Abstract
We asked whether the ability to keep in working memory the binding between a visual object and its spatial location changes with development across the life span more than memory for item information. Paired arrays of colored squares were identical or differed in the color of one square and, in the latter case, the changed color was unique on that trial (item change) or was duplicated elsewhere in the array (color-location binding change). Children (8–10 and 11–12 years old) and older adults (65–85 years old) showed deficits relative to young adults. These were only partly simulated by dividing attention in young adults. The older adults had an additional deficiency, specifically in binding information, which was evident only when item- and binding-change trials were mixed together. In that situation, the older adults often overlooked the more subtle, binding-type changes. Some working-memory processes related to binding undergo life-span development in an inverted U shape, whereas other, bias- and salience-related processes that influence the use of binding information seem to develop monotonically.
Older adults have a memory deficiency compared to young adults, specifically in the retention of binding information. When items are presented in pairs, the items can be remembered normally but there is difficulty in remembering which ones were paired with which others. This appreciable decline occurs for the binding of focal items to contextual elements (Bayen, Phelps, & Spaniol, 2000; Chalfonte & Johnson, 1996; Mitchell, Johnson, Raye, Mather & D’Esposito, 2000; Naveh-Benjamin, 2000; Naveh-Benjamin & Craik, 1995) or the binding of different focal elements to each other (Naveh-Benjamin, 2000; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003 a; Naveh-Benjamin, Guez, Kilb, & Reedy, 2004 a). Naveh-Benjamin (2000), extending the work by Chalfonte & Johnson (1996), suggested an associative deficit hypothesis, which focuses on the distinction between memory for single units and memory for associations among units. The present study examines the generality of the associative deficit hypothesis for children as well as the older adults, and for a task examining working memory rather than long-term memory. In this task, two arrays of colored squares must be compared, with the arrays sometimes identical and sometimes differing in the color of a single square. This task has been extensively researched in young adults (Luck & Vogel, 1997; Morey & Cowan, 2004, in press; Todd & Marois, 2004; Vogel & Machizawa, 2004; Vogel, Woodman, & Luck, 2001) and has been examined in infants (Ross-Sheehy, Oakes, & Luck, 2003) and children (Cowan et al., in press).
In our version of the task, haphazardly-placed squares of different colors form a sample array that is soon replaced by a blank interval and then a test array identical to the sample array or differing in the color of a single square. A circle surrounding one square in the test array indicates which square changed color, if any square did, and the required response is a judgment as to whether a color change occurred. Inasmuch as colors are selected for the sample array from a small set, randomly with replacement, a given color can appear more than once in each array. For that reason, a changed color may be unique in the array (an item change) or may be duplicated at some other location in the array (a binding change). These types of changes are illustrated in Figure 1. In the case of item changes, the response would be correct if the participant noticed that a new color had been introduced whereas, in the case of binding changes, the response could be correct only if the participant noticed that an already-present color was now present also at the cued location. In other words, the change in binding between colors and locations would have to be noticed. The task requires brief but vivid retention of the sample array in memory, to be compared to the test array. Performance levels in young adults are excellent with up to about 4 squares per array and quickly drop as a function of array set sizes beyond 4. We have found that it is possible to examine performance on this task in participants from the early years of elementary school through old age; children younger than the elementary school years often do not use the response keys consistently or do not tolerate the relatively large number of trials needed.
Figure 1.
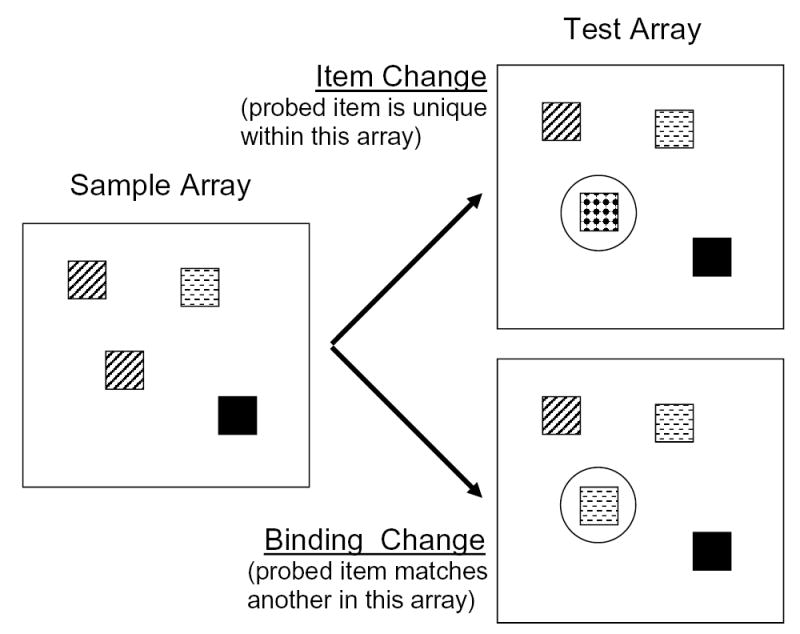
An illustration of two types of stimulus change. Patterns represent colors. In item changes, a new color appears in the test array in the encircled location whereas, in binding changes, the color that appears in the encircled location matches a color already present elsewhere in the array. The drawing is not to scale.
Four key questions about performance in this task are to be investigated here: (1) whether there are age-group deficits in binding in this working memory task, (2) whether the development of binding is shaped as an inverted U across the life span or is monotonic, (3) whether developmental deficits in binding can be mimicked by dividing attention in young adults, and (4) how associative deficits in binding should be described according to a signal-detection analysis. The rationale for these questions follows.
1. Deficits in Binding in a Working-Memory Task
Can an associative deficit in the older adults be found in this visual working memory task, which involves only a short (1-s) retention interval between the two arrays to be compared? We expected that it could, given that working memory presumably underlies long-term learning (Baddeley, Gathercole, & Papagno, 1998; Baddeley, 2000; Cowan, 1995, 2001). In older adults there are few studies of the development of immediate memory for objects in an array and, to our knowledge, none using the procedure of Luck and Vogel (1997). This procedure may provide a direct method of examining the basic capacity of visual working memory, which should change across ages according to the suggestions of Cowan (2001). Mitchell et al. (2000) found a deficit in older adults in both items and locations in a spatiotemporal array.
Olson et al. (2004, Experiment 1) found no such effect in memory for spatial configuration of up to 6 objects but, then, it may have been possible to combine the items into a single spatial design, removing the load from working memory. Specifically, their stimuli were pairs of arrays with 3 or 6 identical squares in each array, with a cued item in the second array. This second array was identical to the first or differed in the location of the cued item (which should lead to a “different” response) and/or differed in the locations of all of the other items in the array. Both younger and older adults found it easier to determine the location of the cued item when the other items remained fixed between the two arrays. Given that each array forms a spatial design that might be remembered in terms of multi-item patterns, it is understandable that no age difference resulted.
In light of the existing literature, the present direct test is still necessary.
2. Life Span Developmental Course of Binding
Is the associative deficit symmetrical across the life span, such that aging reverses the course of child development, as J. Hughlings Jackson expected (Jackson, 1860, reprinted in Taylor, 1958); or is binding only a problem for older adults? When one develops tasks that can be used across a wide age range, from elementary-school children through older adults, it is possible to assess the form of the life-span development of psychological processes (e.g., Gulya, Rossi-George, Hartshor, Vieira, Rovee-Collier, & Johnson, 2002; Cepeda, Kramer, & De Sather, 2001; Massaro, Thompson, Barron, & Laren, 1986; Williams, Ponesse, Schachar, Logan, & Tannock, 1999; Zelazo, Craik, & Booth, 2004).
We have found little recent research specifically on item versus binding memory in children. Most of the relevant recent work has focused on children too young to be tested with the same methods as adults (e.g., see Bauer, Burch, & Kleinknecht, 2002; Moore & Lemmon, 2001). There were some studies in an earlier era with more comparable methods in children and adults (e.g., Goulet, 1968; Lynch & Rohwer, 1972; Keppel, 1964). In general, the research suggests that relatively young children have a deficit in richly encoding pictorial stimuli using covert verbalization. However, the question of whether children have a more basic deficit in binding items to their context, which could be observed in an array-comparison task, remains open.
An expectation of inverted-U-shaped developmental growth comes from the general notion that nervous system decline in old age is a mirror image of nervous system development in childhood. However, specific rates of decline may not mirror the rates of development exactly. For example, the hippocampal system, which is necessary for the storage of episodic memories in a way that allows subsequent explicit recall, declines noticeably in old age but seems to be relatively intact even in elementary-school children. In contrast, the frontal lobes, which largely account for the role of attention in creating rich, explicit memories (for reviews see Cowan, 1995; Kane & Engle, 2002), appear to function more similarly in elementary-school children and older adults, below young adults in both cases (for young children, Nelson, 2002; Rabinowicz, 1980; Saitoh, Karns, & Courchesne, 2001; Smith, Kates, & Vriezen, 1992, for older adults, Raz, 2000; Raz, Rodrigue, Head, Kennedy, & Acker, 2004). If this is so, different profiles of abilities would be predicted in children versus older adults. Children would be less likely than older adults to have memory deficits that are independent of attention (caused by hippocampal deficits) but no less likely to have deficits in the mnemonic use of attention (caused by frontal lobe deficits).
