Despite the fact that trans-meridian travel and shift work are commonplace in our 24/7 society, few controlled studies have addressed the health effects of repeated phase shifts of the biological clock. Shift work [1] and chronic jet-lag [2] reduce mental acuity and increase the risk of a number of medical problems including cancer, digestive diseases including peptic ulcers, and sleep disorders. Some of these problems become more severe with the number of years on the job, the result either of cumulative damage or the increased age of the subjects [3]. In general, morbidity associated with many organic disorders is increased in the aged; however, the role played by age-associated alterations in the circadian clock is poorly understood. In particular the effect of repeated schedule changes is largely unaddressed.
We were led to the current experiment by an observation in an unrelated study where we found that 3 of 8 aged transgenic rats exposed to a 6h advance of the light cycle died following the light schedule change. In contrast, no deaths were observed if the light cycle was delayed. In order to explore whether the effects of light schedule changes on longevity were reproducible in a larger study and observable in another rodent species, we placed young (8-12 month old) and aged (27-31 month old) C57BL/6 male mice on one of three lighting regimens for eight weeks. Nine young and 30 aged mice were maintained on a normal 12:12 light-dark cycle. A second group of young (n=9) and old (n=30) mice was exposed to a 6h advance of the light-cycle once every seven days. The third group of young (n=9) and old (n=28) mice was phase-delayed by 6h once every 7 days. The rotating light schedules were chosen to effect large phase adjustments of the circadian system each week, such as would be expected to occur during flight across time zones or in some situations during rotating shift work cycles.
While younger mice fared well on this 8 week schedule (only one death occurred), aged mice were significantly affected by light schedule changes (Fig. 1 A & B). At the end of the 8 week period of light schedule rotations there was 47% survival in animals whose light cycle was advanced each week, 68% in those experiencing delays of the light cycle and 83% in unshifted aged mice (Chi square, all groups, p<0.05 on Day 54). Importantly, chronic stress was not implicated in this phenomenon as total daily fecal corticosterone levels did not increase in aged mice undergoing phase advances or phase delays (see Supplementary Figure 1). To determine whether the effects of phase advances on mortality might be related to the duration between schedule changes, mice were shifted more rapidly, every 4 days. On this schedule, advancers died faster than with weekly shifts (Fig. 1C; 60% survival on Day 24). Delayers fared much better than advancers (chi square p< 0.05 on Day 32). The data suggest that the asymmetry in mortality rates between animals exposed to light schedule advances and delays persists and is possibly enhanced with the shorter inter-shift interval of 4 days.
Figure 1.
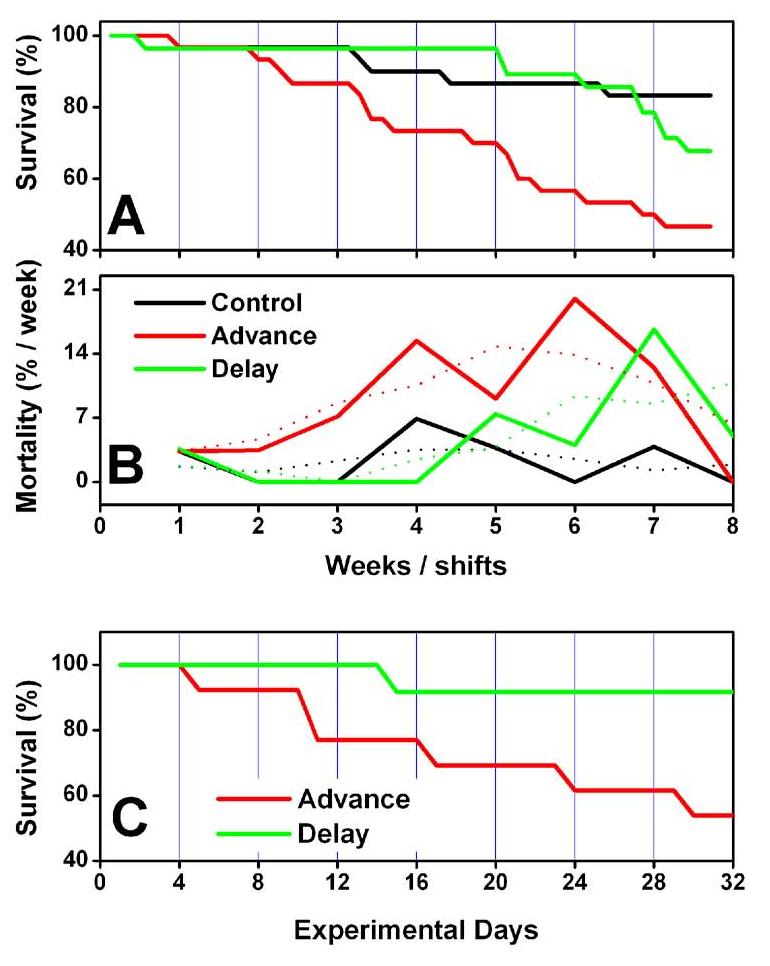
Survival of aged mice undergoing weekly phase shifts of the light-cycle (A) Survival curves of aged mice undergoing a weekly 6h advance or delay adjustment of the light cycle, compared with unshifted aged controls. On Day 56 survival was 47% in advancers, 68% in delayers, and 83% in unshifted aged mice (group sizes are n=30 for controls and advancers and n=28 for delayers). The distribution of surviving mice at the end of Week 4 (p<0.05) Week 5 (p<0.025), Week 6 (p<0.01), Week 7 (p<0.01) and Week 8 (p<0.05) of the protocol is significantly different than chance (Chi square). Advancers died faster than controls (pairwise Chi square; p<0.01, Day 54) but were only different from Delayers at the ends of Weeks 6 (p<0.01) and 7 (p<0.025) (B) Death rate per week of the protocol. % mortality of remaining mice is plotted for each week in bold. Trend-lines (3-point moving average) for each dataset are shown with dotted lines of the same color. Advancers began dying sooner (all 3 groups chi-square; Weeks 3-4, p<0.025; Weeks 5-6 p<0.05) and the death rate remained higher than the other groups until the final week of the protocol. The death rate in unshifted animals was flat for the duration of the experiment. (C) Survival curves for mice shifted every 4 days. We found that advancers still died at a faster rate (p<0.05 on Day 32; group sizes: 13 advancers, 12 delayers).
