Abstract
This study was conducted to assess the stress response of Southern chamois (Rupicapra pyrenaica) to capture and physical restraint and the effects of acepromazine (a short-acting neuroleptic) on this response. Forty free-ranging Southern chamois were captured, injected intramuscularly with acepromazine (19 animals, randomly selected) or saline (the other 21 animals), and physically restrained for 3 h. Heart rate and body temperature were monitored with telemetric devices, and blood samples were obtained at capture and every hour thereafter to determine hematologic and serum biochemical parameters. The lower heart-rate variability, temperature, erythrocyte count, hemoglobin concentration, packed cell volume (PCV), and serum creatine kinase activity in the animals treated with acepromazine indicated that this agent reduced the adverse effects of stress. According to the differences in heart rate, erythrocyte count, hemoglobin concentration, PCV, lymphocyte count, and serum concentrations of glucose, creatinine, chloride, and potassium, α-adrenergic stimulation by catecholamines seemed to be stronger in females, whereas the adrenal-cortex reaction seemed to be stronger in males. The differences in erythrocyte parameters, temperature, serum creatine kinase activity, and serum concentrations of potassium and chloride indicated that acepromazine’s beneficial effects were greater in females.
Résumé
Cette étude visait à évaluer la réponse au stress de chamois (Rupicapra pyrenaica) suite à leur capture et contention physique et les effets de l’acépromazine (un agent neuroleptique de courte durée) sur cette réponse. Quarante chamois en liberté ont été capturés, injectés par voie intra-musculaire avec de l’acépromazine (19 animaux sélectionnés au hasard) ou de la saline (les 21 autres animaux), et retenus physiquement pendant 3 h. Le rythme cardiaque et la température corporelle ont été suivis à l’aide d’appareils de mesures télémétriques et des échantillons de sang prélevés lors de la capture et à chaque heure subséquente afin de déterminer les paramètres hématologiques et biochimiques sériques. La variabilité moindre du rythme cardiaque, de la température, du comptage érythrocytaire, de la concentration en hémoglobine, de l’hématocrite (PCV), et de l’activité de la créatine kinase sérique chez les animaux traités avec l’acépromazine indiquait que cet agent réduisait les effets néfastes du stress. Selon les différences notées pour le rythme cardiaque, le comptage érythrocytaire, la concentration en hémoglobine, le PCV, le comptage lymphocytaire et les concentrations sériques de glucose, créatinine, chlorure et potassium, la stimulation α-adrénergique par les cathécholamines semblait plus forte chez les femelles, alors que la réaction du cortex des surrénales semblait plus forte chez les mâles. Les différences observées dans les paramètres érythrocytaires, de la température, de l’activité de la créatine kinase sérique et des concentrations sériques de potassium et chlorure indiquaient que les effets bénéfiques de l’acépromazine étaient plus marqués chez les femelles.
(Traduit par Docteur Serge Messier)
Introduction
Wild ungulates are captured for population restocking, game translocation, or scientific purposes. Capture may result in death and has animal-welfare implications, so it is increasingly a cause of concern (1–5). One of the main factors underlying the development of physiological disorders is stress. A number of studies evaluating stress and its effects on animal health and well-being have been carried out in different species of wild ungulates (1–4,6). The Pyrenean population of Southern chamois (Rupicapra pyrenaica) increased from more than 35 000 in 1997 to around 50 000 in 2001, but 1 subspecies, the Apennine chamois (R. pyrenaica ornata), is considered vulnerable, with an estimated population of only 400 to 700 (7). Capture, restocking, and translocation of both Southern chamois and the closely related Northern chamois (R. rupicapra) were carried out during the last century in France, Italy, Spain, and New Zealand (7,8). However, the stress response to capture and handling has not been studied in either species. Handlers should be familiar with the normal responses to stress, including the physiological values, in the species for which they are responsible (5).
When an animal is captured and physically restrained, a stress response is initiated that involves first the sympathetic– adrenal-medulla axis (which releases catecholamines) and later the hypothalamic–pituitary–adrenal-cortex axis (which releases corticosteroids) (9). This response induces changes in hematologic, serum biochemical, and clinical parameters, which have been proposed as useful stress indicators (1–4,10). Stress related to capture and physical restraint has been reported to increase heart rate, body temperature, erythrocyte count, hemoglobin concentration, hematocrit, and serum concentrations of glucose, lactate, creatinine, urea, bilirubin, chloride and potassium (1–4,6,11–17). The leukocyte response has a biphasic pattern: lymphocytic leukocytosis is followed by corticosteroid-induced leukocytosis, lymphopenia, and neutrophilia. Monocytosis depends on the species (18). Serum concentrations of cholesterol or triglycerides, or both, have been reported to increase, to decrease, or not to change with stress (3,19,20). Serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), and l-lactate dehydrogenase (LDH) also have been reported to increase during capture and handling owing to increased muscle-cell permeability or damage (4,18). Finally, an increase in the serum concentration of cortisol has traditionally been considered a good indicator of stress (10,21). However, because great interindividual differences exist in stress-induced changes in corticosteroid concentrations, and it is difficult to relate physiological changes and animal welfare, some believe that cortisol is a poor indicator of animal welfare (9,14).
It is important to establish the most accurate way to measure stress to reduce the mortality rate and improve the well-being of wild animals undergoing capture and handling (21). Since animals do not show a consistent response to all stressors, and since physiological changes vary with the species and the stressor (2), stress indicators must be established for each species and circumstance (9).