3. The Role of Attention in Developmental Deficits in Binding
A rationale for this question about the role of attention has been established directly above. To the extent that a difference from the performance of young adults is due to poorer attention in some other group, the performance of that group should be mimicked by dividing attention in adults. The different states of neural development in childhood versus aging could lead to an especially informative pattern in which divided attention resembles some aspects of binding (or, perhaps, binding in some age groups) and not others. Indeed, some researchers have proposed that older adults have depleted attentional resources, and have used divided-attention conditions in young adults to simulate the effects of that attentional depletion (e.g., Craik, 1983; 1986; Craik & Byrd, 1982; Rabinowitz, Craik, & Ackerman, 1982).
In the domain of long-term episodic memory, dividing attention in young adults between a tone discrimination task and a long-term memory task has not produced deficits in binding compared to item memory (Naveh-Benjamin, 2000; Naveh-Benjamin, Guez, & Marom, 2003 b; Naveh-Benjamin et al., 2004 a; Naveh-Benjamin, Guez, & Shulman, 2004 b). Instead, dividing attention has caused roughly equal deficits in item and associative recognition. According to some theoretical considerations, though, insufficient attention might be expected to hinder the detection of binding changes more than item changes in working-memory procedures. Treisman and Gelade’s (1980) view suggests that the construction of a mental representation with features bound together into objects should require attention. The basis for this statement is that searches for conjunctions of features take much longer than searches for individual features, with a much higher search slope as a function of the array set size in the search for conjunctions. On the other hand, for the sake of working memory it may be that one can remember only objects, not features per se, in which case a test of feature memory versus memory for bindings between those features should produce similar results. In one recent study on visual working memory for arrays, Wheeler and Treisman (2002) obtained ambiguous results, including a disadvantage for binding trials in some circumstances but not in others.
Given the evidence on brain development cited above, one might expect that the pattern found in children would resemble the divided-attention pattern. It might not be expected in older adults, for whom an associative deficit has been observed (e.g., Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003a, 2003b). A deficit in binding within long-term memory may be linked to the use of hippocampal regions of the brain that are not dependent on attention beyond what is needed for adequate stimulus encoding (e.g., Cohen & Eichenbaum, 1993; Johnson, 1994; Rolls, 1996). The question of what aspects of performance in children or older adults resemble divided-attention conditions in young adults is addressed in the present Experiments 1b and 2b.
4. A Signal Detection Analysis of Binding Deficits
How should the associative deficit be described according to a signal-detection analysis (e.g., Green & Swets, 1966)? That analysis includes the concept of sensitivity, or in our study how well a participant could distinguish between trials in which the two arrays changed versus stayed the same; and bias, or in our study a participant’s proclivity, when in doubt, to indicate that a change occurred. It is of interest whether sensitivity, bias, or both change with age, as it sheds light on the psychological processes involved.
Interpretation of signal detection results depends on the test procedure. In the ideal case for a signal detection analysis, a trial block includes only one kind of trial in which a signal is present (or in this case, one kind of change) and a kind of trial in which the signal is absent (i.e., no change occurs). On every trial, the participant presumably perceives a particular signal strength, or in this case the sense that a change has occurred. This signal strength comes from a summation of the actual signal, if one was present, plus the contribution of various sources of internal and external noise. Given that the participant has no way to know for sure whether that perceived signal strength comes from the distribution of signal trials or no-signal trials, the decision can only be made by setting a criterion and responding accordingly; that is, by indicating that a change has occurred only if the signal strength is higher than the criterion. In many actual experiments, however, there are several types of signal trials mixed together in a trial block. In this situation, the criterion setting can be of considerable interest.
To illustrate the latter case, Figure 2 depicts the situation in which item-change, binding-change, and no-change trials are mixed together in the same trial block. The horizontal axis represents the amount of change sensed, and the vertical axis represents the proportion of trials for each amount of change. The top and bottom panels of the figure depict two theoretical situations with identical sensitivities but different criterion settings. In the top panel, a liberal bias results in correct responses for most trials of all types. In the bottom panel, a conservative bias results in correct responses for most no-change and item-change trials, but incorrect responses for most binding-change trials. In this panel, a small improvement in performance on no-change trials has been obtained at a much larger cost on binding-change trials. Therefore, criterion setting can be of considerable practical importance. To explore the consequences of task demands, we used stimuli with item and binding changes mixed together in the same blocks in Experiments 1a and 1b, and in separate blocks in Experiments 2a and 2b.
Figure 2.
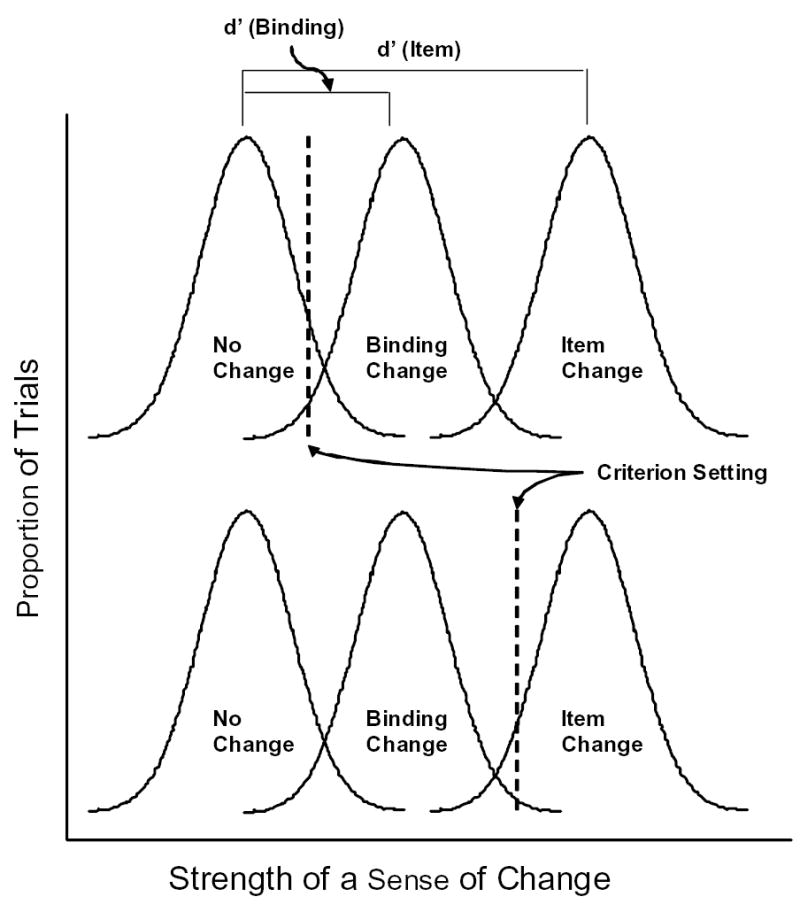
An illustration of the effect of criterion bias on performance in a situation with item-change, binding-change, and no-change trials mixed together. Top panel: a conservative bias allows detection of most item changes and few binding changes. Bottom panel: a liberal bias allows detection of most item changes and also most binding changes. See text for details.
At first glance, one might suppose that performance deficits in older adults in mixed trial blocks could be obtained as a result of deficits in task-switching (e.g., Kramer, Sowon, & Gopher, 1999; Smith et al., 2001). However, participants were not informed whether item or binding changes might occur. Therefore, from the participant’s point of view, even in the mixed-block situation there is only one task, which is to determine if the probed item in the second array has changed color compared to the first array. On every trial, the participant must assume that both item and binding information could be relevant, until the second array arrives and the comparison can be made. Given that the participant has no way to know which type of trial is in process during the retention interval, we assume that task-switching cannot play a role.
Expectations
All plausible theoretical frameworks are likely to make the prediction that children and older adults will display lower levels of sensitivity than young adults. Where theoretical views might diverge is in whether any of the age groups differ from others in the difference in sensitivity between item and binding trials.
In the mixed-block design of Experiments 1a and 1b, any difference between item and binding trials must, in principle at least, produce both sensitivity differences and bias differences. That is because the performance level on no-change trials is the same in the evaluation of both item and binding changes. (Subdividing no-change trials into two sets cannot alter the situation, inasmuch as they are all the same from the participant’s point of view.) Lower performance on binding changes than on item changes, with a common no-change trial type, would result in both lower d’ values for binding and a lower relative tendency to say that there was a change, in the evaluation of binding trials. In contrast, in the separate-block design of Experiments 2a and 2b, there is the opportunity to do better on no-change trials in item blocks than in binding blocks, if participants happen to learn implicitly that all of the changes in the item blocks are more salient than the ones encountered in the binding blocks. That would allow an easier decision in item-change blocks for both change and no-change trials (even though the instructions do not include a discussion of the different types of changes that are possible, in any experiment). Thus, sensitivity and bias can be considered independent of one another in Experiments 2a and 2b.
