Abstract
Objective: To construct a novel kind of nonviral gene delivery vector based on polyethylenimine (PEI) conjugated with polypeptides derived from ligand FGF with high transfection efficiency and according to tumor targeting ability. Methods: The synthetic polypeptides CR16 for binding FGF receptors was conjugated to PEI and the characters of the polypeptides including DNA condensing and particle size were determined. Enhanced efficiency and the targeting specificity of the synthesized vector were investigated in vitro and in vivo. Results: The polypeptides were successfully coupled to PEI. The new vectors PEI-CR16 could efficiently condense pDNA into particles with around 200 nm diameter. The PEI-CR16/pDNA polyplexes showed significantly greater transgene activity than PEI/pDNA in FGF receptors positive tumor cells in vitro and in vivo gene transfer, while no difference was observed in FGF receptors negative tumor cells. The enhanced transfection efficiency of PEI-CR16 could be blocked by excess free polypeptides. Conclusion: The synthesized vector could improve the efficiency of gene transfer and targeting specificity in FGF receptors positive cells. The vector had good prospect for use in cancer gene therapy.
Keywords: FGF receptors, Polyethylenimine, Targeting, Gene delivery, Gene therapy
INTRODUCTION
At present, the choice of gene delivery vectors is the bottleneck in gene therapy (El-Aneed, 2004). Great concern on the safety and immunogenecity of viral vectors make nonviral vectors more attractive. Among kinds of nonviral vectors, polyethylenimine (PEI) has shown high transfection efficiency both in vitro and in vivo and attracted great attention (Boussif et al., 1995; Neu et al., 2005). But compared with viral vectors, PEI shows lower gene delivery efficiency, higher toxicity and lack of tumor cells targeting ability.
There are several strategies to modify PEI such as PEGylation, link to ligands and so on (Wagner, 2004; Read et al., 2005a). Among these strategies, linking ligands which bind specific receptor on cell membrane to PEI appear most attractive. Ligand-receptor mediated gene delivery enhances the targeting ability and transfection efficiency of PEI/pDNA polyplexes. There are researches on the linkage of ligands such as EGF, NGF, mannose, galactose, transferrin, folate, monoclonal antibody, etc. (Wagner, 2004). But to our knowledge, there is little attention on the linkage of FGF onto PEI.
In this study, the FGF receptor-targeting polypeptide CR16 (CYRSRKYTSWYVALKR) was linked to the sequence of FGF molecules (Plotnikov et al., 2000) onto PEI to form PEI-CR16 polymer. Chemical characterization, gene transfection efficiency and the tumor targeting ability of PEI-CR16 polymers in vitro and in vivo were investigated.
MATERIALS AND METHODS
Materials
Polyethylenimine (PEI, 25 kDa), SPDP ((N-Succinimidyl-3-(2-pyridyldithio))pro-pionate), DMF and DMSO were obtained from Sigma-Aldrich (St. Louis, MO, USA). The polypeptides CR16 (CYRSRKYTSWYVALKR, MW=2080) were synthesized by GL Biochem (Shanghai) Ltd. Plasmid pCAG-Luc encoding luciferase was bought from Promega. HepG2 (FGF receptors positive), a human hepatocellular carcinoma cell line and PC-3 (FGF receptors negative), a human prostate adenocarcinoma cell line, were obtained from American Type Culture Collection ATCC (Rockville, MD). Female BALB/C athymic nude mice (4~6 weeks old) were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China).
Synthesis of PEI-CR16
PEI (4.5 mg/ml, 5.6 ml) was reacted with SPDP (1.25 mg) in a mixture of NS and DMSO at room temperature for 1 h under the protection of nitrogen in the dark. When the reaction was finished, CR16 (1.56 mg) was mixed with the product of the first reaction at room temperature for 4 h under the protection of nitrogen gas. The product was dialyzed with the dialysis membrane (MW cutoff 10000) for 24 h and lyophilized for 48 h. The product was preserved at −20 °C.
1H nuclear magnetic resonance (1H NMR) assay
Five milligrams of the product was subjected to 8 scans 1H NMR analysis with Bruker 400 MHz NMR spectrometer at room temperature.
Fourier transformed infrared spectroscopy (FT-IR) assay
FT-IR spectra were examined on an FT-IR spectrometer (Spectrum 2000, PERKIN ELMER).