Acepromazine is a short-acting phenothiazine with central nervous system effects (blocking postsynaptic dopamine receptors), as well as anticholinergic, antihistaminic, antispasmodic, and α-adrenergic-blocking effects (22). This neuroleptic has been widely used as a preanesthetic or sedative agent in wild ungulates (4,23,24). The recommended intramuscular dose for sedation is 0.05 to 0.1 mg/kg for red deer (Cervus elaphus), domestic sheep (Ovis aries), domestic goats (Capra hircus), and large wild animals in general (22–24). Acepromazine has been reported to decrease heart rate, body temperature, erythrocyte count, hemoglobin concentration, packed cell volume (PCV), counts of leukocytes, lymphocytes, monocytes, and band neutrophils, activity of ALT, AST, CK, and LDH, and serum creatinine concentration in stressed wild ungulates (2,4).
The objectives of our study were to determine the stress response of Southern chamois to capture and physical restraint, to establish the best indicators of stress related to capture with drive-nets and physical restraint in this species, and to assess the effect of acepromazine on the stress response as indicated by hematologic, biochemical, and clinical parameters.
Materials and methods
Forty free-ranging Southern chamois, 25 males (20 adults and 5 yearlings) and 15 females (13 adults and 2 yearlings), were captured in drive-nets with a mesh of 10 × 10 cm (Ziboni Ornitecnica, Bergamo, Italy) in the national game reserves of Cadí (42°18′ N, 1°54′ E) and Freser-Setcases (42°22′ N, 2°09′ E), Spain. The 18 capture operations were carried out in spring (March and April) and summer (June and July) in 2000 to 2003. All captures were carried out in early morning, under similar environmental conditions, as far as could be standardized in the field. Once trapped in the net, the animals were blindfolded and freed from the net. Then, with their legs restrained, they were placed in a sack net with a mesh of 4 × 4 cm (Ziboni Ornitecnica) and kept out of direct sunlight until their release 3 h later, at the place of capture.
At the moment of capture, 19 randomly selected Southern chamois, 13 males (9 adults and 4 yearlings) and 6 adult females, were injected intramuscularly (IM) with 2.5 mg of acepromazine maleate in 0.5 mL volume (Calmo Neosan, 5 mg/mL; SmithKlineBeecham, Madrid, Spain), receiving a dose of 0.11 ± 0.02 mg/kg (mean ± standard deviation). The other 21 animals, 12 males (11 adults and 1 yearling) and 9 females (7 adults and 2 yearlings), received 0.5 mL of saline and were used as controls. Once the capture operation was finished, 17 animals in the control group, all 12 males and 5 of the females (4 adults and 1 yearling), as well as 18 animals in the treated group, all 13 males and 5 of the 6 adult females, were fitted with heart rate recording devices designed for human athletes (Polar Vantage NV and Polar S710i; Polar Electro Oy, Kempele, Finland), as previously described (2). All the animals were fitted with telemetric body- temperature recording devices (Mätman datalogger; Eltex of Sweden AB, Almut, Sweden), as previously described (2). Both heart rate and rectal temperature were measured and recorded, by means of the device software, at 60-s intervals for at least 2 h, starting 56 ± 23 min after capture and lasting until the end of the study period. For statistical analysis, the mean value for every 5-min period was calculated.
Blood samples were collected from the jugular vein at the moment of capture (time 1) and each hour thereafter for 3 h (times 2, 3, and 4), with the use of disposable 10-mL syringes and 21-gauge 1′′ needles. Of each sample, 2 mL was placed in commercial tubes with anticoagulant (ethylene diamine tetraacetic acid K3) for hematologic analyses, and the remaining blood was placed in serum collection tubes and allowed to clot at room temperature, after which the sample was centrifuged and the serum frozen at −20°C until biochemical analyses were performed.
Erythrocyte, leukocyte, and platelet counts, as well as hemoglobin concentration, were determined by means of an electric-impedance semiautomated analyzer (Sysmex F-800; Toa Medical Electronics, Kobe, Japan). The PCV was measured by the standard microhematocrit method after centrifugation at 14 000 × g for 6 min in a micro-hematocrit centrifuge (Hawksley, Lancing, England). Mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin amount (MCH) were calculated from the data obtained. The differential leukocyte count was performed by identifying 200 leukocytes in blood smears stained with a commercial Diff-Quick-like stain (Química Clínica Aplicada, Amposta, Spain). All biochemical parameters were determined by means of an automated analyzer (Cobas Mira; Roche, Nutley, New Jersey, USA) except for sodium and potassium, which were measured by flame photometry (Corning 410C; Corning Medical, Medfield, Massachusetts, USA), and cortisol, which was analyzed with a commercial enzyme-linked immunosorbent assay kit (EIA-1887; DRG Instruments, Marburg, Germany). Because the MCHC values in the treated and the control groups of females differed significantly at the time of capture, we assessed differences in the trend by converting MCHC to MCHC ratio: all means were divided by the initial value for the corresponding sex and treatment group.
We performed repeated-measures analysis of variance to detect significant differences among groups and over time within groups, using the PROC MIXED procedure of SAS System for Windows, version 8 (SAS Institute, Cary, North Carolina, USA). The main factors were treatment (acepromazine or saline) and sex; the repeated factor was time. Four groups were considered according to the 2 main factors: control males, treated males, control females, and treated females. Season, age, and interactions among factors were also included in the model. Least-square means were used owing to the unbalanced distribution of animals in the groups. The minimum accepted significance level was at least P < 0.05 for all parameters.
This study was approved by the Animal Welfare Committee of the Universitat Autònoma de Barcelona.
Results
No differences in behavior during physical restraint (as far as could be assessed) were observed.