There are several lines of research leading to different expectations regarding the development of response bias. Young children have a tendency to respond “yes” regardless of the nature of the question, but that trend typically disappears by 3 or 4 years of age (Fritzley & Lee, 2003). In the medical decision literature, older adults have a tendency to avoid risk by deciding in favor of treatment quickly or, if possible, deferring to a physician’s judgment (Curley, Eraker, & Yates, 1984; Leventhal, Leventhal, Schaefer, & Easterling, 1993). However, no difference between young and older adults was obtained in a study in which the decisions were of much smaller practical consequence (Dror, Katona, & Mungur, 1996). In any case, it is difficult to know how to apply the concept of risk to the present task, in which there is no difference in the consequence of incorrect responses on change versus no-change trials.
A concept that seems more relevant is false recognition, the tendency to recognize as old the items that are new, but share some features with old items (e.g., , Kausler, 1994; Rankin & Kausler, 1979). Related to this tendency is the notion that two varieties of memory contribute to responses: familiarity and recollection (cf. Jacoby, 1991). Items that share only some of the features of an old item may still seem familiar, which can result in a positive recognition response even though they cannot be completely recollected. The research literature suggests that older adults do rely on familiarity rather than recollection, more than do younger adults (Hay & Jacoby, 1999; Jennings & Jacoby, 1997). That is true of young children, as well (Anooshian, 1999), with an inverted-U-shaped change in recollection across the life span (Zelazo et al., 2004). The notion of false recognition could apply to the present procedure if a participant thought that the changed item in the second array looked familiar, and therefore judged it to be unchanged. This would be expected to occur most frequently on binding-change trials, because the color of the cued item is familiar in that it was present elsewhere in the first array. Reliance on familiarity cues in older adults therefore would be expected to increase the tendency to miss binding changes. That tendency theoretically could affect the sensitivity, the bias, or both, depending on how no-change trial performance was affected.
Finally, some of the literature suggests that bias does not change independently with age but that it may be a byproduct of age differences in sensitivity, with sensitivity and bias being correlated (Danziger, 1980; Harkins, Chapman, & Eisdorfer; 1979; Le Breck & Baron, 1987). Children can be of use in evaluating that hypothesis if, as expected, they perform at levels comparable to older adults. If bias is a byproduct of sensitivity, then the bias should be similar in groups with similar sensitivities. If, on the other hand, older adults have a specific tendency to engage in false recognition, they could show large differences in bias from children with levels of sensitivity similar to theirs.
In sum, the array-comparison task will be used to examine the life span development of binding in visual working memory with particular scrutiny of the potential roles of attention, sensitivity, and criterion-setting. Experiment 1a examines the life-span development of binding, and Experiment 2a is a modification that allows sensitivity and criterion bias to be examined separately. Experiments 1b and 2b examine the effects of dividing attention in adults, in tasks comparable to 1a and 2a, respectively.
Experiment 1a: Developmental Study Using Mixed Blocks of Conditions
In this first experiment, we assess detection of both item changes (i.e., trials in which the color changed to a new color that was not present in the sample array) and binding changes (i.e., trials in which the color changed to a color already present elsewhere in the sample array, so that a new binding of color and location had to be detected) against a common set of no-change control trials. The relative rate of occurrence of item and binding changes was left up to chance, and about 46% of all changes were item changes. We included children in two age groups for the sake of a life-span comparison because, on the basis of past research related to perceptual memory across the life span (e.g., Gulya et al., 2002; Zelazo et al., 2004), we expected that performance in older adults would be at a level bracketed by these two age groups.
Method
Participants
The participants were 41 third-grade children (19 male, 22 female; mean age: 8.82 years, SD = 0.40), 43 fifth-grade children (17 male, 26 female; mean age: 10.75 years, SD = 0.48), 53 young adults (29 male, 24 female; mean age: 20.43 years, SD = 2.03), and 33 older adults. Child data in only this first experiment came from a larger study (see author notes). Five older females failed to follow instructions, sometimes advancing to the next trial without making a forced response, an option permitted because of a programming mistake corrected in later experiments. The remaining 28 older adults (4 male, 24 female) had a mean age of 71.11 years (SD = 4.56). The younger and older adults were equated on the number of years of formal education (M = 13.92 and 14.39, respectively; SD = 1.74 & 1.59).
The children and the older adults were residents of Columbia, Missouri, whereas the young adults were students at the University of Missouri. The older adults lived independently in the community, reported having good health, and were able to come to the laboratory. They passed a screening questionnaire to eliminate those with memory or thinking problems, stroke, and any degenerative chronic disease (Parkinson, Alzheimer, MS, etc) and were readily able to follow the instructions. All participants reported having good hearing and normal or corrected-to-normal vision. The children were 85% White, 4% Hispanic, 4% Black, 4% Asian, and 2% other or unknown. Information about family structure or siblings is unavailable and is assumed to be unimportant for the present study. The young adults were 88% White, 4% Hispanic, 4% Black, and 4% Asian. Exact figures are unavailable for the older adults but they were approximately comparable to the other groups.
Stimuli, apparatus, and procedure
Testing took place one participant at a time in a sound-attenuated booth equipped with a computer. As in the study of Luck and Vogel (1997), an array of colored squares was presented on a gray screen on each trial, followed by a second array identical to the first or differing in the color of one square. One square was cued (encircled) in the second array and either the two arrays were alike or they differed in the color of the cued square. A single key press response was to indicate whether there was a change or not. Each trial began with a fixation cross that appeared after the participant pressed a key to indicate that the trial should begin. The fixation cross lasted 1 s and was replaced by the first array for 250 ms. At an estimated viewing distance of 50 cm, the array encompassed 9.8 degrees horizontal × 7.3 degrees vertical visual viewing angle, and each colored square within it took up 0.75 × 0.75 degrees. Spatial locations of the squares in the array were random except that the minimum separation between their centers was 2.0 degrees, and no square appeared within 2.0 degrees of the center of the viewing area.
The square colors, red, blue, violet, green, yellow, black, and white, were assigned randomly with replacement so that the same color could appear more than once in an array. Interpolated between arrays was a 1-s gray screen, the same shade as the background behind the color squares. The cue was a 1-pixel-thick, black circle 1.5 degrees in diameter, surrounding one square in the second array. The required response was a computer key press indicating whether or not the color changed between arrays or not (the “/” and “z” keys, respectively). The second array persisted on the screen until a response was made. Eight practice trials were presented, followed by 128 test trials. These included equal numbers of trials with 4, 6, 8, or 10 squares per array, Half of the trials at each array size were change trials. Array sizes were randomly ordered across trials. Each trial ended with response feedback (to keep motivation up and provide an index of maximal performance), and breaks between trials were offered and allowed as needed.
Results and Discussion
Proportion correct
A 4 × 3 × 4 ANOVA was carried out with age group (third grade, fifth grade, young adults, and older adults) as the between-subject factor and two within-subject factors: change condition (item change, binding change, or no change) and the number of items in the array, or array size (4, 6, 8, or 10). Predictably, there were main effects of the age group, F(3, 161) = 24.23, MSE = 0.10, p < .001, change condition, F(2, 322) = 43.77, MSE = 0.07, p < .001, and array size, F(3, 483) = 92.92, MSE = 0.03, p < .001. The age-group trend was curvilinear as expected, with M (and SEM) for third-graders, fifth-graders, young adults, and older adults of .70 (.01), .73 (.01), .83 (.01), and .69 (.02), respectively. Newman-Keuls post-hoc tests showed that the young adults differed from all other groups, which did not differ significantly from one another. Performance in the item-change, binding-change, and no-change trials averaged .78, .65, and .78, respectively (SEM = .01 in each case), so that the effect of change condition was due to the relatively poor performance on binding trials. Performance generally decreased with increasing array set sizes as expected (M = .85, .71, .73, & .66 for set sizes 4, 6, 8, and 10, respectively; SEM = .01 in each case). All pairwise differences were significant by Newman-Keuls tests except for the reversal between Set Sizes 6 and 8.
Most importantly, there was an interaction of Age Group X Change Condition, F(6, 322) = 12.07, MSE = 0.07, p < .001. One can see from Figure 3 that older adults had a different response profile than other participants: they displayed especially low performance on binding-change trials, combined with excellent performance on no-change trials. This same interaction was found in an ANOVA in which no-change trials were omitted, F(3, 161) = 5.72, MSE = 0.07, p < .001. Thus, there is evidence that older adults, but not the children, showed a binding deficit compared to the young adults. Although the item - binding difference was significant in all age groups, it was more than twice as large in older adults as in any other group, extending to working memory the associative deficits observed by Naveh-Benjamin and colleagues in long-term memory procedures (Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003 a; 2004 a,b).
Figure 3.

Proportions correct for every age group and change condition in Experiment 1a (which involved mixed trial blocks including item and binding trials), collapsed across set size. Error bars are standard errors.