Cell viability assay
HepG2 and PC-3 cells were grown in DMEM supplemented with 10% FCS at 37 °C, 5% CO2 in 96-well plates (Falcon, Becton Dickison, USA) at an initial density of 5000 cells/well; 24 h later, the growth medium was replaced by 200 μl serum-free DMEM containing PEI or PEI-CR16 with different concentrations (0, 1, 2, 4, 5, 10, 20, 50 and 100 μg/ml). After 36 h incubation, the cells viability was measured by MTT assay. The relative cell growth (%) of control cells cultured in media without PEI or PEI-CR16 was calculated by ([A] test/[A] control)×100.
Agarose gel electrophoresis assay
Varying amounts of polymers were mixed with 0.2 μg of pDNA in 5% glucose solution. Gel electrophoresis was carried out at room temperature in TAE buffer in 1% (w/w) agarose gel at 100 V for 30 min. pDNA bands were visualized by a UV illuminator.
Particle size test
Polyplexes of PEI/pDNA and PEI-CR16/pDNA were prepared at DNA concentration of 0.333 μg/ml, N/P ratio was 5, 10, 20 and 30, respectively for samples. Sizes were measured with 90 Plus Particle Size Analyzer (Brookhaven Instruments Corporation, USA) at 25 °C temperature. Scattering light was detected at 90° angle.
In vitro gene transfer
HepG2 cells were cultured in 48-well plates (Falcon, Becton Dickison, USA) at a density of 2.5×104/well with 0.8 ml DMEM containing 10% FCS. Twenty-four hours later, the culture medium was replaced with 0.8 ml serum-free DMEM and the polyplexes of PEI/pCAG-Luc or PEI-CR16/pCAG-Luc with different N/P ratios (5, 10, 20 and 30) and containing 1 μg pCAG-Luc was added to each well. The polyplexes were incubated with the cells for 36 h at 37 °C. After removing the incubation medium, cells of each well were rinsed with PBS and frozen thawing in 200 μl PBS at −80 °C. The luciferase activity in cells extracts was measured using a luciferase assay kit (Promega). Measurements were made in a single-well luminometer (Berthold Lumat LB 9507, Germany) for 10 s. The relative light units (RLUs) were normalized by determining the total protein of the extracts. PC-3 cells which express no FGF receptors on the cell membrane were used as negative control. Different concentrations of CR16 (0, 10, 50 and 100 nmol/L) were employed to investigate the free oligopeptide competitive inhibition effect by 3 h co-incubation before the procedure of gene transfer.
In vivo gene transfer
Female BALB/C athymic nude mice (4~6 weeks of age) were used to in vivo gene transfer. Two million HepG2 or PC-3 cells (in 100 μl saline) were locally injected into the dorsal site of the mice. When the size of tumor reached to 8 mm, the polyplexes of PEI/pDNA or PEI-CR16/pDNA containing 50 μg pCAG-Luc with N/P ratio of 20 (in a volume of 100~150 μl) were injected into the tumors. After 36 h, the mice were sacrificed and the tumors tissue samples were taken out and homogenized with 1 ml PBS and then subjected to three freeze-thaw cycles. The RLUs of the luciferase in the lysate were determined as the methods in in vitro experiments.
Statistical analysis
Unless noted otherwise, results from in vitro experiments represented at least two independent experiments whereas in vivo experiments utilized six animals per group. All results are expressed as means±SD. A mean with P<0.05 was considered statistically significant. The analysis method was one way ANOVA (SPSS, Version 12.0).
RESULTS
Synthesis and characterization of PEI-CR16
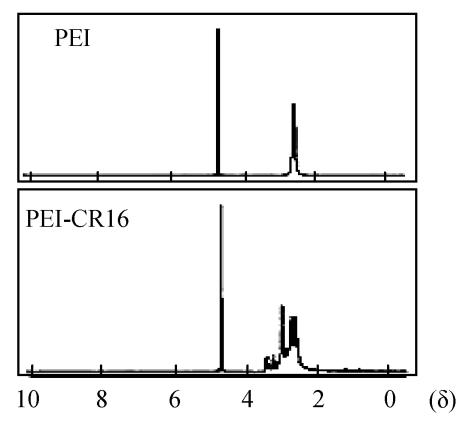
The polymer of PEI-CR16 was synthesized by linking CR16 to the primary amino groups of branch PEI (25 kDa). The molar ratio of PEI to CR16 was 1:0.75. The peaks in the δ range of 3.2~3.5 were assigned to CR16 and the peaks in the δ range of 2.5~3.2 were assigned to PEI in 1H-NMR assay. All of these demonstrated that CR16 was linked to PEI (Fig.1).
Fig. 1.