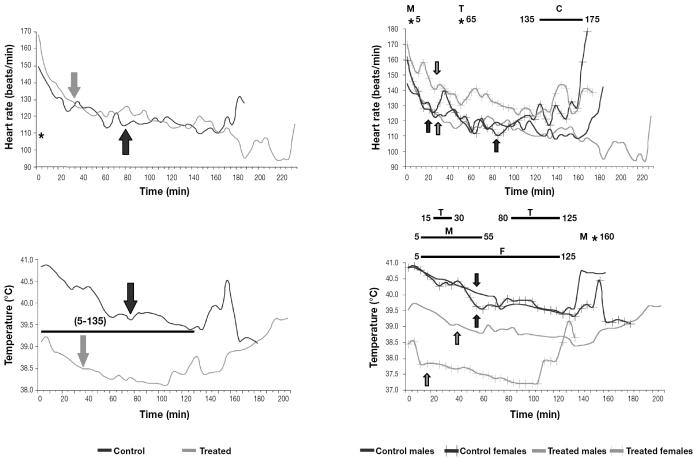
Heart rate and rectal temperature decreased from the beginning of the monitoring, 56 ± 23 min after capture (Figure 1). No treatment differences in heart rate were observed during the study, although heart rate stabilized (i.e., no significant differences were found between 2 consecutive means) earlier in the males and the treated females (at 25 to 30 min) than in the control females (at 85 min). Control females had higher heart rates than control males at the end of the study. Individual variability of heart rate did not differ significantly between the control and treated groups (16.41% ± 4.92% [mean ± standard deviation] and 16.09% ± 4.09%, respectively). However, the interindividual coefficient of variation was higher in the control animals (22.07% ± 4.21%) than in the treated animals (18.30% ± 5.28%). The body temperature was lower and stabilized earlier in the treated animals than in the control animals (Figure 1). Treated females had significantly lower temperatures than treated males from minute 15 to minute 30 and from minute 80 to minute 125.
Figure 1.
Mean heart rate and rectal temperature of 40 captured and physically restrained Southern chamois. Arrows indicate stabilization. Asterisks and bars indicate significant differences (P < 0.05) between groups. In all the figures, C — differences between control groups; T — differences between treated groups; M — differences between male groups; F — differences between female groups.
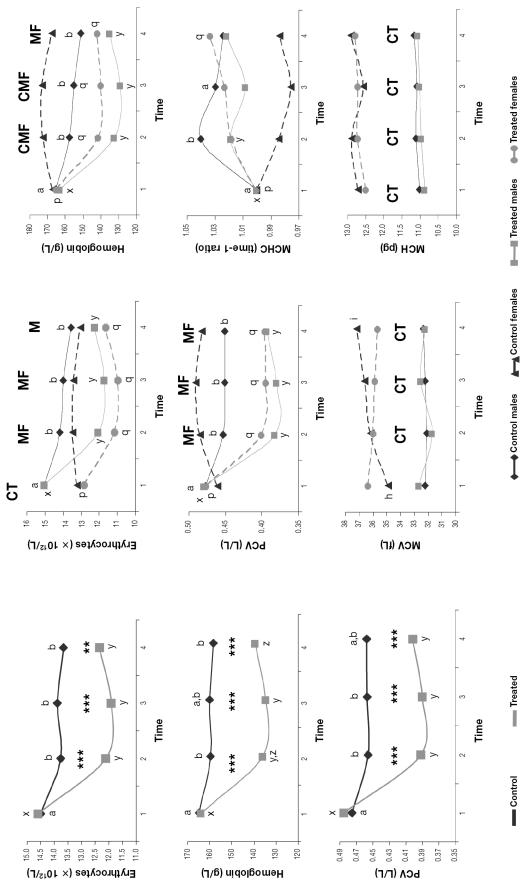
The erythrocyte count, hemoglobin concentration, and PCV decreased and the MCHC (expressed as the time-1 ratio owing to the significant differences between the groups at time 1) increased over time in all the groups except the control females (Figure 2). The erythrocyte count, hemoglobin concentration, and PCV were significantly lower in both treated groups than in the respective control groups from time 2 onward. The MCV and MCH were higher in females than in males throughout the study (Figure 2).
Figure 2.
Mean erythrocyte parameters for the 40 animals. Asterisks indicate significant differences between groups, double asterisks at P < 0.01, triple asterisks at P < 0.005. Different lower-case letters (a,b; x,y,z; h,i; p,q) indicate significant differences (P < 0.05) between means in the respective groups, and C, T, M, and F indicate significant differences (P < 0.05) between the control, treated, male, and female groups, respectively. PCV — packed cell volume; MCV — mean corpuscular volume; MCHC — mean corpuscular hemoglobin concentration; MCH — mean corpuscular hemoglobin amount.
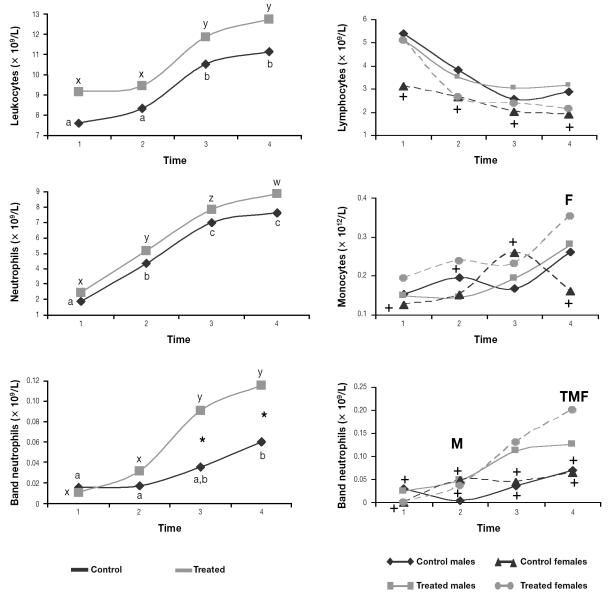
The total leukocyte and neutrophil counts increased over time in all the groups (Figure 3). The lymphocyte count decreased and the monocyte count increased over time in all the groups except the control females, whose means were considered stable. The band neutrophil count increased in the treated groups and was significantly higher at the end of the study in the treated Southern chamois than in the controls.
