Abstract
Background: Alternaria alternata is one of the most common fungi associated with allergic disease. However, Alternaria exposure in indoor environments is not well characterized.
Objective: The primary goals of this study were to examine the prevalence of Alternaria exposure and identify independent predictors of Alternaria antigen concentrations in U.S. homes.
Methods: Data for this cross-sectional study were obtained from the National Survey of Lead and Allergens in Housing. A nationally representative sample of 831 housing units in 75 different locations throughout the U.S. completed the survey. Information on housing and household characteristics was obtained by questionnaire and environmental assessments. Concentrations of Alternaria antigens in dust collected from various indoor sites were assessed with a polyclonal anti-Alternaria antibody assay.
Results: Alternaria antigens were detected in most (95-99%) of the dust samples. The geometric mean concentration, reflecting the average Alternaria concentration in homes, was 4.88 μg/g (SE=0.13 μg/g). In the multivariable linear regression analysis, the age of the housing unit, geographic region, urbanization, poverty, family race, observed mold and moisture problems, use of dehumidifier, and presence of cats and dogs were independent predictors of Alternaria antigen concentrations. Less frequent cleaning and smoking indoors also contributed to higher Alternaria antigen levels in homes.
Conclusion: Exposure to Alternaria alternata antigens in U.S. homes is common. Antigen levels in homes are not only influenced by regional factors but also by residential characteristics. Preventing mold and moisture problems, avoiding smoking indoors, and regular household cleaning may help reduce exposure to Alternaria antigens indoors.
Keywords: Alternaria alternata, allergen, antigen, indoor, exposure, asthma, allergy
INTRODUCTION
Exposure to the fungus Alternaria alternata is an important risk factor for asthma and allergic rhinitis.1-5 Severe asthma and acute, sometimes life-threatening exacerbations of asthma have been associated with Alternaria sensitivity and increased airborne concentrations of Alternaria spores.6-10
Alternaria spores are common aeroallergens in many regions of the world, especially in warm inland climates, but also in arid regions.9,11 Alternaria exposure is often assessed by outdoor spore counts, because most intense exposure is likely to occur outdoors.12–14 Nonetheless, fungal spores can enter a home from outdoor air via ventilation or infiltration, or they can be carried in by occupants.15,16 Infiltration may have less importance for Alternaria spores because of their large size (23-34 μm x 7-10 μm).17 The indoor environment may also become a secondary source of exposure, if fungal spores colonize interior or building materials.15,16 Although indoor fungal levels tend to reflect the levels found outdoors, housing characteristics and occupants' behavior can affect exposure levels considerably.15,16,18,19
Because of the complexity of fungal exposure assessment, few studies have assessed exposure to Alternaria or other fungal allergens in indoor environments.20 Fungal allergen extracts have largely remained uncharacterized and non-standardized, unlike other common allergens derived from cat, dog, dust mite, cockroach, or pollens.21,22 Exposure to fungal allergens is traditionally estimated by indirect methods, considering spores as indicators of the presence of allergens.23 However, allergen content in spores may vary and fungal allergens may also be carried by means other than intact spores (e.g. hyphael fragments).24-26 Therefore, spore counts may not accurately reflect allergen exposure levels. Recent advances in molecular biology and immunology have facilitated progress in qualifying and quantifying fungal allergens, especially Altermaria allergens.16,22,27 The National Survey of Lead and Allergens in Housing (NSLAH) is the first population-based study that measured antigenic components of Alternaria alternata, including allergens, in U.S. homes using a polyclonal anti-Alternaria antibody assay.
This article presents nationally representative estimates of dustborne Alternaria alternata antigen levels at multiple household sites and identifies independent predictors of Alternaria antigen concentrations in U.S. homes.
METHODS
Study data
The data for this study were collected as part of the National Survey of Lead and Allergens in Housing (NSLAH). This cross-sectional study, which was conducted from 1998 to 1999 by the NIEHS and the U.S. Department of Housing and Urban Development, used a complex, multistage design to sample the U.S. population of permanently occupied, non-institutional housing units that permit children. The study protocol was approved by the NIEHS Institutional Review Board in 1998. The sampling frame of 1,404 primary sampling units (PSUs) consisted of Metropolitan Statistical Areas (MSAs), counties, or groups of counties. MSAs included areas with a large population nucleus and adjacent communities having a high degree of economic and social integration with the area. Every area in the 50 states and the District of Columbia was assigned to a PSU. A nationally representative random sample of housing units was drawn from 75 randomly selected primary sampling units. In all, 831 housing units were surveyed. A detailed description of the methodology for the survey has been previously published.28
At each home, a trained interviewer obtained information on housing characteristics and the occupants' household via questionnaire. A copy of the questionnaire can be found on-line at http://www.niehs.nih.gov/airborne/research/risk.html. Environmental data were also acquired by inspection and sample collection. Detailed, well-defined protocols for all aspects of data and sample collection were utilized throughout the study.28 Briefly, single surface dust samples were collected from a bed (all bedding layers, pillow, and mattress or mattress cover), a sofa or a chair, and from bedroom, living room, and kitchen floors as previously described.28 Vacuumed dust samples were collected using a Eureka Mighty-Might® 7.0-ampere vacuum cleaner (Eureka Company, Bloomington, Illinois) modified to collect dust into a 19 × 90 mm cellulose extraction thimble (Whatman International, Ltd., England). Each sampling site was vacuumed for 5 minutes. For bedding samples, all bedding layers were vacuumed for a total of 2.5 minutes, the primary sleeping pillow for 30 seconds, and the mattress or mattress cover for 2 minutes.