Last, there was an interaction of Age Group X Array Size, F(9, 483) = 2.30, MSE = 0.03, p < .02, modified by a three-way, Age Group X Array Size X Change Condition interaction, F(18, 966) = 1.65, MSE = 0.03, p < .05. The means corresponding to these interactions are shown in Figure 4. One potential basis of these interactions is particularly germane. One can see that, for the three younger age groups, performance levels at the lowest set size were uniformly high in all three change conditions. As set size increased, performance losses differed across change conditions. In older adults, however, a different pattern was observed. The binding-change trials resulted in markedly lower performance levels than the other change conditions, even at the smallest set size. Thus, the binding deficit in older adults may not be based on conditions that overwhelm capacity limits; it may be more ubiquitous.
Figure 4.
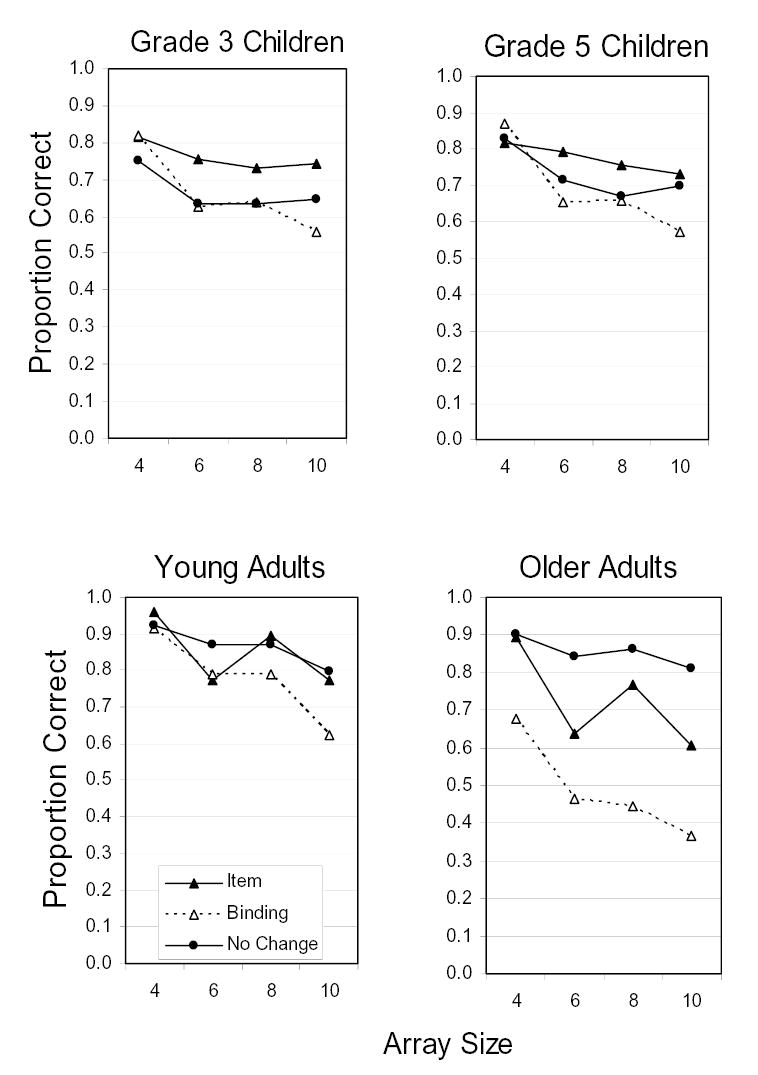
Proportions correct for every age group, change condition, and array set size in Experiment 1a.
In all of the analyses, interaction effects that do not have age group as a factor are deemed irrelevant to the aims of the research and, for the sake of simplicity, will not be discussed.
We were concerned that most of the older adults in this sample were females. Therefore, we conducted the analyses again using only females in each age group. All of the same significant effects were obtained on ANOVAs and Newman-Keuls tests. This rules out gender as the basis of age effects observed. Of course, it remains possible that a different pattern would emerge in males if we had a larger sample of them.
It is also noteworthy that there was a developmental change from children to adults in performance levels. The aging deficit in the binding condition cannot be attributed to developmental level alone because children had a similar level of performance on item-change trials (lower than the young adults by about the same amount in both cases). Yet, for binding-change trials, children’s deficit was similar to their deficit on item-change trials, not specifically more impaired as were the binding-change trials in older adults (see Table 1).
Table 1.
Mean Proportions Correct on Trials In Which the Sample and Test Arrays Differed
| Test Condition
|
|||
|---|---|---|---|
| Group (& Attention Condition) | Item | Binding | Difference |
| Experiment 1a (Mixed Item and Binding Blocks) | |||
| Grade 3 | 0.76 | 0.66 | 0.10 * |
| Grade 5 | 0.77 | 0.69 | 0.09 * |
| Young adults | 0.85 | 0.78 | 0.07 * |
| Older adults | 0.73 | 0.49 | 0.24 ** |
| Experiment 1b (Mixed Blocks as in 1a) | |||
| Young adults (Full Attention) | 0.84 | 0.70 | 0.14 ** |
| Young adults (Divided Attention) | 0.68 | 0.61 | 0.08 * |
| Experiment 2a (Separate Item and Binding Blocks) | |||
| Grade 3 | 0.71 | 0.65 | 0.06 * |
| Grade 5 | 0.83 | 0.77 | 0.06 * |
| Young adults | 0.84 | 0.88 | −0.04 |
| Older adults | 0.65 | 0.59 | 0.06 * |
| Experiment 2b (Separate Blocks as in 2a) | |||
| Young adults (Full Attention) | 0.86 | 0.87 | −0.01 |
| Young adults (Divided Attention) | 0.71 | 0.71 | 0.00 |
Note. Standard errors (for both item and binding conditions except where otherwise noted) are, in Experiment 1a, 0.02 for Grades 3 and 5, 0.01 for young adults, and 0.03 for older adults; in Experiment 1b, 0.03 for the full-attention condition and, for the divided-attention condition, 0.06 (item) and 0.04 (binding); in Experiment 2a, 0.03 for second and fifth-grade children and young adults, and 0.02 for older adults ; and in Experiment 2b, 0.02 for full attention and, for the divided attention condition, .03 (item) and .02 (binding).
p < .05, post-hoc Newman-Keuls test
p < .01
Signal-detection analysis
Table 2 presents d’ estimates of sensitivity, or the ability to distinguish change- from no-change trials regardless of the bias to respond “change” or “no change.” These estimates are calculated as z(proportion hits) - z(proportion false alarms), with a hit defined as detection of a change and a false alarm defined as a “change” response in the absence of any actual change. For the sake of the analysis, individual mean hit rates of 1.0 were lowered to 0.99 and individual mean false-alarm rates of 0.0 were raised to 0.01. The table shows that, for both item-change trials and binding-change trials, there was an inverted-U-shaped developmental change. That was born out in an ANOVA of d’ scores with age group and trial type (item or binding) as factors, which yielded effects of age group, F(3, 166) = 26.53, MSE = 0.99, p < .001, and trial type, F(1, 166) = 51.17, MSe = 0.28, p < .001, but no interaction. Newman-Keuls tests indicated that the young adults were significantly above all other groups, and additionally that older adults were above third-grade children. For all groups, sensitivity was significantly higher on item changes than on binding changes.
Table 2.
d’ Estimates of Sensitivity and β Estimates of Bias
| Condition and Measure
|
||||
|---|---|---|---|---|
| Group (& Attention Condition) | Item d’ | Item β | Binding d’ | Binding β |
| Experiment 1a (Mixed Item and Binding Blocks) | ||||
| Grade 3 | 1.21 | −0.15 | 0.89 | 0.01 |
| Grade 5 | 1.46 | −0.08 | 1.17 | 0.07 |
| Young adults | 2.44 | 0.01 | 2.07 | 0.19 |
| Older adults | 1.79 | 0.30 | 1.08 | 0.65 |
| Experiment 1b (Mixed Blocks as in 1a) | ||||
| Young adults (Full Attention) | 2.29 | −0.08 | 1.63 | 0.25 |
| Young adults (Divided Attention) | 1.11 | −0.13 | 0.75 | 0.05 |
| Experiment 2a (Separate Item and Binding Blocks) | ||||
| Grade 3 | 1.19 | −0.01 | 1.10 | 0.12 |
| Grade 5 | 1.96 | −0.10 | 1.87 | 0.14 |
| Young adults | 2.56 | 0.18 | 2.62 | 0.03 |
| Older adults | 1.63 | 0.38 | 1.60 | 0.54 |
| Experiment 2b (Separate Blocks as in 2a) | ||||
| Young adults (Full Attention) | 2.40 | 0.04 | 2.55 | 0.04 |
| Young adults (Divided Attention) | 1.33 | 0.07 | 1.25 | 0.05 |
Note. Higher d’ means better discrimination and higher β means a greater tendency to answer “no change.” Standard errors for d’ ranged from .10 – .16 in Grade 3 children, .09 – .17 in Grade 5 children, .11 – .19 in young adults, and .11 – .24 in older adults. For the full versus divided attention groups, they ranged from .11 – .17 with full attention and .12 – .21 with divided attention. Standard errors for β ranged from .04 – .07 in Grade 3 children, .04 – .08 in Grade 5 children, .04 – .05 in young adults, and .06 – .17 in older adults. For the full versus divided attention groups, they ranged from .05 – .08 with full attention and .07 – .13 with divided attention.