1H NMR analysis of PEI and PEI-CR16
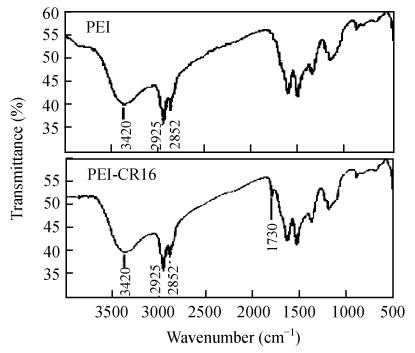
The PEI-CR16 was then determined by FT-IR. The absorbance peak of C=O at 1730 cm−1 showed that CR16 had conjugated to PEI (Fig.2).
Fig. 2.
FT-IR analysis of PEI and PEI-CR16
Toxicity of PEI-CR16
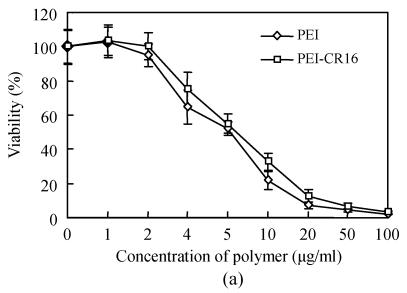
The cytotoxicity of PEI-CR16 was investigated in HepG2 and PC-3 cells by MTT assay. Fig.3 shows that the cytotoxicity of PEI-CR16 was similar to that of PEI. When the concentration increased up to 10 μg/ml or more, the viability of cells was less than 50% (Fig.3).
Fig. 3.
Toxicity of PEI and PEI-CR16 in (a) HepG2 cells and (b) PC-3 cells
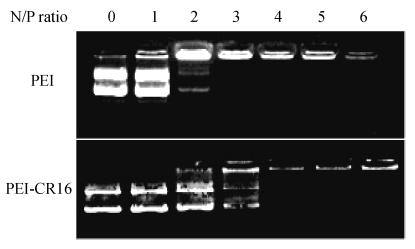
Gel retardation and particle size assay of PEI-CR16
The plasmid DNA condensing ability of PEI-CR16 was detected by gel retardation assay. DNA was fully retarded at N/P ratio of 3 for PEI, and for PEI-CR16, it was at N/P ratio of 4 (Fig.4).
Fig. 4.
Gel retardation assay of PEI and PEI-CR16
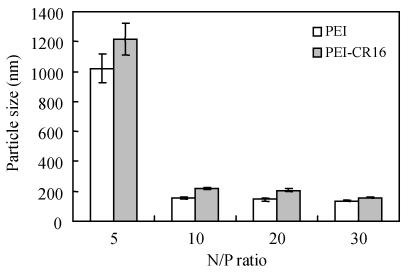
The particle size is shown in Fig.5. At N/P ratio of 10, the particle size was about 200 nm. At the same N/P ratio, the particle size of the polyplexes formed by PEI/pDNA was less than that of PEI-CR16 (P<0.05, n=10).
Fig. 5.
Particle size of the polyplexes complexed with pDNA by PEI and PEI-CR16
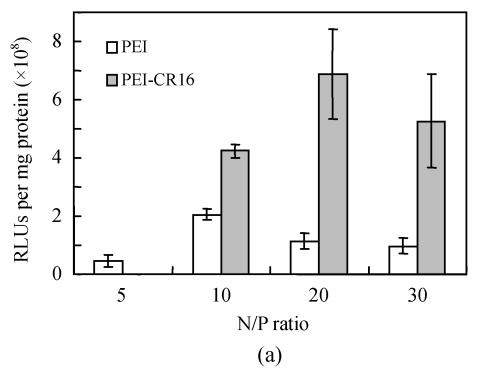
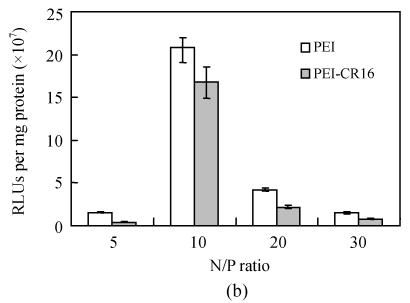
PEI-CR16 mediated gene transfer in vitro
Fig.6 shows the transgene efficiency of PEI and PEI-CR16 in HepG2 cells. It was twice that of PEI at N/P ratio of 10, and when the N/P ratio was 20, the efficiency was at least 6 times, but in PC-3 cells, it was lower than PEI.
Fig. 6.