At the laboratory, dust samples were sieved through 425 μm pore grating and divided into 100 mg aliquots of fine dust. Dust aliquots were extracted in borate buffered saline (pH 8.5), 2 ml per 100 mg dust extracted. After extracts were centrifuged, supernantants were decanted and stored at -20°C. Concentrations of the Alternaria alternata antigens were measured with a competitive inhibition enzyme-linked immunosorbent assay (ELISA) using a commercially prepared polyclonal rabbit anti-Alternaria antibody (Greer Laboratories, Inc., Lenoir, North Carolina; lot# ZA4-4L) and Alternaria antigen standard (Greer Laboratories, Inc., Lenoir, North Carolina; lot# XPM1-X10).20,24 Briefly, antigen standard at 1 μg/ml in bicarbonate buffer, pH 9.6, was added to 96-well Immunlon 4HBX plates (VWR Scientific) overnight at 4°C. Unbound antigen was washed away (with phosphate buffered saline, pH 7.4) and the plate was blocked with bovine serum albumin. After washing, anti-Alternaria antibody, along with either dilutions of unknown samples or dilutions of the antigen standard, were combined in the wells and incubated overnight at 4°C. Unbound material was washed away and peroxidase-labeled goat anti-Rabbit IgG (Sigma Chemical, St. Louis, Missouri) was added to the wells and incubated for 1 hour. Excess antibody was washed away and substrate added; color change was measured kinetically at 405 nm using an OptiMax plate reader (Molecular Devices, Sunnyvale, California). Reaction rates of the unknown sample were plotted against those of the antigen standard to determine concentration. Optimal assay dilutions were determined empirically using dilution matrices. The assay detects major Alternaria antigens, including the most common allergen, Alt a1.29 For most samples, the lower limit of detection of the assay was 0.14 μg/g/sieved dust.
Statistical analyses
Statistical analyses were conducted using SUDAAN (Version 8.0, Research Triangle Institute, Research Triangle Park, NC), and Taylor series linearization methods were used to adjust standard errors for the complex survey design. Sample weights were applied to all estimates to account for housing selection probabilities, non-response, and poststratification. A detailed description of statistical weighing for the NSLAH is described elsewhere.28
In the statistical analysis, Alternaria antigen concentrations were log-transformed because the distributions were skewed to the right. Samples with concentrations less than the detection limit were assigned one-half of the value of the detection limit. Samples having insufficient amount of dust for analysis were considered missing (see Fig 1). We used Spearman rank correlation coefficients to evaluate associations between antigen concentrations. We calculated a house index (i.e. the mean of all sampling location concentrations) to represent the average Alternaria antigen concentration in the household.
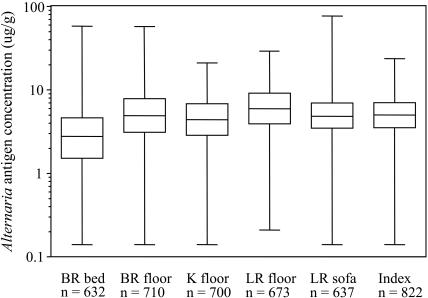
FIG 1.
Distributions of Alternaria antigen concentrations in U.S. homes. Box plots display the minimum and maximum values and the 25th, 50th, and 75th percentiles. Bedroom is abbreviated as BR, kitchen as K, and living room as LR. Index refers to the house index.
Descriptive statistics of Alternaria alternata antigen concentrations were generated. Median and mean (geometric) concentrations were estimated for each level of selected household characteristics. Comparisons of the log-transformed means were assessed with ANOVA using Wald's F statistics. Characteristics in Tables I and II with p-values less or equal to 0.25 were selected to multivariable linear regression. In the data driven modeling approach, backward elimination strategy was used for model selection. All remaining predictors in the final model had p-values less than or equal to 0.05. For each level of independent predictor, geometric mean of Alternaria antigen concentration was computed by adjusting for other predictors in the model.
