Abstract
This study investigated whether the abnormal prion protein (PrPSc) in tissues from sheep with scrapie would be destroyed by composting. Tissues from sheep naturally infected with scrapie were placed within fiberglass mesh bags and buried in compost piles for 108 d in experiment 1 or 148 d in experiment 2. The temperature in the compost piles rose quickly; it was above 60°C for about 2 wk and then slowly declined to the ambient temperature. Before composting, PrPSc was detected in all the tissues by Western blotting. In experiment 1, PrPSc was not detected after composting in the tissue remnants or the surrounding sawdust. In experiment 2, 1 of 5 specimens tested negative after composting, whereas PrPSc was detected in the other 4 bags, though in reduced amounts compared with those before composting. Tissue weights were reduced during composting. Analysis of the tissue remnants for microbial 16S ribosomal DNA demonstrated that there were more diverse microbes involved in experiment 1 than in experiment 2 and that the guanine and cytosine content of the microbial 16S DNA was higher in the specimens of experiment 1 than in those of experiment 2, which suggests greater dominance of thermophilic microbes in experiment 1. These results indicate that composting may have value as a means for degrading PrPSc in carcasses and other wastes.
Résumé
Afin de déterminer si la protéine prion anormale (PrPSc) retrouvée dans les tissus de moutons atteint de tremblante serait détruite par le compostage, des tissus provenant de moutons infectés naturellement par la tremblante ont été placés à l’intérieur de sacs en filet de fibre de verre et enterrés dans des tas de compost pour 108 j lors de la première expérience ou 148 j lors de la deuxième expérience. La température dans le tas de compost s’est élevée rapidement; elle s’est maintenue au dessus de 60 °C pendant environ 2 semaines et déclina lentement par la suite jusqu’à température ambiante. Avant le compostage, la PrPSc a été détectée par immunobuvardage dans tous les tissus. Lors de l’expérience 1, la PrPSc n’a pas été détectée après compostage dans les restes de tissus ou le brin de scie à proximité. Lors de l’expérience 2, 1 des 5 spécimens s’est avéré négatif après le compostage alors que la PrPSc a été détectée dans les quatre autres sacs, mais en quantité moindre qu’avant le compostage. Le poids des tissus a diminué durant le compostage. Une analyse des restants de tissus pour la présence d’ARN 16S microbien a permis de démontrer qu’il y avait une plus grande variété de microbes impliqués dans l’expérience 1 que dans l’expérience 2 et que le contenu en guanine et cytosine de l’ADN 16S était plus élevé dans les spécimens de l’expérience 1 comparativement à l’expérience 2, ce qui suggère une plus grande dominance des agents thermophiles dans l’expérience 1. Ces résultats indiquent que le compostage pourrait avoir une certaine efficacité comme moyen pour dégrader la PrPSc dans les carcasses et autres résidus.
(Traduit par Docteur Serge Messier)
Introduction
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative diseases that include scrapie in sheep and goats, chronic wasting disease (CWD) in deer and elk, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease in humans (1). The TSEs are characterized by vacuolation and loss of neurons in the central nervous system (CNS) and by the accumulation of abnormal prion protein (PrPSc), the disease isoform of the host’s normal cellular prion protein (PrPc) in animals with scrapie (1). Although the exact nature of the agent causing TSEs is controversial, PrPSc is associated with pathogenesis and is believed to be a major component of the causative agent (1).
The PrPSc is highly resistant to conventional procedures for inactivation and destruction that would destroy microorganisms, nucleic acids, and proteins (2). Incineration and burial are the 2 methods acceptable for disposal of animal carcasses affected by TSEs in many countries, including Canada (3). However, these procedures have limitations due to cost, availability of relevant facilities, and local regulations. Therefore, an alternative, safe, and economical procedure for disposing of animal carcasses and other biologic wastes of animals affected or potentially affected by TSEs is needed. Recent reports indicate that microbial activity could have a role in destroying abnormal prions. The infectivity of the BSE agent was reduced by treatment with thermophilic proteases (4). In addition, treatment of tissues with keratinase from Bacillus licheniformis in the presence of detergents and heating to more than 100°C degraded PrPSc in the tissues to levels undetectable by an immunologic test (5). Also, proteases from anaerobic thermophilic prokaryotes and Streptomyces subspecies degraded PrPSc in brain homogenates of mice infected with a BSE strain (6).