The criterion bias was estimated in a common way as −.5[z(proportion hits) + z(proportion false alarms)]. The estimates are shown in Table 2. As one can see from the table, the pattern was quite different from the sensitivities and appears more as a monotonic as opposed to an inverted-U-shaped developmental trend. An ANOVA comparable to the one conducted for sensitivities revealed an effect of age group, F(3, 166) = 10.68, MSE = 0.39, p < .001, and trial type, F(1, 166) = 51.17, MSe = 0.17, p < .001, but no interaction. Newman-Keuls tests showed that older adults had a tendency to respond “no change” significantly more than in any other participant group. The difference in measured bias between item and binding trials was significant in every group except the fifth-grade children.
In the youngest age group, the bias toward responding that there was a change was manifest in better performance on item-change trials than on no-change trials, as Figure 3 shows. It is possible that these children were so concerned with detecting changes that they overlooked the importance of not producing many false positives.
In sum, this experiment suggests that although both children and older adults had poorer working-memory performance than young adults, the tendency for this problem to be exaggerated for binding trials was observed only in older adults.
Experiment 1b: Attention Manipulation Using the Mixed-Blocks Design
A second experiment was conducted with young adults to determine whether their performance under divided attention can simulate the results obtained in children and/or older adults. This should be the case if the only difference between age groups were in the ability to use attention in visual working memory.
Method
The participants were 24 college students (10 male, 14 female) with a mean age of 22.1 (S.D. = 2.7) and an average level of education of 14.8 years (S.D. = .9), who did not participate in Experiment 1a. Their exact ethnic distribution of participants is unavailable but is approximately comparable to the young adults in Experiment 1a. The procedure was identical to that of experiment 1a, except that participants completed 2 similar blocks of the visual array task, one under full attention and one under divided attention, each consisting of 72 trials. Between these blocks, they also completed two baseline trials for the secondary task alone. The secondary task, carried out throughout the block, was a continuous 3-choice reaction time task that involved a sequential presentation of auditory tones by the computer, one at a time, and a manual response on a computer keyboard to each tone. One of three tones, which differed from each other in frequency, was presented at random and the participants’ task was to press a pre-designated corresponding key on the keyboard. A response to a tone caused the immediate presentation of any of the three tones at random. The order of the visual array blocks and of the attention condition was counterbalanced.
Results and Discussion
The results for all conditions are shown in Figure 5. It can be seen from the figure that dividing attention lowered the proportion correct but did not do so differentially for the item, binding, and no-change trials or for the different array sizes. A 2 × 3 × 4 ANOVA with the attention condition, change condition, and set size as within-subject variables produced only an effect of attention condition, F(1, 27) = 136.27, MSE = 0.04, p < .001, and array size, F(3, 81) = 71.02, MSE = 0.02, p < .001. Thus, the differential deficit in binding trials seen in older adults in Experiment 1a (Table 1) cannot be explained on the basis of poor attention. However, the general pattern with divided attention is similar to what is seen in children (cf. Figures 4 & 5).
Figure 5.

Proportions correct for the full and divided attention conditions in Experiment 1b. Standard errors for individual means in the figure were always below 0.08 for the full-attention condition and 0.10 for the divided-attention condition.
Analyses of signal-detection results (Table 2) showed that attention had an effect on sensitivity, F(1, 23) = 30.33, MSe = 0.84, p < .001, but not on bias, F(1, 23) = 1.27, MSe = 0.29. The difference between item and binding trials did not interact with attention.
It can be observed that dividing attention in young adults appears to provide a reasonable facsimile of children’s task deficits. In particular, dividing attention lowered the sensitivity for both item and binding trials. However, dividing attention in young adults did not provide a close model of performance in older adults, who displayed not only an overall decrease in sensitivity but also a strong bias detrimental to the detection of changes, and especially binding changes.
Experiment 2a: Developmental Study Using Separate Blocks of Item and Binding Trials
In this experiment, we continue to pursue the question of what the life-span developmental differences observed in Experiment 1a mean. In that experiment, item and binding changes were mixed with no-change control trials in common trial blocks. The advantage for detecting item changes as compared to binding changes, especially in older adults, could reflect a deficit in sensitivity to binding changes. However, it also could reflect a judgment issue in which older adults more conservatively respond “no change” to a possible change that is not very salient. In the present experiment, item and binding changes occurred in separate trial blocks, each with its own set of no-change control trials. That way, the sensitivity and bias for both kinds of changes could be assessed independently.
Method
Participants
The participants were 24 children about to enter third grade (13 male, 11 female; mean age: 8.69 years, SD = 0.46), 24 fifth-grade children (11 male, 13 female; mean age: 11.09 years, SD = 0.37), 24 young adults (9 male, 15 female; mean age: 18.94 years, SD = 0.52), and 32 older adults (9 male, 23 female; mean age: 71.4, SD = 4.8). Older adults fit the same description as in Experiment 1a. They had a mean of 13.9 years of education (SD = 1.70), whereas the young adults, who were drawn from an introductory psychology class, had a mean of 12.4 years of education (SD = 0.72); the age-related difference in number of years of education was significant, t(54) 4.12, p<.05) so, if anything, the true aging deficit is slightly underestimated in the present results. Clearly, as well, the introductory psychology students are expected to go on to acquire a few more years of education on average, and therefore appear to be fair representatives of the same type of individual as our aging population. The children were 90% White, 1% Hispanic, 5% Black, and 3% other or unknown. Information about the family structure was not available and is assumed to be unimportant for this study. The young adults were 87% White, 9% Black, 1% Asian, and 3% other or unknown. Exact ethnic information was unavailable for the older adults but it was approximately comparable to the other groups.
Apparatus, stimuli, and procedure
These aspects of the experiment were the same as in Experiment 1a except that, in separate blocks of trials, the changes always were item changes (with the encircled square changing to a color that is unique in the trial) or always were binding changes (with the encircled square changing to a color already present elsewhere in the array). Additional alterations were made in the structure of the arrays in order to prevent participants from guessing the correct response based on the test array alone, without reference to the sample array. Given that item changes always produced a unique color, arrays in item-change blocks were constructed in such a way that the encircled square was a color that occurred nowhere else in the array, even on no-change trials. Conversely, given that binding changes always produced a non-unique color (by definition), arrays in binding-change blocks were constructed in such a way that the encircled square was a color that also occurred elsewhere in the array, even on no-change trials. The item and binding trial blocks were presented in a counterbalanced order and there was a total of 8 practice trials and 64 trials in each kind of block, evenly divided among conditions and randomly ordered.
Results and Discussion
Proportions correct
The data are shown collapsed across array set sizes and presence versus absence of a change (Figure 6; cf. Figure 3 for Experiment 1a), and for the complete data set (Figure 7; cf. Figure 4 for Experiment 1a). Unlike Experiment 1a, there was no longer a dramatically larger binding deficit in older adults than in the children. Figure 6 suggests that there was a small deficit that now emerged in the children as well as in older adults. Figure 7 shows that, in older adults, there was also a very pronounced advantage of no-change trials from both blocks over change trials from both blocks, indicating a conservative response bias as in Experiment 1a.
Figure 6.

Proportions correct for every age group and change condition in Experiment 2a (which involved separate trial blocks for item and binding trials), collapsed across set size. Error bars are standard errors.
Figure 7.

Proportions correct for every age group and condition in Experiment 2a (which involved separate trial blocks for item and binding trials).
These suggestions were supported by statistical analyses, beginning with an ANOVA in which the factors were the age group, the type of trial block (item versus binding), the change status (change or no change), and the array size (4, 6, 8, & 10). There were significant main effects of age group, F(3, 100) = 23.70, MSE = 0.10, p < .001, change status F(1, 100) = 21.68, MSE = 0.11, p < .001, and array set size, F(3, 300) = 126.69, MSE = 0.02, p < .001. The age group effect was again curvilinear, with means (and SEMs) of .70, .80, .88, and .74 for the four age groups, respectively (SEM = 0.02 for the younger three age groups and 0.01 for older adults). According to Newman-Keuls post-hoc tests, all group mean differences were significant. The mean performance level was not as high on change trials (M = .74, SEM = .01) as on no-change trials (M = .82, SEM = .01), and performance decreased with increasing array set size, M = .88, .80, .74, and .70 for sets of 4, 6, 8, and 10 items, respectively; SEM = .01 in each case. There was no overall item versus binding difference.
There was no interaction of Age Group X Trial Block, F < 1 (see Figure 6). There were interactions of Age Group X Array Size, F(9, 300) = 1.99, MSE = 0.02, p < .05, and Age Group X Change Status, F(3, 100) = 13.53, MSE = 0.11, p < .001. These interactions were qualified by a three-way, Age Group X Change Status X Array Size interaction, F(9, 300) = 3.07, MSE = 0.03, p < .01. As Figure 7 indicates, the large Age Group X Change Status interaction is clearly a result of the shift in criterion in older adults, strongly favoring no-change responses at the expense of fewer correct responses on change trials, unlike the other age groups. The basis of the other interactions may be that no-change trial performance also dropped off across set sizes more rapidly in older adults than in the other groups, as the figure shows.