Figure 3.
Mean leukocyte parameters for the 40 animals. Asterisks indicate significant differences (P < 0.05) between the control and treated groups. Crosses indicate that the means were stable for the group, and T, M, and F indicate significant differences between the treated, male, and female groups, respectively.
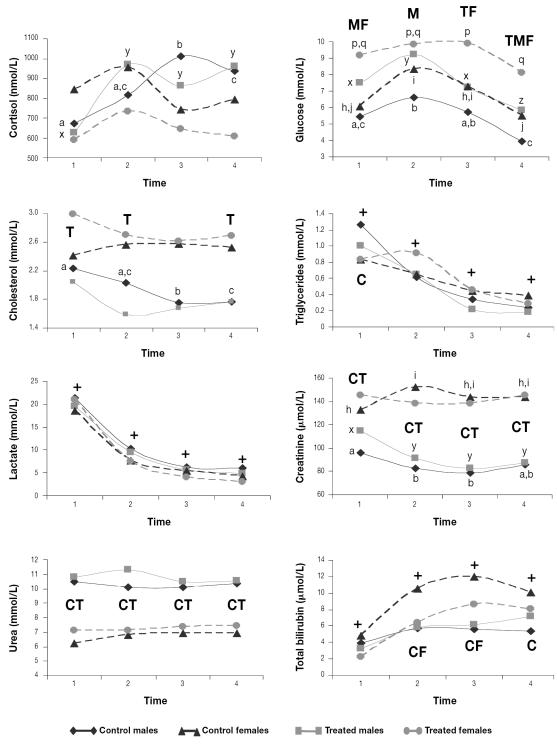
The serum cortisol concentration increased over time only in the 2 groups of males. The serum glucose concentration increased at first, then decreased until the end of the study in all 4 groups; both treated groups had significantly higher means than the respective control groups throughout the study. The serum cholesterol concentration decreased during the study only in the control males; the concentration was higher in the treated females than in the treated males during most of the study period. The serum triglyceride and lactate concentrations decreased over time in all the groups. The serum creatinine concentration was higher and the urea concentration lower in females than in males throughout the study. The serum creatinine concentration decreased during the study in both groups of males and increased in the control females. The serum bilirubin concentration increased over time in all the groups, reaching highest values in the control females (Figure 4).
Figure 4.
Mean serum biochemical parameters for the 40 animals. Crosses indicate means that changed significantly throughout the study in all 4 groups. Different lower-case letters (a,b,c; x,y,z; h,i,j; p,q) indicate significant differences (P < 0.05) between means among the control males, the treated males, the control females, and the treated females, respectively, whereas C, T, M, and F indicate significant differences between the control, treated, male, and female groups, respectively.
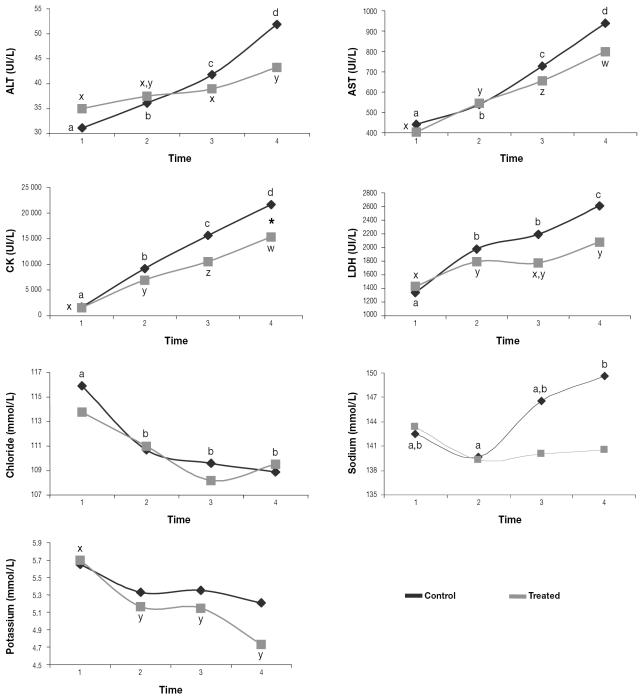
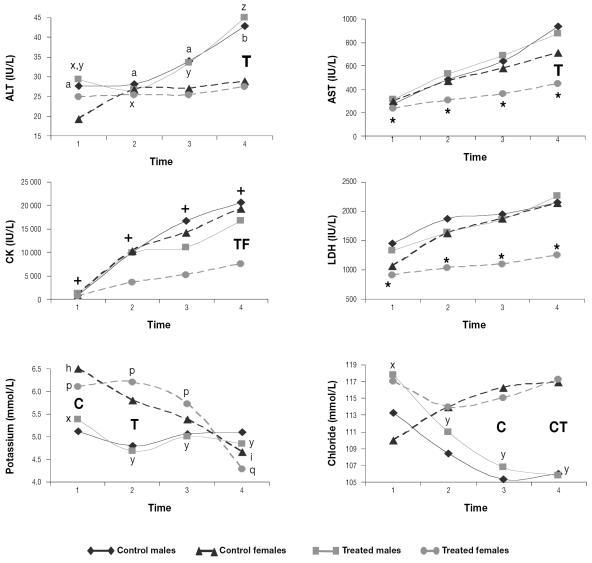
The serum activity of ALT, AST, CK, and LDH increased during the study in both the treated and control Southern chamois. However, the treated females had stable means for AST and LDH and lesser increases for ALT and CK; in addition, the treated females had lower ALT, AST, and CK activity than the treated males and lower CK activity than the control females at the end of the study (Figures 5 and 6). During the study, the serum chloride concentration decreased in the control animals and the serum potassium concentration decreased in the treated animals, whereas the serum sodium concentration increased in the control animals (Figures 5 and 6).