Table I.
Geometric means of Alternaria concentrations (house index* ) by demographic characteristics
| Characteristic | Number of homes | GM (SE)† (μg/g) | p-value‡ |
|---|---|---|---|
| Total | 822 | 4.88 (0.13) | -- |
| Construction year | <0.001 | ||
| 1978-1998 | 216 | 4.21 (0.17) | |
| 1977 or earlier | 606 | 5.22 (0.17) | |
| Census region | <0.001 | ||
| Northeast | 151 | 4.59 (0.46) | |
| Midwest | 195 | 5.42 (0.30) | |
| South | 276 | 5.21 (0.22) | |
| West | 200 | 4.06 (0.19) | |
| Urbanization | <0.001 | ||
| MSA§ | 684 | 4.53 (0.14) | |
| Non-MSA | 138 | 6.16 (0.20) | |
| Housing unit type | <0.001 | ||
| Multi-family | 124 | 4.01 (0.24) | |
| Single family | 698 | 5.04 (0.15) | |
| Tenure | 0.036 | ||
| Renter occupied | 282 | 4.58 (0.17) | |
| Owner occupied | 537 | 5.01 (0.16) | |
| Number of persons in the household | 0.870 | ||
| 1-3 | 543 | 4.89 (0.14) | |
| 4-9 | 279 | 4.85 (0.24) | |
| Children (< 18 years) | 0.188 | ||
| No | 423 | 4.97 (0.16) | |
| Yes | 396 | 4.74 (0.16) | |
| Census poverty | 0.002 | ||
| No | 643 | 4.76 (0.16) | |
| Yes | 136 | 5.67 (0.26) | |
| Race | <0.001 | ||
| White | 603 | 5.10 (0.16) | |
| Other | 219 | 4.11 (0.16) | |
| Education | 0.008 | ||
| Above high school | 553 | 4.67 (0.17) | |
| High school or less | 255 | 5.38 (0.19) |
House index is the mean of the sample location concentrations
GM indicates geometric mean, (SE) standard error of the mean
Wald F-test on difference of means across levels of the characteristic
Metropolitan Statistical Area
Table II.
Geometric means of Alternaria concentrations (house index) by allergen-related housing and behavioral characteristics
| Characteristic | Number of homes | GM (SE)* (μg/g) | p-value† |
|---|---|---|---|
| Total | 822 | 4.88 (0.13) | -- |
| Main heating source | 0.003 | ||
| Gas/Electric forced air | 556 | 4.68 (0.15) | |
| Radiator | 74 | 4.77 (0.56) | |
| Other | 189 | 5.64 (0.24) | |
| Air filtration device used | 0.547 | ||
| No | 702 | 4.87 (0.15) | |
| Yes | 101 | 5.04 (0.27) | |
| Mold or moisture problems‡ | <0.001 | ||
| No | 398 | 4.43 (0.15) | |
| Yes | 424 | 5.38 (0.18) | |
| Dehumidifier in the home | 0.001 | ||
| No | 676 | 4.70 (0.15) | |
| Yes | 130 | 5.87 (0.34) | |
| Cats or dogs currently | 0.016 | ||
| No | 455 | 4.66 (0.18) | |
| Yes | 359 | 5.17 (0.15) | |
| Windows/doors kept open in the past month | 0.920 | ||
| No | 132 | 4.91 (0.23) | |
| Yes | 685 | 4.88 (0.14) | |
| Season | 0.682 | ||
| Summer | 285 | 4.90 (0.26) | |
| Fall | 344 | 4.75 (0.23) | |
| Winter | 193 | 5.05 (0.28) | |
| Smoking inside the home | 0.027 | ||
| No smoking indoors | 536 | 4.61(0.19) | |
| Light smoking§ | 75 | 4.89 (0.34) | |
| Heavy smoking∥ | 208 | 5.60 (0.28 ) |
GM indicates geometric mean, (SE) standard error of the mean
Wald F-test on difference of means across levels of the characteristic
Assessed by observation (occupants, field team)
Tobacco products smoked indoors < 4 times a day
Tobacco products smoked indoors 4 or more times a day
RESULTS
Prevalence and distribution of Alternaria antigen levels in U.S. homes
The majority (≥ 95%) of the dust samples had detectable levels of Alternaria antigens. Figure 1 shows a statistical summary of Alternaria antigen concentrations in U.S. homes. Spearman rank correlation coefficients between Alternaria concentrations at the five sampling locations ranged from 0.16 (bedroom bed vs. kitchen floor) to 0.47 (living room floor vs. upholstery). Correlations between Alternaria concentrations at each sampling locations and the house index (which reflects the average Alternaria antigen concentration across all sampling locations in a home) were between 0.59 and 0.73 (p<0.0001). In our analyses, we used the house index as our primary exposure measure.
