgY 4 h were protected against challenge with A. hydrophila while no protection was seen in the control fishes injected with PBS or n-IgY during the 8-day period. The death of fishes treated with s-IgY ceased at the 3rd day post-challenge. Moreover, the number of A. hydrophila in kidney samples of dead fishes treated with s-IgY was substantially lower (4.6×103 cfu/g) than the control dead fishes (2.9×106 cfu/g) and n-IgY group (3.1×106 cfu/g). These results suggest that s-IgY was protective against infection by agglutinating, and/or killing the bacteria in fishes. For treatment of fishes pre-exposed to A. hydrophila, 3 out of 10 fishes injected with s-IgY survived with an efficacy of 30% and none of the fishes in the PBS and n-IgY group survived (Table 1). Death of fishes in the s-IgY treated group ceased at the 4th day, and the count of A. hydrophila in kidney samples of fishes treated with s-IgY was also less than that from the PBS and n-IgY treated fishes. Lee et al.(2000) reported that intraperitoneal injection of anti-Yersinia ruckeri at a dose of 4 mg was effective against an immersion challenge with 108 cfu/ml of the homologous strain. However, Korbsrisate et al.(2002) found that the polyclonal antibodies against A. hydrophila strains 76 and 236 could agglutinate 21 different O antigen serogroups with no specific serogroups preference and rough strains as well as untypable Aeromonas strains.
Table 1.
Efficacy of specific IgY (s-IgY) and non-specific IgY (n-IgY) against A. hydrophila infections when used before challenge (Prevention) or after challenge (Treatment) as evaluated by protection rate and average bacterial numbers in the kidney in the fishes died of the infections
| Groups | Days post-challenge |
Protection (%) | Average bacterial No. in died fishes’ kidney (cfu/g) | ||||
| 1 | 2 | 3 | 4~8 | ||||
| Prevention | PBS | 8 | 2 | 0 | 0 | 0/10 (0) | 2.9×106 |
| s-IgY | 3 | 1 | 0 | 0 | 6/10 (60) | 4.6×103 | |
| n-IgY | 9 | 1 | 0 | 0 | 0/10 (0) | 3.1×106 | |
| Treatment | PBS | 8 | 2 | 0 | 0 | 0/10 (0) | 3.2×106 |
| s-IgY | 5 | 1 | 1 | 0 | 3/10 (30) | 5.6×104 | |
| n-IgY | 9 | 1 | 0 | 0 | 0/10 (0) | 4.5×106 | |
The above findings reveal that specific IgY was more effective against A. hydrophila infections in fishes when used before challenge with the virulent strain than when applied after challenge. Direct inhibition of the challenged bacteria by specific IgY present in the body might have contributed to the efficacy as shown by the in vitro growth inhibition study (Fig.4). The antibody might also function to prevent the bacterial adhesion or attachment to the host cells, an essential step for the establishment of bacterial infections. However, the protection rate was generally low either in the prevention or treatment experiments. This might be due to the fact that the challenge or pre-exposure dose with virulent A. hydrophila was too high with respect to the antibody concentrations used. Therefore, optimization of the challenge dose and specific IgY concentrations used for passive immunization is expected to generate dose- or concentration-dependent responses. Another factor may be due to the intrinsic bacteriostatic nature of the immunoglobulins, which might give the surviving bacteria chances of re-growth upon continuing decrease of the specific antibody.
In conclusion, the present study clearly indicates that the specific IgY obtained from hens immunized by inactivated A. hydrophila may provide a novel approach to the management of A. hydrophila including of various serotypes infections in aquatic species. However, there are still a number of issues remaining to be addressed before it can be used in the field, such as the mechanisms behind the effects of egg-yolk antibodies (Sugita-Konishi et al., 1996; Carlander et al., 2000), persistence of the antibodies with the body, its efficacy after oral administration and formulations suitable for oral application through feeds.
Acknowledgments
We thank technicians Z.C. Zhou, W.D. Zhou, and Z.D. Jiang at School of Animal Science, Zhejiang University for their assistance during the experiments.