Our data show that phase-advancing the light cycle hastens the death of aged mice. The mechanism underlying the deleterious effects of phase-advances of the light cycle is unclear. It appears not be stress-related. Other possibilities include sleep deprivation and disruption of the immune system. There is significant complexity in the resetting behavior of the mammalian timing system to phase advances in the light schedule[4] which might play a role in the increased mortality that we observed. In future experiments it will be important to explore how the length of the interval between shifts affects longevity and whether there is reduced longevity in animals that experience light cycle changes when younger.
Non-standard lighting cycles have repeatedly been shown to hasten death in animals. Fruit flies [5] and blowflies [6] have shorter lifespan when housed in L:D cycles with a period shorter than 21h or longer than 27h. Cardiomyopathic hamsters exhibited a median life expectancy that was 11.3% shorter if they were housed on a light schedule that was inverted once per week compared with a stationary 14:10 L:D cycle. However the same shifting schedule did not affect lifespan in CD2F1 mice [7]. A 6h phase-shift in the light-cycle every two days increased the growth rate of Glasgow osteosarcoma in mice [8]. Our report is the first study indicating differential mortality based on the direction of the shift in the light schedule.
Endogenous circadian oscillations have been detected in nearly all mammalian tissues. Our results lead us to speculate that the internal desynchrony among these functional oscillations that accompanies readjustment to an advanced light schedule may have serious health consequences which are exacerbated in the aged. There is evidence that the circadian system of aged animals is altered in significant ways [9]. These age-related circadian changes may have an adverse effect on health during phase advances. Alternately, the general frailty of older animals rather than age-related changes in the circadian system may make them less able to tolerate changes in the light schedule. Whatever the precise mechanism, the dramatic differences in morbidity associated with phase advances of the biological clock raises important issues about the safety of counter-clockwise rotating shift work and the potential long-term health consequences for airline crews regularly crossing time zones.
Supplementary Material
Acknowledgements
The authors wish to thank Denise Holmes and Naomi Ihara for technical assistance and Debbie Roach for her helpful comments on the manuscript. This work was supported by NIA grant F32 AG22741-01 to AJD, NINDS grant RO1 NS051278 to SY, NSBRI grant NCC9-58-167 and NIMH grant RO1 MH56647 to MM, and NIMH grant RO1 MH062517 to GDB.
References
- 1.COSTA G. SHIFT WORK AND OCCUPATIONAL MEDICINE: AN OVERVIEW. OCCUP MED (LOND) 2003;53:83–88. doi: 10.1093/occmed/kqg045. [DOI] [PubMed] [Google Scholar]
- 2.RAFNSSON V, TULINIUS H, JONASSON JG, HRAFNKELSSON J. RISK OF BREAST CANCER IN FEMALE FLIGHT ATTENDANTS: A POPULATION-BASED STUDY (ICELAND) CANCER CAUSES CONTROL. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 3.LISKOWSKY DR. FROM THE CONGRESSIONAL OFFICE OF TECHNOLOGY ASSESSMENT. JAMA. 1992;268:3047. doi: 10.1001/jama.268.21.3047. [DOI] [PubMed] [Google Scholar]
- 4.NAKAMURA W, YAMAZAKI S, TAKASU NN, MISHIMA K, BLOCK GD. DIFFERENTIAL RESPONSE OF PERIOD 1 EXPRESSION WITHIN THE SUPRACHIASMATIC NUCLEUS. J NEUROSCI. 2005;25:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PITTENDRIGH CS, MINIS DH. CIRCADIAN SYSTEMS: LONGEVITY AS A FUNCTION OF CIRCADIAN RESONANCE IN DROSOPHILA MELANOGASTER. PROC NATL ACAD SCI U S A. 1972;69:1537–1539. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VON SAINT PAUL U, ASCHOFF J. LONGEVITY AMONG BLOWFLIES PHORMIA TERRAENOVA R.D. KEPT IN NON-24-HOUR LIGHT-DARK CYCLES. J. COMP. PHYSIOL. 1978;127:191–195. [Google Scholar]
- 7.NELSON W, HALBERG F. SCHEDULE-SHIFTS, CIRCADIAN RHYTHMS AND LIFESPAN OF FREELY-FEEDING AND MEAL-FED MICE. PHYSIOL BEHAV. 1986;38:781–788. doi: 10.1016/0031-9384(86)90043-0. [DOI] [PubMed] [Google Scholar]
- 8.FILIPSKI E, DELAUNAY F, KING VM, WU MW, CLAUSTRAT B, GRECHEZ-CASSIAU A, GUETTIER C, HASTINGS MH, LEVI F. EFFECTS OF CHRONIC JET LAG ON TUMOR PROGRESSION IN MICE. CANCER RES. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 9.YAMAZAKI S, STRAUME M, TEI H, SAKAKI Y, MENAKER M, BLOCK GD. EFFECTS OF AGING ON CENTRAL AND PERIPHERAL MAMMALIAN CLOCKS. PROC NATL ACAD SCI U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