Alternaria antigen levels and demographic, household, and behavioral characteristics
We investigated associations between Alternaria antigens and various demographic factors by comparing the geometric mean concentration of the house index across levels of the characteristics presented in Table I. Higher Alternaria antigen concentrations were present in older homes, homes in the Midwest and South census regions, non-urban homes, single family homes, owner occupied homes, homes in impoverished census areas, homes inhabited by white individuals, and homes inhabited by individuals with less education. In contrast to most of the demographic characteristics, the presence of children and the number of occupants were not associated with higher concentrations of Alternaria antigens. Table II shows geometric mean concentrations (house index) by additional housing and behavioral characteristics that are thought to be associated with antigen levels. Concentrations of Alternaria antigens were considerably lower (p=0.003) in homes that used forced air heating systems or radiators as the main heating source than in homes which used other heating sources (e.g. kerosene space heaters, wood burning stoves/fireplaces). Alternaria antigen concentrations were consistently higher in homes where either residents or the field team observed signs of mold or moisture related problems, such as mold or water stains, musty or mildew odor, or dampness in the home (p<0.001). The use of a dehumidifier was strongly associated with higher Alternaria levels (p=0.001). The presence of cats or dogs in the home was also associated with higher Alternaria antigen levels (p=0.016). Smoking indoors increased mean antigen concentrations significantly (p=0.027). Antigen concentrations increased with increasing smoking frequency.
We also examined associations between Alternaria antigen levels and the household characteristics in each sampling location separately because some of the characteristics are site-specific. Less frequent cleaning was associated with higher Alternaria antigen levels in all locations. In particular, Alternaria concentrations were significantly (p<0.05) higher in living rooms where the floor cleaning frequency was less often than weekly. Alternaria levels were also higher in beds where bedding had not been washed within the past week (p=0.01). Type of flooring affected Alternaria levels differently depending on the site. Alternaria concentrations were significantly higher in kitchens with carpeting than in kitchens without carpeting (p=0.001). On the contrary, carpeting in bedrooms predicted much lower Alternaria antigen concentrations in beds (p=0.004).
Independent predictors of Alternaria antigen levels in U.S. homes
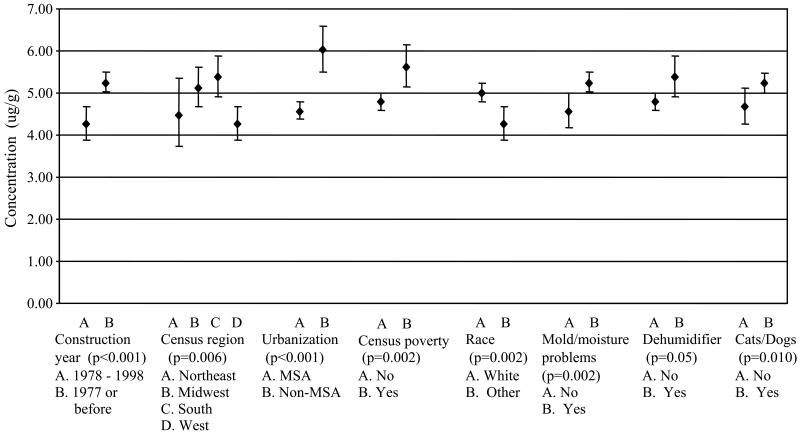
Multivariable linear regression was used to identify independent predictors of higher mean concentrations of Alternaria antigens in U.S. homes. The following predictors remained in the final model: construction year, region, degree of urbanization, poverty, family race, signs of mold and moisture problems, use of dehumidifier, and presence of cats or dogs in the home. The results for the house index are summarized in Figure 2, which shows adjusted mean concentrations of the independent predictors.
FIG 2.
Adjusted mean (geometric) concentrations of Alternaria antigens (μg/g) for the house index (N=822) and 95% confidence intervals for levels of the independent predictors identified in the multivariable linear regression model. MSA indicates Metropolitan Statistical Area.
DISCUSSION
This survey is the first study that assessed dustborne Alternaria alternata antigen concentrations in the U.S. housing stock. Alternaria antigens were present in virtually all homes. Both regional and residential characteristics influenced Alternaria antigen concentrations. Based on our data driven prediction model, the age of the housing unit, census region, degree of urbanization, poverty, race of residents, observed mold and moisture problems in the home, use of dehumidifier, and presence of cats and dogs contributed independently to Alternaria levels. Our results suggest that antigens of Alternaria may deposit in house dust from various sources and via multiple mechanisms.