Footnotes
Project (No. 2004C26026) supported by the Science and Technology Department of Zhejiang Province, China
References
- 1.Akita EM, Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J Immunol Methods. 1993;160(2):207–214. doi: 10.1016/0022-1759(93)90179-B. [DOI] [PubMed] [Google Scholar]
- 2.Asha A, Nayak DK, Shankar KM, Mohan CV. Antigen expression in biofilm cells of Aeromonas hydrophila employed in oral vaccination of fish. Fish Shellfish Immunol. 2004;16(3):429–436. doi: 10.1016/j.fsi.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Austin B, Austin DA, Dalsgaard I, Gudmundsdottir BK, Hoie S, Thornton JM, Larsen JL, O′Hici B, Powell R. Characterization of atypical Aeromonas salmonicida by different methods. Syst Appl Microbiol. 1998;21(1):50–64. doi: 10.1016/s0723-2020(98)80008-8. [DOI] [PubMed] [Google Scholar]
- 4.Camenisch G, Tini M, Chilov D, Kvietikova I, Srinivas V, Caro J, Spielmann P, Wenger RH, Gassmann M. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. FASEB J. 1999;13(1):81–88. doi: 10.1096/fasebj.13.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Carlander D, Kollberg H, Wejaker PE, Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res. 2000;21(1):1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HQ, Lu CP. Study on pathogen of bacterial hemorrhagic septicemia of rice eel. Chinese Journal of Zoonoses. 1991;7(4):21–23. (in Chinese) [Google Scholar]
- 7.Chowdhury MA, Yamanaka H, Miyoshi S, Shinoda S. Ecology of mesophilic Aeromonas spp. in aquatic environments of a temperate region and relationship with some biotic and abiotic environmental parameters. Zentralbl Hyg Umweltmed. 1990;190(4):344–356. [PubMed] [Google Scholar]
- 8.Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217–223. doi: 10.1007/978-3-7091-6553-9_23. [DOI] [PubMed] [Google Scholar]
- 9.Elwitigala JP, Higgs DS, Namnyak S, White JW, Yaneza A. Septic arthritis due to Aeromonas hydrophila: case report and review of the literature. Int J Clin Pract. 2005;59(s147):121–124. doi: 10.1111/j.1368-504X.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez CJ, Santos JA, Garcia-Lopez ML, Gonzalez N, Otero A. Mesophilic aeromonads in wild and aquacultured freshwater fish. J Food Prot. 2001;64(5):687–691. doi: 10.4315/0362-028x-64.5.687. [DOI] [PubMed] [Google Scholar]
- 11.Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, Hirasawa M, Katz J, Childers NK, Michalek SM. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31(4):268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- 12.Hennig-Pauka I, Stelljes I, Waldmann KH. Studies on the effect of specific egg antibodies against Escherichia coli infections in piglets. Dtsch Tierarztl Wochenschr. 2003;110(2):49–54. [PubMed] [Google Scholar]
- 13.Ikemori Y, Kuroki M, Peralta RC, Yokoyama H, Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli . Am J Vet Res. 1992;53(11):2005–2008. [PubMed] [Google Scholar]
- 14.Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding Panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27(2):332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 15.Kampfer P, Christmann C, Swings J, Huys G. In vitro susceptibilities of Aeromonas genomic species to 69 antimicrobial agents. Syst Appl Microbiol. 1999;22(4):662–669. doi: 10.1016/S0723-2020(99)80019-8. [DOI] [PubMed] [Google Scholar]
- 16.Kariyawasam S, Wilkie BN, Gyles CL. Resistance of broiler chickens to Escherichia coli respiratory tract infection induced by passively transferred egg-yolk antibodies. Vet Microbiol. 2004;98(3-4):273–284. doi: 10.1016/j.vetmic.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Korbsrisate S, Dumnin S, Chawengkirttikul R, Gherunpong V, Eampokalap B, Gongviseisoog C, Janyapoon K, Lertpocasombat K, Shimada T. Distribution of Aeromonas hydrophila serogroups in different clinical samples and the development of polyclonal antibodies for rapid identification of the genus Aeromonas by direct agglutination. Microbiol Immunol. 2002;46(12):875–879. doi: 10.1111/j.1348-0421.2002.tb02775.x. [DOI] [PubMed] [Google Scholar]