Figure 5.
Mean serum enzyme activity and ion concentrations for the 40 animals. The asterisk indicates a significant difference (P < 0.05) between the control and treated groups. Different lower-case letters (a,b,c,d; x,y,z,w) indicate significant differences (P < 0.05) between means in the control group and in the treated group, respectively. ALT — alanine aminotransferase; AST — aspartate aminotransferase; CK — creatine kinase; LDH — l-lactate dehydrogenase.
Figure 6.
Mean serum enzyme activity and ion concentrations for the 40 animals. Asterisks indicate means that were stable throughout the study for the group. Crosses indicate means that changed significantly throughout the study in all 4 groups. Different lower-case letters (a,b; x,y,z; h,i; p,q) indicate significant differences (P < 0.05) between means among the control males, the treated males, the control females, and the treated females, respectively, and C, T, and F indicate significant differences between the control, treated, and female groups, respectively.
Discussion
When an animal is captured and physically restrained, changes in hematologic, serum biochemical, and clinical parameters follow a biphasic pattern, depending first on catecholamines and then on corticosteroids (9). All the hematologic, serum biochemical, and clinical parameters that we analyzed are potential stress indicators (1–4,6,11–17). The stress response to capture in Southern chamois that we observed followed the general pattern previously described for stressed wild ungulates. The heart rate and rectal temperature trends agreed with those previously reported for wild ungulates (4). The increase in heart rate at capture is due to catecholamines and has been used as an indicator of acute stress, since it is an objective sign of the response of the autonomic nervous system to stressors (11,25). Captured animals may experience an increase in body temperature not only because of physical activity prior to capture but also because of stress-induced hyperthermia. The latter is time-dependent: the temperature increases in the first 10 min after capture, then returns to its baseline value after 60 to 90 min (12); thus, when our monitoring started (at 56 ± 23 min after capture), the body temperature was already increased because of stress and was starting to decline.
The erythrocyte count, PCV, hemoglobin concentration, and serum lactate and triglyceride concentrations also decreased after capture, probably indicating a return to baseline values during physical restraint after reaching their maximum at capture, as previously reported for wild ungulates (2–4,16). The erythrocyte count, hemoglobin concentration, and hematocrit increase during capture because of the effect of catecholamines on α-adrenergic receptors in the splenic capsule, inducing smooth muscle contraction and the release to the circulation of erythrocytes stored in the spleen (13).
Overall, the changes in leukocyte, lymphocyte, and neutrophil counts followed the biphasic pattern typical of stress response, with lymphocytic leukocytosis due to epinephrine and then corticosteroid-induced leukocytosis, lymphopenia, and neutrophilia (18). Corticosteroid-induced monocytosis, which depends on the species (18), seems to be characteristic of the stress response of Southern chamois.
In domestic animals, the serum glucose concentration has been reported to increase for 2 h after injection of adrenocorticotropin (26), which agrees with the trend we observed in the captured Southern chamois. The serum bilirubin concentration and the serum activity of ALT, AST, CK, and LDH increased consistently in the captured and physically restrained Southern chamois. Bilirubin increases have previously been reported in wild ungulates (3) and may be attributed to stress-related hemolysis, since ruminant hyperbilirubinemia is related to hemolytic conditions rather than to hepatic problems (18). The increases in serum ALT, AST, CK, and LDH activity are due to increased muscle-cell permeability or damage related to poor tissue perfusion induced by catecholamine vasoconstriction, decreased heat dissipation, and hypoxia (4,5,18).
The remaining parameters (cortisol, cholesterol, triglycerides, creatinine, urea, potassium, and chloride) did not show a consistent trend during the study in all the groups and therefore do not seem to be good indicators of stress induced in Southern chamois by capture and physical restraint. Nevertheless, and owing to the differences observed during the study, they can be useful in assessing physiological differences among groups. Although cortisol has traditionally been considered a good stress indicator (10,21), other authors believe that it is a poor welfare indicator because of the great interindividual differences in stress-induced corticosteroid concentrations and the difficulty of relating physiological changes and animal welfare (9,14). Lack of variation in cortisol concentration in stressed wild ungulates has been reported previously (3,4). The cholesterol concentration has been reported to increase, to decrease, or not to change with stress, so it is not considered a good welfare indicator (3,11,19,20). The serum creatinine concentration is directly related to the muscle mass of the animal, and the serum urea concentration depends largely on the diet (17,18), which could explain the lack of a specific pattern in the stress response of Southern chamois. Differences in diet and habitat could explain the consistently different urea values in both sexes, since male and female Southern chamois use different habitats (27), and different reference ranges apply to males and females (28). Catecholamines may cause an increase (α-adrenergic effect) followed by a decrease (β2 effect) in the serum potassium concentration, and short-term administration of corticosteroids causes transitory hyperkalemia (29). Chloride is exchanged by potassium in the kidney (30). This biphasic and multifactorial cause of variation could explain why potassium and chloride are not useful as stress indicators in Southern chamois.