Regional factors were strongly associated with Alternaria levels. Homes in non-metropolitan statistical areas had significantly higher Alternaria antigen concentrations than homes in metropolitan statistical areas. Alternaria alternata is a ubiquitous saprophyte that is found in the soil and on plants, especially on decaying vegetation.12, 30,31 Therefore, Alternaria antigen levels likely reflect the abundance of local vegetation. The regional variation in Alternaria antigen concentrations was also substantial; antigen concentrations were clearly higher in Midwestern and Southern homes. Region is considered as an important determinant for fungal concentrations, both indoors and outdoors.32 Our results are consistent with previous findings that Alternaria spores are plentiful in grain-growing areas in the Midwest.6
Alternaria antigen concentrations were significantly higher in older homes than in newer homes. Because new homes are more tightly built than older ones, fewer spores may be able to penetrate to indoor environments. Furthermore, newer homes are more likely to be better equipped to provide improved control of environmental factors (e.g. temperature, humidity) indoors. Of the demographic factors, race and poverty had the greatest influence on Alternaria levels, both white race and low income contributing consistently to higher Alternaria levels in U.S. homes.
Mold and moisture related problems in the home were strongly associated with higher Alternaria antigen levels. Although outdoor air is considered the dominant source for indoor fungal spores, indoor sources may increase Alternaria antigen levels considerably.15,16 Fungal growth indoors is influenced by various environmental factors. However, the most important factor controlling fungal growth is water availability.33 Water leaks, defective drainage, inadequate ventilation and moisture condensation resulting from faulty thermal insulation, and heating, cooling and ventilation (HVAC) systems have been major contributors to moisture related fungal problems in buildings.34,35 Prolonged high relative humidity has been shown to increase both dust- and airborne fungal populations in indoor environments.36-38 Alternaria antigen concentrations were significantly higher in homes that used dehumidifiers. The presence of a dehumidifier is likely an indication of an ongoing humidity or moisture problem, although dehumidifiers may also become reservoirs for fungi. In this study, increased humidity per se was not associated with Alternaria antigen levels (data not shown). However, this is not necessarily surprising because relative humidity was measured at only one point in time. Dehumidifier use may reflect long-term humidity levels or water availability better than a single relative humidity measurement.
Outdoor allergenic spores such as those of Alternaria can be carried on residents' hair, skin, clothing, or shoes as well as on their pets' fur. Chew and coworkers found that the presence of a dog increased fungal populations in floor dust.39 Our results agree with their findings. The presence of dogs was associated with higher Alternaria concentrations especially in living room floor and upholstery dusts. Dogs may spend more time in living rooms than in other rooms in the home. In our data, the presence of cats was also associated with higher Alternaria antigen levels.
Predictors of Alternaria antigen concentrations may vary by location because the activities of occupants, humans and pets, can affect each location in the home differently. For example, having carpeting in bedrooms predicted lower Alternaria antigen concentrations in beds, whereas kitchens with carpeting had significantly higher Alternaria antigen levels than kitchens without carpeting. Carpeting in the kitchen may provide favorable environment for fungal growth because of availability of nutrients (e.g. food and beverage residues). Frequent cooking may also result in higher temperature and humidity levels in kitchens. While carpeting may reduce particle resuspension, possibly explaining the reduced levels in the beds of carpeted bedrooms, it can be a reservoir or an amplification site for fungi.19, 39 Furthermore, the presence of children predicted higher Alternaria antigens levels in beds (p=0.015) but not in floor or upholstery dusts (data not shown). Children are more likely exposed to potential outdoor sources of the fungus than adults, because they usually spend more time outdoors than adults.40 Spores and fungal fragments could be carried into bed on children's hair and/or clothing. Although indoor smoking did not reach statistical significance in the final model for the house index, it was a strong predictor for higher Alternaria levels in bed dust. Smoking was also significantly (p=0.03) associated with higher Alternaria antigen levels in living rooms where smoking most likely occurs.41
Less frequent cleaning contributed to higher Alternaria antigen levels in floor and upholstery dusts, especially in living rooms. Correspondingly, levels of Alternaria antigens in beds were lower if bedding was washed more frequently. Washing temperature (cold, warm, hot) did not influence antigen levels (data not shown).