Transfection efficiency of PEI and PEI-CR16 in (a) HepG2 cells and (b) PC-3 cells
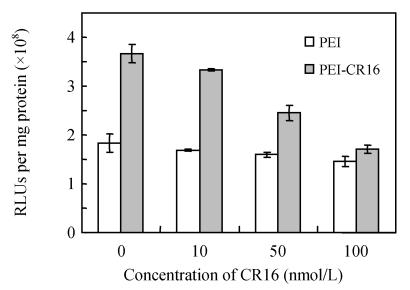
The results of free CR16 competitive inhibition (Fig.7) indicated that free CR16 showed the inhibition effect on the transgene activity of PEI-CR16 dependent on the concentration of free peptides, while no inhibition effect was observed on the transgene activity of uncoupled PEI.
Fig. 7.
Transfection inhibition effect of free oligopeptide CR16 in HepG2 cells
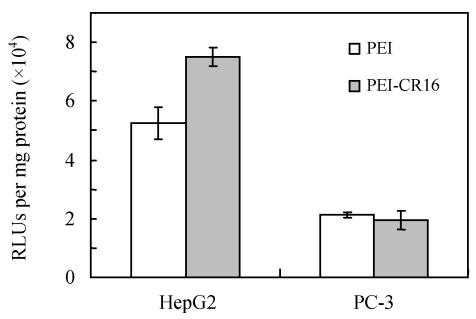
PEI-CR16 mediated gene transfer in vivo
In the HepG2 tumor model, which highly expressed FGF receptors, the luciferase activity in the tumor tissues after intratumoral injection of the PEI-CR16/pCAG-luc complexes was significantly higher than that in PEI/pCAG-luc (P=0.003, n=6). On the contrary, in PC-3 tumor model which expressed FGF receptors weakly, no significant difference of luciferase activity was observed between the PEI-CR16 group and the PEI control group (Fig.8).
Fig. 8.
Transfection efficiency of PEI and PEI-CR16 in vivo with tumor model with HepG2 cells or PC-3 cells
DISCUSSION
Since Boussif et al.(1995) developed PEI as the hope for non-viral vector in gene delivery, PEI has become the golden criteria in the field of non-viral gene transfer vectors (Read et al., 2005b). To make PEI more suitable for gene therapy, modifying PEI with ligands is one of the useful strategies, targeting specially to the receptors on cell membrane to improve the targeting ability of PEI. It is the most prospective future in gene therapy (Jeong et al., 2005).
FGF receptors are highly expressed on most tumor cells and up-regulated in endothelial cells proliferating during angiogenesis and are attractive targets for cancer gene therapy (Chandler et al., 1999). Though some papers reported FGF receptors targeting viral vectors with results coinciding with the retargeting of the vectors and the improvement of gene expression (Chandler et al., 2000), there is little research on the modification of PEI with FGF. In this study, the oligopeptides were designed from the same sequence of the FGF molecule’s core fragment in order to diminish the steric hindrance of the whole FGF molecule.
PEI has abundant amine groups, so in the synthesis system, the reaction was nearly complete between PEI and CR16. The molar ratio of CR16 to PEI in PEI-CR16 complexes could be obtained by referring to the theoretically calculated results (0.75:1).
The particle size of the polyplexes for gene delivery was about 200 nm and was affected by the type of polymer, size, modification of the carrier, and the protocol of complex formation. Considering the balance of factors the suitable diameter of polyplexes with more than 100 nm and less than 300 nm is suitable for the transfection (Munier et al., 2005). Our results indicated the diameter of polyplexes prepared with PEI-CR16 and pDNA was suitable for transfection.
HepG2 cells and PC-3 cells were used to verify the gene transfer ability of PEI-CR16 and explore the role of the oligopeptide CR16. In our preliminary experiments, cell immunocytochemistry was used to screen the FGF receptors expression conditions in different cells including HepG2, PC-3 cells. The results showed that the positive expression of FGF receptors in HepG2 cells and the negative expression of FGF receptors in PC-3 cells, accorded with the results of Chandler et al.(1999) (data not showed). In vitro gene delivery experiments, we found that the most suitable N/P ratio was 10 for PEI and 20 for PEI-CR16. But even at N/P ratio of 10, the transfection efficiency of PEI-CR16 was higher than that of PEI. The difference between PEI-CR16 and PEI might have resulted from the relatively looser pDNA condensing ability and larger particle size. The relatively lower efficiency of PEI-CR16 on PC-3 cells was due to the fact that the linkage of CR16 had no receptor-mediated effect on PC-3 cells. But the effect of linkage on the steric hindrance of the peptide might decrease the efficiency. And, the free peptides CR16 added to the cell culture before the transfection showed efficient inhibition effect on the transgene ability of PEI-CR16, indicating that the combination of CR16 and FGF receptors mediated the enhanced transfection activity of PEI-CR16 in HepG2 cells. When PEI was modified with ligands, the pathway of polyplexes binding to cells will be receptor mediated replacement of simple static electricity process partly and thus enhances the efficiency (Grosse et al., 2004). The mechanism of receptors-mediated gene transfer is very complex and not elucidated completely, including extracellular and intracellular pathways, but according to some papers, the extracellular part may play a more important role (Wagner, 2004).