Most importantly, there was a three-way interaction of Age Group X Trial Block X Change Status, F(3, 100) = 3.51, MSE = 0.03, p < .05. Separate analyses for the no-change and change trials showed that there was an Age Group X Trial Block interaction for the change trials, F(3, 100) = 2.84, MSE = 0.04, p < .05, but not for the no-change trials, F < 1. For trials in which there was a change, Table 1 shows that there was an overall disadvantage for the binding changes as compared to item changes for children and older adults, but not for the young adults.
Notice that in Experiment 1, but not Experiment 2, even the young adults had a deficit on binding changes compared to item changes, for change trials examined separately (Table 1). This suggests that the salience of binding trials may be diminished when they are mixed with item trials, as they were in Experiments 1a and 1b. This diminished salience of binding changes helps to explain why older adults were especially poor at detecting those changes whereas, in Experiment 2a, they detected the changes as well as the children did.
Signal detection analysis
Table 2 presents d’ estimates of sensitivity. It shows that, for both item-change and binding-change trials, there was an inverted-U-shaped developmental change. That was born out in an ANOVA of d’ scores with age group and trial block (within subject) as factors, which yielded only an effect of age group, F(3, 100) = 21.01, MSE = 0.85, p < .001. Newman-Keuls tests indicated that all pairs of age groups differed significantly except for the fifth-grade children and older adults.
For β, the criterion bias (Table 2), the pattern was quite different from the sensitivities and appears more as a monotonic as opposed to an inverted-U-shaped developmental trend. Moreover, there were differences between the patterns in the item and binding-change trial blocks. An ANOVA comparable to the one conducted for sensitivities revealed not only an effect of age group, F(3, 100) = 13.17, MSE = 0.19, p < .001, but also an effect of trial block, F(1, 100) = 9.03, MSE = 0.05, p < .01, and an Age Group X Trial Block interaction, F(3, 100) = 7.10, MSE = 0.05, p < .001. Newman-Keuls tests for the age group main effect showed only that the older adults differed significantly from all of the other groups. As in Experiment 1a, older adults had a much stronger tendency to respond “no change,” and thus minimized both hits and false alarms. Moreover, the type of trial block tended to modulate the biases. The difference between item- and binding-trial blocks was significant in the fifth-grade children and in older adults. Whereas the children and older adults tended to respond “no change” more frequently in the binding-change than in the item-change trial blocks, for young adults there was a slight trend in the opposite direction.
Thus, overall, there is some support for a Jacksonian inverted-U function of development regarding the difficulty of remembering binding information, and it is combined with a monotonic change in the criterion bias that differentiates older adults from everyone else.
Experiment 2b: Attention Manipulation with the Separate-Blocks Design
The purpose of this final experiment was to determine whether the developmental effects observed in Experiment 2a can be modeled on the basis of poor attention in the children, older adults, or both, much as Experiment 1b did for Experiment 1a.
Method
The participants were 28 college students (10 male, 18 female) with a mean age of 19.2 (S.D. = 1.3) and an average level of education of 12.7 years (S.D. = 1.1), who did not participate in the previous experiments. The exact ethnic distribution is unavailable but is approximately comparable to the young adults in the prior experiments. The procedure was identical to that of Experiment 2a, except that the visual array trial blocks (for item and binding) were performed under either full or divided attention. The secondary task was the continuous auditory 3-choice reaction time task used in Experiment 1b. The order of trial blocks for change type and attention were counterbalanced.
Results and Discussion
Proportion correct
The results of this experiment are shown in Figure 8. An analysis was conducted with attention condition, the change-type block (item versus binding), the change status (change or no change), and the array size (4, 6, 8, & 10) as within-subject factors. This analysis produced main effects of attention condition, F(1, 27) = 136.27, MSE = 0.04, p < .001, and array size, F(3, 81) = 71.02, MSE = 0.02, p < .001. No other effects were significant. Thus, although attention interfered with performance, it did not alter the pattern of performance. It produced no hint of an effect favoring item changes over binding changes (as was seen in both children and older adults in Experiment 2a), and it produced no hint of an advantage of no-change trials over change trials (as was seen in older adults in Experiment 2a). Poor attention thus cannot explain the pattern of responses observed in Experiment 2a, a result also found in Experiment 1.
Figure 8.

Proportions correct for the full and divided attention conditions in Experiment 2b. Standard errors for individual means in the figure were always below 0.05.
Signal detection analysis
Figures 8 and 9 show the d’ and bias scores for this experiment, as for the previous one. An ANOVA of d’ with attention condition and trial blocks as factors yielded only a main effect of dividing attention, which consistently lowered d’, F(1, 27) = 145.66, MSE = 0.27, p < .001. A comparable analysis on β, the criterion bias, yielded no effects, however. Thus, it appears that dividing attention provides a useful model of effects of age on sensitivity, but not of effects of age on response bias.
General Discussion
The present study was designed to address several questions about the life span development of performance on a working memory task (after Luck & Vogel, 1997) in which memory for item color information and the binding between an item and its location in an array both were examined in two-array comparisons. The questions were (1) whether there are age-group deficits in binding in this working memory task, (2) whether the development of binding is shaped as an inverted U across the life span or is monotonic, (3) whether developmental deficits in binding can be mimicked by dividing attention in young adults, and (4) how associative deficits in binding should be described according to a signal-detection analysis.
The results were clear. Consider first Experiment 1a, in which trials with changes in item and in binding (see Figure 1) were intermixed, along with no-change trials. The results indicated that item changes were noticed more easily than binding changes in all age groups (two ages of children, young adults, and older adults). However, performance was lower in children and older adults than it was in young adults. Moreover, older adults were especially poor at detecting changes in binding. Experiment 1b showed that the particular pattern observed in older adults could not be modeled by dividing attention in young adults (Figure 4 & Table 2). It resulted in equal decrements in item and binding information, a pattern more similar to the children. This suggests that there could be an attentional basis of developmental changes in sensitivity, but that there is some additional deficit in older adults unlikely to be based on attention deficits (involving poor detection of the lower-salience, binding changes when intermixed with item changes).
In Experiment 2a, trials with item and binding changes were segregated into separate trial blocks, each with its own no-change control trials. This arrangement again reproduced a disadvantage of binding changes over item changes, though the pattern was now significant only in a separate analysis of change trials. This small binding deficit (shown in Table 1) was found in about equal magnitudes in children and older adults, with no effect in young adults. Moreover, older adults differed from all other groups in once more showing a large response bias advantageous for no-change trials at the expense of change trials (Figure 5 & Table 2). The difference between item and binding trials emerged as a shift in response bias in children and older adults, with a slight trend in the opposite direction in young adults. Experiment 2b, similar to 1b but with separate item and binding trial blocks, showed that the pattern of developmental differences in older adults was not mimicked when attention was divided in young adults. It mimicked the decrement in sensitivity (d’), but not the shift in bias (β) observed in older adults or the change in bias between item and binding trials observed in both fifth-grade children and older adults (Table 2). On the basis of these results, the key questions raised above can be addressed, and then the question of life span change in binding can be considered more generally.
Key Questions
1. Are there age-group deficits in binding?
The largest age-group deficits in binding could be seen for older adults. This finding was clearest in Experiment 1a, in which item and binding change trials were mixed together. In Experiment 2a, with item and binding change trials in separate blocks, there was no difference between fifth-grade children and older adults. Both groups differed from young adults in that they were less likely to notice binding changes than were the young adults. However, the binding deficits per se were not really sensitivity decrements compared to young adults, but rather bias shifts.
We cannot truly characterize the binding deficit in any group as a decrement in sensitivity. Instead, it appears that participants who have a lower sensitivity to changes in general also are reticent to respond that a stimulus has changed, when that change is small in magnitude. In Experiment 2a, the magnitude of changes was essentially held constant within a trial block (given that item and binding changes were not intermixed in this experiment). This could have allowed participants to adapt to a certain magnitude of change. Yet, the difference between item and binding trials in this circumstance remained in older adults (as in Experiment 1a), and emerged in this experiment also in fifth-grade children, even though older adults had overall a much stronger bias toward not noticing changes.
2. Is the life span development of binding shaped as an inverted U?
Based on Experiment 2a, the answer is clearly that an inverted U shape adequately describes development of binding. Both fifth-grade children and adults shifted in their bias toward fewer change detections in binding trial blocks, whereas young adults did not shift in that manner. In terms of sensitivity and bias measured separately in this experiment, that is, the hypothesis of Jackson (1860, reprinted in Taylor, 1958) seems correct.