In cross-sectional studies, the temporal sequence of cause and effect cannot necessarily be determined. It is possible that the results from a dust sampling conducted at a single point in time may not represent exposure throughout the entire year because fungal exposure is prone to temporal, particularly seasonal, and spatial variations.37,39 However, settled dust samples are often used as surrogate measures for long-term exposure because they are considered less influenced by temporal and spatial variability than air samples.42,43 Sampling in the study was conducted throughout summer, fall, and winter months in each geographic region in order to capture the seasonal variability in the data. We acknowledge that one limitation of the study is that we cannot determine the variablity in Alternaria antigen exposure in different geographic regions in the U.S. because of limited number of homes in each region. Although our approach does not allow for assessments of seasonal variability in antigen levels in individual homes, this is the most cost-effective method of sampling for a large-scale national survey that requires in-person home visits. For other common indoor allergens, measurement of allergen concentration in reservoir dust has generally been used as the standard index of exposure. The presence of missing values is another limitation of the study. Insufficient amount of dust to assay Alternaria alternata antigens contributed most to the missing values. We maximized the number of samples in our analyses by using imputed values for the samples that had concentrations less than the detection limit. To evaluate potential bias, we conducted our analyses excluding the imputed values. The final prediction model remained the same in both analyses.
Although monoclonal antibody-based assays are more sensitive and specific for a single allergenic protein, for example for Alt a 1, an important advantage of a polyclonal assay is that it captures the allergen variability that is characteristic to fungal allergen exposure. Allergenic fungi, including Alternaria alternata, express great variability in allergen profiles depending on the environmental conditions under which they grow.24, 29 For example, Barnes et al.24 observed substantial discrepancies in GP70 and Alt a1 levels over time when these two glycoproteins were measured in air samples. The life cycle of fungi can also affect the different patterns of allergen release; germination has been shown to increase allergen release from Alternaria spores.44 We acknowledge that polyclonal antibody-based assays have their disadvantages; polyclonal antibodies are directed only toward antigens recognized by source species, they can be variable in composition and potency, and cross-reactivity is possible.16 Because there is a finite supply of the polyclonal antibody that we used, it may not be possible to reproduce the findings of this study in the future. However, some previous studies that have used monoclonal antibody-based assays have had difficulties to detect Alternaria allergens in environmental samples,27 even among populations where Alternaria sensitivity and exposure to Alternaria spores are known to be common.27,45,46 Furthermore, Alternaria species -sensitive subjects elicit positive skin test reactions to other Alternaria allergens,47,48 not only to Alt a 1.
The major strength of this study is that the survey sample is nationally representative. The weighted characteristics of the survey sample, including distributions of housing characteristics, socioeconomic, and demographic factors, did not differ significantly from nationwide characteristics obtained from other national surveys,28 which strengthens the external validity of our findings. To improve the internal validity, quality assurance was integrated into all components of the study; sampling procedures, data collection and analysis followed detailed, well-defined protocols.28 The NSLAH is the first study to estimate levels of Alternaria antigens in the U.S. housing stock. The NSLAH data provide valuable new information on exposure to Alternaria antigens in indoor environments, although further validation of fungal immunoassays is warranted in order to determine clinically relevant exposure levels in the future. Immunoassays that are used to assess fungal allergen exposures have not yet achieved the same reliability as have similar assays for other allergens.21
In conclusion, Alternaria antigens are commonly detected in U.S. homes. Antigen levels are influenced not only by regional and housing characteristics but also by residents' behavior. The age of the housing unit, census region, urbanization, poverty, family race, signs of mold and moisture problems, use of dehumidifier, and presence of cats and dogs can affect Alternaria antigen levels significantly. In addition, the frequency of cleaning activities in the home contributes to Alternaria antigen concentrations. Smoking indoors may also increase Alternaria antigen levels in home environments. Preventing mold and moisture related problems, avoiding indoor smoking, and regular household cleaning could potentially lower Alternaria antigen concentrations in homes.
ACKNOWLEDGEMENTS
We thank Westat Inc. for their assistance with conduct of the field component of the survey. We acknowledge Drs. Steve Kleeberger and Glinda Cooper for their helpful comments.
Footnotes
This study was funded by the National Institute of Environmental Health Sciences and the U.S. Department of Housing and Urban Development.