Preliminary research on gene delivery efficiency and the targeting ability of PEI-CR16 was investigated in vivo. As we expected, in tumor-bearing nude mice inoculated with HepG2 cells, the transgene expression of reporter gene in tumor tissues transfected by PEI-CR16 was higher than that of PEI mediated transfection. In the PC-3 tumor model, PEI-CR16 showed no enhanced transgene efficiency. But the amplitude of the enhanced transgene efficiency of PEI-CR16 was not so significant compared with the results of in vitro experiments. The reason may be imputed to the interaction of polyplexes with negative charges proteins like matrix proteins and the aggregation of tumors in the parenchyma (Ogris et al., 1999). To decrease such interaction effect, some strategies like PEGylation may be helpful (Tang et al., 2003).
In conclusion, a novel type of nonviral vectors based on PEI and modified with CR16 has been successfully developed which has higher transfection efficiency and FGF receptors retargeting ability. It will hopefully be directly used in gene therapy of cancer whether by local or systemic usage.
Footnotes
Project (Nos. 2001AA217071 and 2003AA216041) supported by the Hi-Tech Research and Development Program (863) of China
References
- 1.Boussif O, Lezoualc′h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandler LA, Sosnowski BA, Greenlees L, Aukerman SL, Baird A, Pierce GF. Prevalent expression of fibroblast growth factor (FGF) receptors and FGF2 in human tumor cell lines. Int J Cancer. 1999;81(3):451–458. doi: 10.1002/(SICI)1097-0215(19990505)81:3<451::AID-IJC20>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, Nesbit M, Crombleholme TM, Herlyn M, Sosnowski BA, et al. FGF2-targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2(2):153–160. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 4.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94(1):1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Grosse S, Aron Y, Honore I, Thevenot G, Danel C, Roche AC, Monsigny M, Fajac I. Lactosylated polyethylenimine for gene transfer into airway epithelial cells: role of the sugar moiety in cell delivery and intracellular trafficking of the complexes. J Gene Med. 2004;6(3):345–356. doi: 10.1002/jgm.515. [DOI] [PubMed] [Google Scholar]
- 6.Jeong JH, Kim SH, Kim SW, Park TG. In vivo tumor targeting of ODN-PEG-folic acid/PEI polyelectrolyte complex micelles. J Biomater Sci Polym Ed. 2005;16(11):1409–1419. doi: 10.1163/156856205774472335. [DOI] [PubMed] [Google Scholar]
- 7.Munier S, Messai I, Delair T, Verrier B, Ataman-Onal Y. Cationic PLA nanoparticles for DNA delivery: comparison of three surface polycations for DNA binding, protection and transfection properties. Colloids Surf B Biointerfaces. 2005;43(3-4):163–173. doi: 10.1016/j.colsurfb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7(8):992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 9.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 10.Plotnikov AN, Hubbard SR, Schlessinger J, Mohammadi M. Crystal structures of two FGF-FGF receptors complexes reveal the determinants of ligand-receptor specificity. Cell. 2000;101(4):413–424. doi: 10.1016/S0092-8674(00)80851-X. [DOI] [PubMed] [Google Scholar]
- 11.Read ML, Logan A, Seymour LW. Barriers to gene delivery using synthetic vectors. Adv Genet. 2005;53:19–46. [PubMed] [Google Scholar]
- 12.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33(9):e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang GP, Zeng JM, Gao SJ, Ma YX, Shi L, Li Y, Too HP, Wang S. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 2003;24(13):2351–2362. doi: 10.1016/S0142-9612(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 14.Wagner E. Strategies to improve DNA polyplexes for in vivo gene transfer: will “artificial viruses” be the answer? Pharm Res. 2004;21(1):8–14. doi: 10.1023/B:PHAM.0000012146.04068.56. [DOI] [PubMed] [Google Scholar]