Composting, the decomposition of once-living materials by the digestive activities of microbes, has been used for disposal of animal carcasses (7). Although studies have suggested that PrPSc could be degraded by bacterial enzymes of selected microbes (4–6), no studies have been done to determine if the intensive microbial activity that results in degradation of animal carcasses during composting would destroy the abnormal prions associated with TSEs. The objective of this study was to investigate the degradation of PrPSc during composting as determined by a sensitive Western blot (WB) procedure.
Materials and methods
Samples
Tissues from the CNS, lymphoid system, and various organs of 3 sheep with natural scrapie infection in Canada were used. The scrapie status was diagnosed by immunochemical and histopathologic study of the CNS and lymphoid tissues (8). The samples were kept at −80°C and were tested for PrPSc by WB before composting to ensure that PrPSc was detectable.
In the 1st of 2 experiments, tissues from the CNS, lymph nodes, ileum, spleen, liver, kidney, heart, and pancreas from 2 of the scrapie-positive sheep were divided into 4 groups (Table I). Three groups were placed in open petri dishes and covered with 2 cm of sawdust, and then each dish was placed inside a separate bag (approximately 13 × 17 cm) made of fiberglass mesh (Bay Mills, Brampton, Ontario) that would allow penetration of air and moisture. The 4th group of tissues was placed in the same type of bag, where the tissues were surrounded with about 2 cm of sawdust. In the 2nd experiment, the CNS tissues from the remaining scrapie-positive sheep were divided among 5 more bags and surrounded with 2 cm of sawdust within the bags.
Table I.
Specimen composition and weight and results of Western blot (WB) testing for abnormal prion protein (PrPSc) in animals with scrapie before and after composting
| Weight (g)b |
WB resultc |
|||||
|---|---|---|---|---|---|---|
| Experiment number | Sheep number | Bag numbera | On day 0 | After composting (and % reduction) | On day 0 | After composting |
| 1 | 1 | 1 | 92.5 | 19.3(79.1) | +++ | − |
| 2 | 54.1 | 7.8(85.6) | +++ | − | ||
| 3 | 109.1 | 8.9(91.8) | +++ | − | ||
| 4 | 388.7 | 10.8(97.2) | +++ | − | ||
| 2 | 1 | 74.4 | 9.0(87.9) | +++ | − | |
| 2 | 59.8 | 8.7(85.4) | +++ | − | ||
| 3 | 83.5 | 19.8(76.3) | +++ | − | ||
| 4 | 319.5 | 19.2(94.0) | +++ | − | ||
| 2 | 3d | 1 | 5.5 | 1.4(74.5) | ++++ | ± |
| 2 | 7.9 | 3.9(50.3) | ++++ | + | ||
| 3 | 12.8 | 2.1(83.6) | ++++ | + | ||
| 4 | 8.5 | 1.4(83.5) | ++++ | + | ||
| 5 | 15.2 | 4.0(73.7) | ++++ | − | ||
For bags 1 to 3 in experiment 1, the samples were placed in open petri dishes and were covered with sawdust, then the dishes were placed within mesh bags. The samples in bag 4 were surrounded with sawdust inside the mesh bag.
Weights (in grams) of individual tissues in experiment 1 were as follows. Sheep 1: bag 1 — central nervous system (CNS) 18.9, lymph node 17.7, kidney 55.9; bag 2 — lung 31, spleen 23.1; bag 3 — heart 73.6, intestine 35.5; bag 4 — CNS 14.2, lymph node 17.1, kidney 58, lung 36.8, intestine 34.8, liver 114, spleen 26.5, heart 87.3. Sheep 2: bag 1 — CNS 15, lymph node 6.8, kidney 46, diaphragm 6.6; bag 2 — lung 43, spleen 16.8; bag 3 — heart 64, intestine 7.7, pancreas 11.8; bag 4 — CNS 15.8, lymph node 8.2, kidney 39, lung 35, intestine 10.5, liver 108, spleen 17, heart 73, pancreas 13.
The testing involved enrichment by precipitation with sodium phosphotungstic acid. The intensity of the bands was rated as ++++ (bands very intense; difficult to clearly differentiate their boundaries), +++ (bands very intense, with identifiable boundaries), ++ (clear, typical bands), + (only 1 or 2 bands visible), ± (suspect: atypical band pattern), or − (no bands visible).
Bags 1 and 2 contained samples of frontal cerebrum, bag 3 middle cerebrum, bag 4 anterior cerebrum, and bag 5 cerebellum and brain stem.