- NIEHS
- National Institute of Environmental Health Sciences
- NSLAH
- National Survey of Lead and Allergens in Housing
- PSU
- Primary sampling unit
- MSA
- Metropolitan Statistical Area
- ANOVA
- Analysis of Variance
REFERENCES
- 1.Licorish K, Novey HS, Kozak P, Fairshter RD, Wilson AF. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol. 1985;76:819–25. doi: 10.1016/0091-6749(85)90755-9. [DOI] [PubMed] [Google Scholar]
- 2.Halonen M, Stern DA, Wright AL, Taussig LM, Marinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care. 1997;155:1356–61. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 3.Committee on the assessment of asthma and indoor air . Clearing the Air: Asthma and Indoor Air Exposures. National Academy Press; Washington, DC: 2000. Indoor Biologic Exposures; pp. 105–222. [Google Scholar]
- 4.Perzanowski MS, Sporik R, Squillace SP, Gelber LE, Call R, Carter M, et al. Association of sensitization to Alternaria allergens with asthma among school-age children. J Allergy Clin Immunol. 1998;101:626–32. doi: 10.1016/S0091-6749(98)70170-8. [DOI] [PubMed] [Google Scholar]
- 5.Andersson M, Downs S, Mitakakis T, Leuppi J, Marks G. Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatr Allergy Immunol. 2003;14:100–5. doi: 10.1034/j.1399-3038.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Eng J Med. 1991;324:359–63. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 7.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, Matteucci RM, Anderson PR, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–35. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999;103:709–11. doi: 10.1016/s0091-6749(99)70247-2. [DOI] [PubMed] [Google Scholar]
- 9.Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leüppi JD, et al. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–9. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 10.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 2002;325:411–4. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sneller MR, Hayes HD, Pinnas JL. Frequency of airborne Alternaria spores in Tuscon, Arizona over a 20-year period. Ann Allergy. 1981;46:30–3. [PubMed] [Google Scholar]
- 12.Corden JM, Millington WM. The long-term trends and seasonal variation of the aeroallergen Alternaria in Derby, UK. Aerobiologia. 2001;17:127–36. [Google Scholar]
- 13.Mitakakis TZ, Tovey ER, Xuan W, Marks GB. Personal exposure to allergenic pollen and mould spores in inland New South Wales, Australia. Clin Exp Allergy. 2000;30:1733–9. doi: 10.1046/j.1365-2222.2000.00966.x. [DOI] [PubMed] [Google Scholar]
- 14.Ripe E. Mould allergy. Acta Allergol. 1962;17:130–59. [PubMed] [Google Scholar]
- 15.Sterling DA, Lewis RD. Pollen and fungal spores indoor and outdoor of mobile homes. Ann Allergy Asthma Immunol. 1998;80:279–85. doi: 10.1016/S1081-1206(10)62971-7. [DOI] [PubMed] [Google Scholar]
- 16.Bush RK, Portnoy JM. The role of and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107:S430–40. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- 17.de Hoog GS. On the Potentially Pathogenic Dematiaceous Hyphomycetes. In: Howard DH, Howard, LF, editors. Fungi Pathogenic for Humans and Animals. Marcel Dekker; New York: 1983. pp. 149–53. [Google Scholar]
- 18.Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998;28:459–67. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 19.Dharmage S, Bailey M, Raven J, Mitakakis T, Thien F, Forbes A, et al. Prevalence and residential determinants of fungi within homes in Melbourne, Australia. Clin Exp Allergy. 1999;29:1481–9. doi: 10.1046/j.1365-2222.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 20.Barnes C, Tuck J, Simon S, Pacheco F, Hu F, Portnoy J. Allergenic materials in the house dust of allergy clinic patients. Ann Allergy Asthma Immunol. 2001;86:517–23. doi: 10.1016/S1081-1206(10)62899-2. [DOI] [PubMed] [Google Scholar]
- 21.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–34. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Horner WE, Helbling A, Salvaggio JE, Lehrer SB. Fungal allergens. Clin Microbiol Rev. 1995;8:161–79. doi: 10.1128/cmr.8.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burge HA, Rogers CA. Outdoor Allergens. Environ Health Perspect. 2000;108(Suppl 4):653–9. doi: 10.1289/ehp.00108s4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes C, Schreiber K, Pacheco F, Landuyt J, Hu F, Portnoy J. Comparison of outdoor allergenic particles and allergen levels. Ann Allergy Asthma Immunol. 2000;84:47–54. doi: 10.1016/S1081-1206(10)62740-8. [DOI] [PubMed] [Google Scholar]
- 25.Flückiger B, Koller T, Monn C. Comparison of airborne spore concentrations and fungal allergen content. Aerobiologia. 2000;16:393–6. [Google Scholar]
- 26.Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol. 2003;111:285–9. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- 27.Vailes L, Sridhara S, Cromwell O, Weber B, Breitenbach M, Chapman M. Quantitation of the major fungal allergens, Alt a1 and Asp f 1, in commercial allergenic products. J Allergy Clin Immunol. 2001;107:641–6. doi: 10.1067/mai.2001.114118. [DOI] [PubMed] [Google Scholar]