Acepromazine has been reported to prevent the adverse effects of stress in captured wild ungulates not only because of its sedative effect but also because of peripheral vasodilation (2,4). The lack of differences between the control and treated males in our study in both mean heart rate and individual variability agrees with previously reported data for wapiti, red deer, and roe deer (Capreolus capreolus); in all cases this finding was attributed to reflex tachycardia secondary to hypotension caused by the drug (4,22,31). The use of heart rate variability has been proposed as a better stress indicator than basic heart rate (32). The earlier stabilization of heart rate in the treated female Southern chamois, as well as the lower interindividual variation of heart rate in the treated group, could be attributed to the tranquilizing and vascular effects of the drug, as previously described in red and roe deer (4,31). A more uniform response may permit a better prediction of the consequences of capture and make it easier to establish corrective measures for deviations from the “normal” response.
We observed that acepromazine shortened the period of capture-related stress-induced hyperthermia, as demonstrated by the earlier temperature decrease and stabilization in the treated Southern chamois, and it reduced the intensity of the hyperthermia: body temperature was significantly lower in the treated animals during most of the study. Hypothermia is an additional pharmacologic action of acepromazine. By depressing the reticular activating system and inducing vasodilation by both central and α-adrenergic effects, acepromazine could help dissipate heat (22). This hypothermic effect may modulate stress-induced hyperthermia and prevent capture myopathy, since hyperthermia and metabolic acidosis are the 2 central factors in the development of this condition (5).
As in our treated Southern chamois, significantly lower values for erythrocyte count, hemoglobin concentration, and hematocrit have been described in sedated or anesthetized wild ungulates (6,13,33). This can be explained by spleen smooth-muscle relaxation, with consequent sequestration of erythrocytes, due to the α-adrenergic-blocking effect of acepromazine, as previously reported (4,13,22,33). Erythrocytes stored in the spleen have a higher MCV (and, consequently, a lower MCHC) than circulating erythrocytes, since the cells mature in the spleen after being released from the bone marrow (13). The MCV increase only in control females further suggests splenic smooth-muscle relaxation as the origin of the lower erythrocyte count, hemoglobin concentration, and hematocrit in the other 3 groups. Higher band neutrophil counts at the end of our study in the treated Southern chamois could be explained by faster evolution of catecholamine-induced lymphocytosis to corticosteroid-induced neutrophilia in this group. Increases in glucose values in wild ungulates medicated with α-adrenergic anesthetics have previously been reported (34,35) and could explain the higher glucose values of the treated Southern chamois. In our study, the later increase in serum ALT activity in the treated males compared with the control males, the lack of increase in serum AST and LDH activity over time in the treated females, and the significantly lower CK values at time 4 in the treated females compared with the control females indicate a protective effect of acepromazine on muscle. This effect has previously been reported in wild ungulates (4) and was attributed to dilation of muscle arterioles by α-adrenergic-receptor blockade or β2-adrenergic-receptor stimulation (15,36). Catecholamine-induced vasoconstriction of renal arterioles and proximal tubules stimulates sodium reabsorption (37). The lack of increase in sodium concentration only in the treated Southern chamois could indicate protection by acepromazine against catecholamine’s effects on the renal arterioles.
We found sex differences in stress response between the Southern chamois. The later stabilization of heart rate and the higher heart rate from minute 135 to minute 175 in the control females compared with the control males seem to point to a greater sympathetic response in females than in males. In humans, a stronger vasodilation effect of corticotropin-releasing hormone (CRH) in females than in males has been reported (38). Similar mechanisms could be the reason for the significantly lower temperatures of the treated females in our study compared with the treated males. The lack of decrease over time in erythrocyte, lymphocyte, band neutrophil, and monocyte counts, PCV, and hemoglobin concentration in the control females suggests stronger and longer-lasting adrenergic stimulation in this group. The higher serum glucose values in the treated females than in the treated males seem to point to a greater and longer-lasting stress response in females. Higher bilirubin values in the control females than in the control males could indicate a higher degree of stress-related hemolysis in the former, and it is probably related to the lack of decrease in circulating erythrocytes over time and the higher catecholamine response in the control females. Creatinine increases related to capture stress have been reported in wild ungulates and were attributed to catecholamine-induced renal vasoconstriction (2,4,17). Since no significant differences were found in live weight between the sexes, the higher serum creatinine values of the Southern chamois females could be related to stronger catecholamine-induced renal vasoconstriction, indicating a stronger stress response, in the females. Both chloride and potassium increases have been related to physical capture or stress (1,6). The higher serum concentrations of potassium at times 1 and 2 and of chloride at times 3 and 4 in the females and the lack of decrease in the former over time only in the control females further suggest a stronger catecholamine-induced vasoconstriction in the muscles and kidneys of females. The increases over time in cortisol concentration only in the males could indicate a more intense response of the hypothalamic–pituitary–adrenal-cortex axis in males than in females. Sex differences in the free serum glucocorticoid concentration, with higher and increasing cortisol values in males, have been reported in polygynous species (38). It has been suggested that cholesterol could be used for corticosteroid synthesis during the stress response, and decreases in serum cholesterol concentration have been related to the corticosteroid stress response (19). Higher cholesterol values in treated females than in treated males and decreasing cholesterol values in control males could be due to the increasing cortisol concentration over time only in males. Higher muscle enzyme values in males have been previously reported in different species of wild ungulates (1,39), but differences between the treated groups in our study were found only for CK.