However, in Experiment 1a, in which item and binding changes were mixed together, clearly an additional deficit in the detection of binding changes showed up in older adults. It appears that the juxtaposition of two different levels of change works against detection of the more subtle, binding-type changes in that age group. One explanation for this difference is that older adults might be lulled more easily into a reliance on familiarity as opposed to recollection (cf. Hay & Jacoby, 1999; Jennings & Jacoby, 1997). In Experiment 1a, the presence of a number of item changes, which can be detected on the basis of familiarity, may allow the participant to use familiarity and still produce a reasonable distribution of “change” and “no change” responses. In contrast, in Experiment 2a, the separation of item and binding trial blocks means that recollection must be used in the binding blocks or else the participant would not produce a reasonable number of “change” responses.
In at least two ways, then, the Jacksonian hypothesis fails. First, one can see in Figure 3 that older adults do far worse than the children on binding trials in Experiment 1a. Second, in both Experiments 1a and 2a, one can see in Table 2 that the bias parameter β gets steadily larger with age, indicating a greater tendency to say that nothing has changed. These effects could occur because older adults may be especially prone to omitting the recollection process, and therefore may often answer “same” on the basis of familiarity, provided that a reasonable proportion of trials present unfamiliar stimuli to which a “different” response can be given on that same basis (i.e., in Experiment 1a and in the item-change block of Experiment 2a).
3. Can developmental deficits in binding can be mimicked by dividing attention in young adults?
Dividing attention did not differentially affect item and binding trials, and did not affect bias, though it did lower task performance, reducing sensitivity. Therefore, although it may provide a model of some aspects of developmental change (inasmuch as children and older adults performed worse overall than young adults), it cannot serve as a model of what causes a binding deficit. In this way it is consistent with other failures to find that dividing attention produces a binding deficit (Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003b, 2004a,b).
4. How should associative deficits in binding be described according to a signal-detection analysis?
It appears to be the response bias, rather than the sensitivity, of the memory process that produces a larger deficit for binding than for item changes in older adults in Experiment 1a (cf. Olson et al., 2004, Figure 2), and in both fifth-grade children and older adults in Experiment 2a. Life span development produces an inverted U shape of overall sensitivity, for both item and binding changes alike. This combination of results, carefully considered, rules out the view that bias is just a correlate of sensitivity (Danziger, 1980); bias changes asymmetrically over the life span. For example, in Experiment 2a, older adults achieve almost identical sensitivity (d’) levels for item and binding blocks, and yet had significantly different response biases in the two types of trial blocks. A better-supported view of response biases is that they depend on the extent to which the process of familiarity is used instead of recollection. Use of familiarity tends to result in false recognition of some changed items as unchanged, primarily in the case of binding changes, whereas it may improve performance on no-change trials. The tendency of older adults often to fall into this pattern of relying on familiarity when trial types are mixed may reflect their variability in the appropriateness of processing, or lack of “processing robustness” (Li et al., 2004).
Interpretation of the Life Span Development of Binding
Given that the severe deficit in binding-change detection seen in older adults in Experiment 1a was not reproduced at all in a divided-attention experiment (1b), it would appear that a non-attentional explanation must be found. Given the aging deficit in the encoding of contextual information (e.g., Bayen et al., 2000; Chalfonte & Johnson, 1996; Mitchell et al., 2000), a likely candidate is the degradation with aging of temporal lobe regions implicated in memory storage (e.g., Raz et al., 2004). In particular, the hippocampus and surrounding structures may be especially important in binding information about an item to the context in which it occurred (e.g., Rolls et al., 1996). It may be that a non-severe deficit of temporal lobe function in aging could result in a type of participant who misses binding changes between arrays when other, more salient changes are present in the same trial block (as in the present Experiment 1a), but who is able to detect those binding changes when no other, more salient changes are included in the trial block (as in the present Experiment 2a). This type of hypothesis seems consistent with the description of aging memory by Mitchell et al. (2000) as entailing “deficits in the efficacy of reflective component processes.”
Various studies have considered a variety of cognitive mechanisms that may change with age across the life span. These may play a role in the effects that were obtained. Some studies have pointed to an inverted U shape of executive and inhibitory processes across the life span (e.g., Cepeda et al., 2001; Comalli, Wapner, & Werner, 1962; Dempster, 1992; Hasher, Stolzfus, Zacks, & Rypma, 1991; Williams, Ponesse, Schachar, Logan, & Tannock, 1999; Zelazo et al., 2004). This may help to explain our sensitivity results, which did follow an inverted U.
However, aspects of the results related to bias were asymmetrical in developmental trend. (Similarly, see Gulya et al., 2002, for asymmetrical developmental change in some explicit memory processes.) It must be considered that, for trial blocks that include some binding-change trials, successful performance can occur only if familiarity cues are inhibited and answers are based instead on recollection (Jacoby, 1991). Similarly, developmental changes in attentiveness to the task (Craik & Byrd, 1982; Craik, Byrd, & Swanson, 1987; Hashtroudi, Johnson, & Chrosniak, 1990; Hess, Donley, & Vandermaas, 1989; Lane & Pearson, 1982; Plude, Enns, & Brodeur, 1994; Rabinowitz et al., 1982) could be important because it takes attention to use recollection processes consistently. Age differences in the speed of processing (Cerella & Hale, 1994; Kail & Salthouse, 1994) could be important if familiarity information is available sooner than recollection, in which case the speed of recollection processes could influence whether that information is used at all on a trial.
This is not the first study in which life-span changes can be decomposed into processes that display U-shaped change and others that change monotonically from childhood to old age. For example, the results of Hommel, K.Z.H. Li, and S.-C. Li (2004) show that pattern in a speeded task in which a circle target, for which participants searched, differed from non-target objects in one feature (color) or only in the conjunction of two features (color and shape). Although there was a U-shaped trend in response times generally, the elderly were especially slow in the conjunction search when there were many targets, and when the target was absent from the display. Similar explanatory factors could apply to Hommel et al. and to the present study, including the factor of neural efficiency changing in a U shape across age groups and, additionally, factors specific to the elderly, including some deficit in detecting feature binding and a compensatory increase in cautiousness.
We found that age differences in the sensitivity to both item and binding trials appear to undergo mirror image change in childhood and old age. There is a condition difference in response bias that also occurs in mirror image (i.e., inverted-U) form, with binding blocks producing a more positive bias than item blocks in children and older adults, versus a less positive bias in young adults (see Table 2). However, when item and binding changes occur in a common block of trials, as in the present Experiment 1a, older adults are particularly vulnerable to the use of a response criterion that is inappropriate for the binding trials. Thus, older adults differ from other groups in how they deal with stimuli differing in salience; the higher salience of item changes compared to binding changes in the same trial block tends to make the older adults overlook the binding changes. In both studies, also, the overall change in response biases across the life span was fairly monotonic, rather than U-shaped. Although we do not have the benefit of longitudinal data, we believe that large cohort effects are unlikely for basic working memory phenomena. The nature of the asymmetrical differences should be investigated in other types of procedure that have been used to test the associative deficit hypothesis in older adults. Our study emphasizes the value of life-span experimentation in improving the understanding of both maturational advances and aging deficits, on the basis of comparative evidence across much of the life span.
Acknowledgments
This research was supported by NIH Grant R01 HD-21338 to Nelson Cowan, and by a University of Missouri Research Board grant to Moshe Naveh-Benjamin. The data for the children in Experiment 1a are drawn from a published study (Experiment 1 of Cowan et al., in press) that included a battery of working-memory and intelligence measures not reported here. We thank Pinky Bomb, Mike Carr, other members of the Working-Memory Development Laboratory, and members of the Memory and Cognitive Aging Laboratory at the University of Missouri for their help in data collection.
References
- Anooshian LJ. Understanding age differences in memory: Disentangling conscious and unconscious processes. International Journal of Behavioral Development. 1999;23:1–17. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in cognitive sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Burch MM, Kleinknecht EE. Developments in early recall memory: Normative trends and individual differences. Advances in Child Development and Behavior. 2002;30:103–152. doi: 10.1016/s0065-2407(02)80040-4. [DOI] [PubMed] [Google Scholar]
- Bayen UJ, Phelps MP, Spaniol J. Age-related differences in the use of contextual information in recognition memory: A global matching approach. Journals of Gerontology: Series B: Psychological Sciences & Social Sciences. 2000;55B:P131–P141. doi: 10.1093/geronb/55.3.p131. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, De Sather JCMG. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Cerella J, Hale S. The rise and fall in information-processing rates over the life span. Acta Psychologica. 1994;86:109–197. doi: 10.1016/0001-6918(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Cohen, N.J., & Eichenbaum, H. (1993). Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press.
- Comalli PE, Jr, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. Journal of Genetic Psychology. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Cowan, N. (1995). Attention and memory: An integrated framework. Oxford Psychology Series, No. 26. New York: Oxford University Press.
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan, N., Elliott, E.M., Saults, J.S., Morey, C.C., Mattox, S., Hismjatullina, A., & Conway, A.R.A. (in press). On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. [DOI] [PMC free article] [PubMed]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London. 1983;302:341–359. [Google Scholar]
- Craik, F. I. M. (1986). A functional account of age differences in memory. In F. Klix & H. Hagendorf (Eds.), Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam: North Holland.