- 28.Vojta PJ, Friedman W, Marker DA, Clickner R, Rogers JW, Viet SM, et al. First National Survey of Lead and Allergens in Housing: Survey design and methods for the allergen and endotoxin components. Environ Health Perspect. 2002;110:527–32. doi: 10.1289/ehp.02110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portnoy J, Pacheco F, Barnes C, Upadrashta B, Crenshaw R, Esch R. Selection of representative Alternaria strain groups on the basis of morphology, enzyme profile, and allergen content. J Allergy Clin Immunol. 1993;91:773–82. doi: 10.1016/0091-6749(93)90197-n. [DOI] [PubMed] [Google Scholar]
- 30.Mitakakis T, Ong EK, Stevens A, Guest D, Knox RB. Incidence of Cladosporium, Alternaria and total fungal spores in the atmosphere of Melbourne (Australia) over three years. Aerobiologia. 1997;13:83–90. [Google Scholar]
- 31.Thomma BPHJ. Alternaria spp.: from general saprophyte to specific parasite. Mol Plant Pathol. 2003;4:225–36. doi: 10.1046/j.1364-3703.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- 32.Shelton BG, Kirkland KH, Flanders WD, Morris GK. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol. 2002;68:1743–53. doi: 10.1128/AEM.68.4.1743-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaughnessy RJ, Morey PR, Cole EC. Prevention and Control of Microbial Contamination. In: Macher J, Ammann HA, Burge HA, Milton DK, Morey PR, editors. Bioaerosols: Assessment and Control. ACGIH; Cincinnati, OH: 1999. [Google Scholar]
- 34.Christian JE, Lstiburek J, Carmody J. A DOE Moisture Control Handbook. In: Bales E, Rose WB, editors. Bugs, Mold & Rot: Proceedings of A Workshop on Residential Moisture Problems, Health Effects, Building Damage, and Moisture Control; Washington, DC: National Institute of Building Sciences. 1991.pp. 59–64. [Google Scholar]
- 35.Salo P. Identifying and preventing fungal contamination problems in new home construction. In: Johanning E, editor. Bioaerosols, Fungi, and Mycotoxins: Health Effects, Assessment, Prevention, and Control. Eastern New York Occupational & Environmental Health Center; Albany, NY: 1999. pp. 561–7. [Google Scholar]
- 36.Wickman M, Gravesen S, Nordvall SL, Pergshagen G, Sundell J. Indoor viable dust-bound microfungi in relation to residential characteristics, living habits, and symptoms in atopic and control children. J Allergy Clin Immunol. 1992;89:752–9. doi: 10.1016/0091-6749(92)90384-e. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T. Airborne fungal colony-forming units in outdoor and indoor environments in Yokohama, Japan. Mycopathologia. 1997;139:23–33. doi: 10.1023/a:1006831111595. [DOI] [PubMed] [Google Scholar]
- 38.Douwes J, van der Sluis B, Doekes G, van Leusden F, Wijnands L, van Strien R, et al. Fungal extracellular polysaccharides in house dust as a marker for exposure to fungi: Relations with culturable fungi, reported home dampness, and respiratory symptoms. J Allergy Clin Immunol. 1999;103:494–500. doi: 10.1016/s0091-6749(99)70476-8. [DOI] [PubMed] [Google Scholar]
- 39.Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 40.Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It's about time: A comparison of Canadian and American time-activity patterns. J Expo Anal Environ Epidemiol. 2002;12:427–32. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 41.Leech JA, Wilby K, McMullen E. Environmental tobacco smoke exposure patterns: a subanalysis of the Canadian Human Time-Activity Pattern Survey. Can J Public Health. 1999;90:244–9. doi: 10.1007/BF03404125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren P, Jankun TM, Leaderer BP. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dust of dwellings in one Northeast American county. J Expo Anal Environ Epidemiol. 1999;9:560–8. doi: 10.1038/sj.jea.7500061. [DOI] [PubMed] [Google Scholar]
- 43.Martyny JW, Marinez KF, Morey PR. Source Sampling. In: Macher J, Ammann HA, Burge HA, Milton DK, Morey PR, editors. Bioaerosols: Assessment and Control. ACGIH; Cincinnati, OH: 1999. [Google Scholar]
- 44.Mitakakis TZ, Barnes C, Roger E. Spore germination increases allergen release from Alternaria. J Allergy Clin Immunol. 2001;107:388–90. doi: 10.1067/mai.2001.112602. [DOI] [PubMed] [Google Scholar]
- 45.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–70. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor GT, Walter M, Mitchell H, Kattan M, Morgan WJ, Gruchalla RS, et al. Airborne fungi in the homes of children with asthma in low-income urban communities: The Inner-City Asthma Study. J Allergy Clin Immunol. 2004;114:599–606. doi: 10.1016/j.jaci.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 47.Portnoy J, Olson I, Pacheco F, Barnes C. Affinity purification of a major Alternaria allergen using a monoclonal antibody. Ann Allergy. 1990;65:109–14. [PubMed] [Google Scholar]
- 48.Bush RK, Sanchez H, Geisler D. Molecular cloning of a major Alternaria alternata allergen, rAlt a 2. J Allergy Clin Immunol. 1999;104:665–71. doi: 10.1016/s0091-6749(99)70340-4. [DOI] [PubMed] [Google Scholar]