- 18.Krüger C, Pearson SK, Kodama Y, Vacca Smith A, Bowen WH, Hammarstrom L. The effects of egg-derived antibodies to glucosyltransferases on dental caries in rats. Caries Res. 2004;38(1):9–14. doi: 10.1159/000073914. [DOI] [PubMed] [Google Scholar]
- 19.Kuroki M, Ohta M, Ikemori Y, Icatlo FCJr, Kobayashi C, Yokoyama H, Kodama Y. Field evaluation of chicken egg yolk immunoglobulins specific for bovine rotavirus in neonatal calves. Arch Virol. 1997;142(4):843–851. doi: 10.1007/s007050050123. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Larsson A, Carlander D. Oral immunotherapy with yolk antibodies to prevent infections in humans and animals. Ups J Med Sci. 2003;108(2):129–140. [PubMed] [Google Scholar]
- 22.Lee SB, Mine Y, Stevenson RM. Effects of hen egg yolk immunoglobulin in passive protection of rainbow trout against Yersinia ruckeri . J Agric Food Chem. 2000;48(1):110–115. doi: 10.1021/jf9906073. [DOI] [PubMed] [Google Scholar]
- 23.Lee EN, Sunwoo HH, Menninen K, Sim JS. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult Sci. 2002;81(5):632–641. doi: 10.1093/ps/81.5.632. [DOI] [PubMed] [Google Scholar]
- 24.Marquardt RR, Jin LZ, Kim JW, Fang L, Frohlich AA, Baidoo SK. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol Med Microbiol. 1999;23(4):283–288. doi: 10.1016/S0928-8244(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 25.Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease. J Med Food. 2002;5(3):159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 26.Patterson R, Youngner JS, Weigle WO, Dixon FJ. Antibody production and transfer to egg yolk in chickens. J Immunol. 1962;89:272–278. [PubMed] [Google Scholar]
- 27.Rosenberger JK, Fries PA, Cloud SS. In vitro and in vivo characterization of avian Escherichia coli. III. Immunization. Avian Dis. 1985;29(4):1108–1117. doi: 10.2307/1590465. [DOI] [PubMed] [Google Scholar]
- 28.Sarker SA, Casswall TH, Juneja LR, Hoq E, Hossain I, Fuchs GJ, Hammarstrom L. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr. 2001;32(1):19–25. doi: 10.1097/00005176-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Shin JH, Yang M, Nam SW, Kim JT, Myung NH, Bang WG, Roe IH. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2002;9(5):1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith DJ, King WF, Godiska R. Passive transfer of immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infect Immun. 2001;69(5):3135–3142. doi: 10.1128/IAI.69.5.3135-3142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugita-Konishi Y, Shibata K, Yun SS, Hara-Kudo Y, Yamaguchi K, Kumagai S. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Biosci Biotechnol Biochem. 1996;60(5):886–888. doi: 10.1271/bbb.60.886. [DOI] [PubMed] [Google Scholar]
- 32.Tini M, Jewell UR, Camenisch G, Chilov D, Gassmann M. Generation and application of chicken egg-yolk antibodies. Comp Biochem Physiol A Mol Integr Physiol. 2002;131(3):569–574. doi: 10.1016/S1095-6433(01)00508-6. [DOI] [PubMed] [Google Scholar]
- 33.van Nguyen S, Umeda K, Yokoyama H, Tohya Y, Kodama Y. Passive protection of dogs against clinical disease due to Canine parvovirus-2 by specific antibody from chicken egg yolk. Can J Vet Res. 2006;70(1):62–64. [PMC free article] [PubMed] [Google Scholar]
- 34.Yokoyama H, Peralta RC, Diaz R, Sendo S, Ikemori Y, Kodama Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect Immun. 1992;60(3):998–1007. doi: 10.1128/iai.60.3.998-1007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]