- Craik, F. I. M., & Byrd, M. (1982). Aging and cognitive deficits: The role of attentional resources. In F. I. M. Craik & S. E. Trehub (Eds.), Advances in the study of communication and affect: Vol. 8. Aging and Cognitive processes. New York: Plenum Press.
- Craik FIM, Byrd M, Swanson JM. Patterns of memory loss in three elderly samples. Psychology and Aging. 1987;2:79–86. doi: 10.1037/0882-7974.2.1.79. [DOI] [PubMed] [Google Scholar]
- Craik, F. I. M. & Simon, E. (1980). Age differences in memory: The roles of attention and depth of processing. In L. W. Poon, J. L. Fozard, L. S. Cermak, D. Arenberg, & L. W. Thompson (Eds.), New directions in memory and aging: Proceedings of the George A. Talland Memorial Conference. Hillsdale, NJ: Erlbaum.
- Curley SP, Eraker SA, Yates JF. An investigation of patients’ reactions to therapeutic uncertainty. Medical Decision Making. 1984;4:501–511. [Google Scholar]
- Danziger, W.L. (1980). Measurement of response bias in aging research. In L. W. Poon (Ed), Aging in the 1980s: Psychological Issues. Washington, D.C.: American Psychological Association. (pp. 552–557)
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Dror IE, Katona M, Mungur K. Age differences in decision making: To take a risk or not? Gerontology. 1998;44:67–71. doi: 10.1159/000021986. [DOI] [PubMed] [Google Scholar]
- Fritzley VH, Lee K. Do young children always say yes to yes–no questions? A metadevelopmental study of the affirmation bias. Child Development. 2003;74:1297 – 1313. doi: 10.1111/1467-8624.00608. [DOI] [PubMed] [Google Scholar]
- Goulet LR. Verbal learning in children: Implications for developmental research. Psychological Bulletin. 1968;69:359–376. doi: 10.1037/h0025713. [DOI] [PubMed] [Google Scholar]
- Green, D.M., & Swets, J.A. (1966). Signal detection theory and psychophysics. New York: Wiley.
- Gulya M, Rossi-George A, Hartshorn K, Vieira A, Rovee-Collier C, Johnson MK, Chalfonte BL. The development of explicit memory for basic perceptual features. Journal of Experimental Child Psychology. 2002;81:276–297. doi: 10.1006/jecp.2001.2654. [DOI] [PubMed] [Google Scholar]
- Harkins SW, Chapman CR, Eisdorfer C. Memory loss and response bias in senescence. Journal of Gerontology. 1979;34:66–72. doi: 10.1093/geronj/34.1.66. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stolzfus ER, Zacks RT, Rypma B. Age and inhibition. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology & Aging. 1990;5:119–126. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- Hay JF, Jacoby LL. Separating habit and recollection in young and older adults: Effects of elaborative processing and distinctiveness. Psychology & Aging. 1999;14:122–134. doi: 10.1037//0882-7974.14.1.122. [DOI] [PubMed] [Google Scholar]
- Hess TM, Donley J, Vandermaas MO. Aging-related changes in the processing and retention of script information. Experimental Aging Research. 1989;15:89–96. doi: 10.1080/03610738908259762. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZH, Li SC. Visual search across the life span. Developmental Psychology. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory & Language. 1991;30:513–541. [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychology & Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Johnson, M.K. (1994). Binding complex memories: The role of reactivation and the hippocampus. In Schacter, D.L & Tulving, E. (Eds). Memory systems 1994. Cambridge, MA, US: The MIT Press. (pp. 311–350).
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–255. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kausler, D.H. (1994). Learning and memory in normal aging. San Diego: Academic Press.
- Keppel G. Verbal learning in children. Psychological Bulletin. 1964;61:63–80. doi: 10.1037/h0043153. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Sowon H, Gopher D. Task coordination and aging: explorations of executive control processes in the task switching paradigm. Acta Psychologica. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Lane DM, Pearson DA. The development of selective attention. Merrill-Palmer Quarterly. 1982;28:317–337. [Google Scholar]
- LeBreck D, Baron A. Age and practice effects in continuous recognition memory. Journal of Gerontology. 1987;42:89–91. doi: 10.1093/geronj/42.1.89. [DOI] [PubMed] [Google Scholar]
- Leventhal EA, Leventhal H, Schaefer P, Easterling D. Conservation of energy, uncertainty reduction, and swift utilization of medical care among the elderly. Journal of Gerontology: Psychological Sciences. 1993;48:P78–P86. doi: 10.1093/geronj/48.2.p78. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Printz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Lynch S, Rohwer WD., Jr Grade interaction with words and pictures in a paired-associate task: A proposed explanation. Journal of Experimental Child Psychology. 1972;13:413–421. doi: 10.1016/0022-0965(72)90101-4. [DOI] [PubMed] [Google Scholar]
- Massaro DW, Thompson LA, Barron B, Laren E. Developmental changes in visual and auditory contributions to speech perception. Journal of Experimental Child Psychology. 1986;41:93–113. doi: 10.1016/0022-0965(86)90053-6. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D’Esposito M. Aging and reflective processes of working memory: Binding and test load deficits. Psychology and Aging. 2000;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Moore, C., & Lemmon, K. (eds.) (2001). The self in time: Developmental perspectives. Mahwah, NJ: Erlbaum.
- Morey CC, Cowan N. When visual and verbal memories compete: Evidence of cross-domain limits in working memory. Psychonomic Bulletin & Review. 2004;11:296–301. doi: 10.3758/bf03196573. [DOI] [PubMed] [Google Scholar]
- Morey, C.C., & Cowan, N. (in press). When do visual and verbal memories conflict? The importance of working-memory load and retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. [DOI] [PMC free article] [PubMed]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik, FIM. Memory for context and its use in item memory—comparisons of younger and older persons. Psychology and Aging. 1995;10:284–293. doi: 10.1037//0882-7974.10.2.284. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Marom M. The effects of divided attention at encoding on item and associative memory. Memory and Cognition. 2003b;31:1021–1035. doi: 10.3758/bf03196123. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative deficit of older adults: Further support using face-name associations. Psychology and Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Shulman S. Older adults’ associative deficit in episodic memory: Assessing the role of decline in attentional resources. Psychonomic Bulletin & Review. 2004;11:1067–1073. doi: 10.3758/bf03196738. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: Further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2003a;29:826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Nelson, C.A. (2002). The ontogeny of human memory: A cognitive neuroscience perspective. In M.H. Johnson, Y. Munakata, & R.O. Gilmore (eds.), Brain development and cognition: A reader. Malden, MA: Blackwell. (pp. 151–178)
- Olson IR, Zhang JX, Mitchell KJ, Johnson MK, Bloise SM, Higgins JA. Preserved spatial memory over brief intervals in older adults. Psychology and Aging. 2004;19:310–317. doi: 10.1037/0882-7974.19.2.310. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Enns JT, Brodeur D. The development of selective attention: A life_span overview. Acta Psychologica. 1994;86:227–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Rabinowicz, T. (1980). The differentiate maturation of the human cerebral cortex. In F. Falkner & J.M. Tanner (Eds.), Human Growth, V. 3: Neurobiology and Nutrition. NY: Plenum Press.
- Rabinowitz JC, Craik FIM, Ackerman BP. A processing resource account of age differences in recall. Canadian Journal of Psychology. 1982;36:325–344. [Google Scholar]
- Rankin JL, Kausler DH. Adult age differences in false recognition. Journal of Gerontology. 1979;34:58–65. doi: 10.1093/geronj/34.1.58. [DOI] [PubMed] [Google Scholar]
- Raz, N. (2000). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In F.I.M., Craik, and T.A. Salthouse, (Eds). The handbook of aging and cognition (2nd ed.). (pp. 1–90). Mahwah, NJ, US: Lawrence Erlbaum.
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe - A study of a five-year change. Neurology. 2004;63:443–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes LM, Luck SJ. The development of visual short-term memory capacity in infants. Child Development. 2003;74:1807–1822. doi: 10.1046/j.1467-8624.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Karns CM, Courchesne E. Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain. 2001;124:1317–1324. doi: 10.1093/brain/124.7.1317. [DOI] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter_Lorenz P, Koeppe RA. The neural basis of task_switching in working memory: Effects of performance and aging. Proceedings of the National Academy of Sciences. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.L., Kates, M.H., & Vriezen, E.R. (1992). The development of frontal lobe functions. In S.J. Segalowitz (ed.), Handbook of neuropsychology, Vol. 7. (pp. 309–330).
- Taylor, J.E. (ed.). (1958). Selected writings of John Hughlings Jackson, Vol. 2. London, UK: Staples.
- Todd JJ, Marois R. Capacity limit of visual short_term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:749–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Treisman AM. Binding in short-term visual memory. Journal of Experimental Psychology—General. 2002;131:48–64. doi: 10.1037//0096-3445.131.1.48. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmetnal Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Craik FIM, Booth L. Executive function across the life span. Acta Psychologica. 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]