Some of the effects of acepromazine seemed to be stronger or were seen only in the female Southern chamois. This could be explained by the higher adrenergic stress response of this sex, which would make the beneficial actions of the drug more evident. The creatinine increase only in the control females suggests that the α-adrenergic-blocking effect of acepromazine on renal arterioles reduced the catecholamine-mediated vasospasm in treated females, promoting vasodilation and allowing normal filtration and excretion of creatinine (4,33). The significantly lower serum ALT, AST, CK, and LDH activity in the treated females compared with the treated males indicates a stronger vasodilation effect of acepromazine in females than in males. The stronger vasodilation effect induced by CRH in females and the attenuated muscle inflammatory response described in human females (40) could also contribute to explaining the lower serum enzyme activity in this sex.
Conclusion
The stress response to capture and physical restraint in Southern chamois follows the general pattern previously described for other species of wild ungulates (1,3,4), and the response can be modulated and its adverse effects partially prevented by acepromazine. The advantages of acepromazine are mainly due not to its sedative effect but, rather, to its α-adrenergic-blocking effects, which include the blockade of smooth-muscle contraction in the spleen and renal and muscle vasodilation. Acepromazine also decreases the intensity and duration of stress-induced hyperthermia and the interindividual variability of the heart rate. Use of acepromazine improves the welfare of captured Southern chamois by decreasing the risk of stress-related conditions, since hyperthermia and the muscle and renal damage related to renal vasoconstriction are involved in the pathogenesis of capture myopathy (5). The stress response differs between males and females, females seeming to be more reactive to α-adrenergic stimulation by catecholamines (as indicated by heart rate, erythrocyte count, hemoglobin concentration, PCV, lymphocyte count, and serum concentrations of glucose, creatinine, chloride, and potassium). The adrenal-cortex reaction, however, seems to be stronger in males, as indicated by their increasing cortisol and decreasing cholesterol concentrations. Acepromazine’s protective effect is stronger in females, as indicated by erythrocytic parameters, temperature, muscle enzyme activity, and serum concentrations of potassium and chloride, which seems a logical consequence of the greater catecholamine stimulation in this sex and the α-adrenergic- blocking mechanism of the beneficial effect of acepromazine. Thus, the stress response may be physiologically more intense and acepromazine’s α-adrenergic effect more protective in females than in males.
Acknowledgments
We thank profusely the rangers and staff of the national game reserves of Cadí, Cerdanya-Alt Urgell, and Freser-Setcases for their invaluable collaboration in the capture of the animals. This work was funded as research project AGF99-0763-C02 of the Comisión Interministerial de Ciencia y Tecnología and by an FPI (Formación de Personal Investigador) grant from the Universitat Autònoma de Barcelona. A native English-speaking instructor in English of the Universitat Autònoma de Barcelona provided editorial assistance. We are also grateful to 2 anonymous reviewers, whose suggestions considerably improved the manuscript.
References
- 1.Kock MD, Clark RK, Franti CE, Jessup DA, Wehausen JD. Effects of capture on biological parameters in free-ranging bighorn sheep (Ovis canadensis): evaluation of normal, stressed and mortality outcomes and documentation of postcapture survival. J Wildl Dis. 1987;23:652–662. doi: 10.7589/0090-3558-23.4.652. [DOI] [PubMed] [Google Scholar]
- 2.López-Olvera JR, Marco I, Montané J, Lavín S. Transport stress in Southern chamois (Rupicapra pyrenaica) and its modulation by acepromazine. Vet J. In press. [DOI] [PubMed]
- 3.Marco I, Viñas L, Velarde R, Pastor J, Lavín S. Effects of capture and transport on blood parameters in free-ranging mouflon (Ovis ammon) J Zoo Wildl Med. 1997;28:428–433. [PubMed] [Google Scholar]
- 4.Montané J, Marco I, López J, Perpinan D, Manteca X, Lavín S. Effects of acepromazine on capture stress in roe deer (Capreolus capreolus) J Wildl Dis. 2003;39:375–386. doi: 10.7589/0090-3558-39.2.375. [DOI] [PubMed] [Google Scholar]
- 5.Williams ES, Thorne T. Exertional myopathy (capture myopathy). In: Fairbrother A, Locke LN, Hoff GL, eds. Noninfectious Diseases of Wildlife. 2nd ed. Ames, Iowa: Iowa State University Press, 1996:181–193.
- 6.Peinado VI, Fernández-Arias A, Viscor G, Palomeque J. Haematology of Spanish ibex (Capra pyrenaica hispanica) restrained by physical or chemical means. Vet Rec. 1993;132:580–583. doi: 10.1136/vr.132.23.580. [DOI] [PubMed] [Google Scholar]
- 7.Shackleton DM, ed; IUCN/SSC Caprinae Specialist Group. Wild Sheep and Goats and Their Relatives. Gland and Cambridge, England: International Union for Conservation of Nature and Natural Resources, 1997.
- 8.Berducou C. Chamois et isards: bilan des captures par filets, pièges et engins divers realisées en France au cour des trente dernières années (1958–1989). In: Dubray D, ed. Techniques de capture et de marquage des ongulés sauvages. Montpellier, France: FDC de l’Hérault, 1993:113–120.
- 9.Moberg GP. Problems in defining stress and distress in animals. J Am Vet Med Assoc. 1987;191:1207–1211. [PubMed] [Google Scholar]
- 10.Waas JR, Ingram JR, Matthews LR. Real-time physiological responses of red deer to translocations. J Wildl Manage. 1999;63:1152–1162. [Google Scholar]
- 11.Broom DM, Johnson KG, eds. Stress and Animal Welfare. London, England: Chapman & Hall, 1993.
- 12.Moe RO, Bakken M. Effects of handling and physical restraint on rectal temperature, cortisol, glucose and leukocyte counts in the silver fox (Vulpes vulpes) Acta Vet Scand. 1997;38:29–39. doi: 10.1186/BF03548505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross JP, Mackintosh CG, Griffin JFT. Effect of physical restraint and xylazine sedation on haematological parameters in red deer (Cervus elaphus) Res Vet Sci. 1988;45:281–286. [PubMed] [Google Scholar]
- 14.Rushen J. Problems associated with the interpretation of physiological data in the assessment of animal welfare. Appl Anim Behav Sci. 1991;28:381–386. [Google Scholar]
- 15.Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 10th ed. Philadelphia, Pennsylvania: WB Saunders, 2000:253–262.
- 16.Hatting J, Pitts NI, Ganhao MF. Immediate response to repeated capture and handling in wild impala. J Exp Zool. 1988;248:109–112. doi: 10.1002/jez.1402480114. [DOI] [PubMed] [Google Scholar]
- 17.Finco DR. Kidney function. In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals. 5th ed. San Diego, California: Academic Press, 1997:441–484.
- 18.Duncan JR, Prasse KW, Mahaffey EA, eds. Veterinary Laboratory Medicine. Clinical Pathology. Ames, Iowa: Iowa State University Press, 1994.
- 19.Locatelli A, Agnes F, Sartorelli P, Tozzi F, Arrigoni C, Dominoni S. Rilievi ematologici sul transporto simulato nel vitello. Summa. 1988;5:21–27. [Google Scholar]
- 20.Montané J, Marco I, López-Olvera J, Manteca X, Lavín S. Transport stress in roe deer (Capreolus capreolus) Anim Welfare. 2002;11:405–417. [Google Scholar]
- 21.Morton DJ, Anderson E, Foggin CM, Kock MD, Tiran EP. Plasma cortisol as an indicator of stress due to capture and translocation in wildlife species. Vet Rec. 1995;136:60–63. doi: 10.1136/vr.136.3.60. [DOI] [PubMed] [Google Scholar]
- 22.Plumb DC, ed. Veterinary Drug Handbook. 3rd ed. Ames, Iowa: Iowa State University Press, 1999:1–3.
- 23.Arnemo JM, Negard T, Soli NE. Deer farming in Norway. A review of the currently available drugs that can be used for immobilization, pain relief and anaesthesia. Norsk Veterinaertidsskrift. 1993;105:517–521. [Google Scholar]
- 24.Nielsen L, ed. Chemical Immobilization of Wild and Exotic Animals. Ames, Iowa: Iowa State University Press, 1999:105.
- 25.Hopster H, Blockhuis HJ. Validation of a heart rate monitor for measuring a stress response in dairy cows. Can J Anim Sci. 1994;74:465–474. [Google Scholar]
- 26.Hartmann H. Critères biochimiques et hématologiques du stress et leurs relations avec les mécanismes de défense. Rec Med Vet. 1988;164:743–750. [Google Scholar]
- 27.Catusse M, Corti R, Cugnase JM, Dubray D, Gibert P, Michellet J. Les chamois et les isards. In: La Grande Faune de Montagne. Paris, France: Haitier Littérature Générale, 1996:17–69.
- 28.López-Olvera JR, Marco I, Montané J, Lavín S. Haematological and serum biochemical values of Southern chamois (Rupicapra pyrenaica) captured by drive-net. Vet Rec. 2006;158:479–484. doi: 10.1136/vr.158.14.479. [DOI] [PubMed] [Google Scholar]
- 29.Bia MJ, DeFronzo RA. Extarrenal potassium homeostasis. Am J Physiol. 1981;240:257–268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 30.Autrain de Morais H. Disorders of chloride. In: DiBartola SP, ed. Fluid Therapy in Small Animal Practice. 2nd ed. Philadelphia, Pennsylvania: WB Saunders, 2000:73–82.
- 31.Diverio S, Goddard PJ, Gordon IJ. Use of long-acting neuroleptics to reduce the stress response to management practices in red deer. Appl Anim Behav Sci. 1996;49:83–88. [Google Scholar]
- 32.Porges SW. Spontaneous oscillations in heart rate: potential index of stress. In: Moberg GP, ed. Animal Stress. Bethesda, Maryland: American Physiological Society, 1985:97–112.
- 33.Marco I, Lavín S. Effect of the method of capture on the haematology and blood chemistry of red deer (Cervus elaphus) Res Vet Sci. 1999;66:81–84. [Google Scholar]
- 34.Jalanka HH. Evaluation of medetomidine and ketamine induced immobilization in markhors (Capra falconeri megaceros) and its reversal by atipamezole. J Zoo Anim Med. 1988;19:95–105. [Google Scholar]
- 35.Arnemo JM, Negard T, Soli NE. Chemical capture of free-ranging red deer (Cervus elaphus) with medetomidine-ketamine. Rangifer. 1994;14:123–127. [Google Scholar]
- 36.Booth NH. Psychotropic agents. In: Booth NH, McDonald LE, eds. Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982:321–345.
- 37.DiBartola SP. Disorders of sodium and water: hypernatremia and hyponatremia. In: DiBartola SP, ed. Fluid Therapy in Small Animal Practice. 2nd ed. Philadelphia, Pennsylvania: WB Saunders, 2000:45–72.
- 38.Boonstra R. Equipped for life: the adaptive role of the stress axis in male mammals. J Mammal. 2005;86:236–247. [Google Scholar]
- 39.Chapple RS, English AW, Mulley RC, Lepherd EE. Haematology and serum biochemistry of captive unsedated chital deer (Axis axis) in Australia. J Wildl Dis. 1991;27:396–406. doi: 10.7589/0090-3558-27.3.396. [DOI] [PubMed] [Google Scholar]
- 40.Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]