Composting
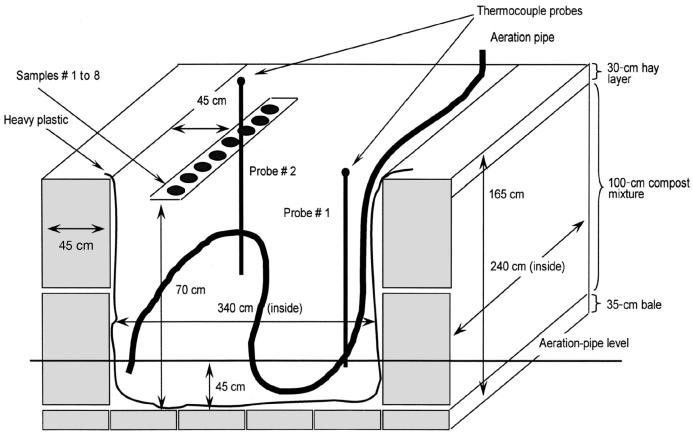
Static-pile passive-aeration composting was done in 2 insulated bins built in a level-2 isolation facility to provide biosecurity (9). The bins were constructed indoors, with rectangular bales of hay 45 cm wide used as walls. The cement floor was covered with about 35 cm of hay, and the interior of the bins was lined with heavy plastic to contain all liquids. Excess plastic was laid over the top of the walls. The internal dimensions of the bins were 340 × 240 × 135 cm (length × width × height).
Manure to be composted was collected from a barn where healthy cattle had been bedded on straw. Manure and straw were mixed in a feed mixer, and water was added to bring the moisture content to about 70%. After each bin was loaded to a depth of 45 cm, a perforated, flexible plastic drainage pipe 10 cm in diameter was laid over the manure and bent back and forth in an “N” shape. One end of the pipe penetrated the wall of hay and plastic at a point about 60 cm above the floor; the other end came out at the top of the pile on the opposite side to provide aeration. The bin was then filled slightly above the height of the walls, and 2 probes containing thermocouples were inserted into the bins to monitor the temperature. The contents of both bins weighed approximately 3000 kg.
The sample bags were buried about 20 to 40 cm from the top of the compost piles and were at least 10 cm apart in experiment 1 and 45 cm apart in experiment 2 (Figure 1). All specimens were at least 45 cm from the edge of the piles. The top of each pile was then covered with vapor-barrier fabric that helped to preserve moisture, and the fabric was covered with a 30-cm-thick layer of straw to provide insulation.
Figure 1.
Diagram of compost-pile structure for experiment 1; the structure for experiment 2 was similar. The walls and floor of the bin were bales of hay, and the interior was lined with heavy plastic. A perforated, flexible plastic drainage pipe, 10 cm in diameter, was laid towards the bottom of the pile to provide aeration. The pile was covered with vapor-barrier fabric. The fiberglass bags containing the specimens were placed 20 to 40 cm below the surface of the pile, which was a mixture of cow manure and straw. Two thermocouple probes were located close to the specimens to enable temperature monitoring by a computerized system.
The piles were not disturbed until the sample bags were removed for testing after 108 or 148 d in the 1st and 2nd experiments, respectively. The bags were stored in plastic bags at −20°C and later thawed for testing. Remnants of tissues were collected and weighed. The darkened sawdust immediately around the tissues was also collected for testing.
Sample preparation for detection of PrPSc
Specimens tested for the presence of PrPSc by WB were control tissues that were not composted, the remnants of tissues after composting, and the dark-stained sawdust that surrounded the tissues. A WB lysis buffer containing 0.5% Nodinet P-40, 0.5% sodium deoxycholate in 10 mM Tris buffer, pH 7.5 (8), was added to each remnant and to the sawdust to yield 20% and 40% homogenates, respectively. The composted samples were blended with a Stomacher 80 Laboratory Blender (Seward Medical, London, England) at high speed for 5 min, then centrifuged at 1300 × g for 15 min to form a pellet of the debris, which largely consisted of wood and plant fiber. The supernatants were homogenized with Zirconia microbeads 1 mm in diameter (BioSpec Products, Inc., Bartlesville, Oklahoma, USA) by means of a FastPrep FP120 cell disrupter (Bio-101, Thermo Savant; Q-Biogene, Carlsbad, California, USA) at a speed setting of 6.5 for 45 s (8). The homogenate was incubated at room temperature for 30 min and further processed for detection of PrPSc or microbial DNA or stored at −20°C. The control tissues that were not composted were homogenized in WB lysis buffer as a 20% homogenate with the FastPrep FP120 cell disrupter and then incubated at room temperature for 30 min.
Western blot analysis
The WB test was conducted on samples enriched for PrPSc by means of sodium phosphotungstic acid (PTA) precipitation, as described previously (8). Briefly, a 20% homogenate was treated with 4% N-lauroyl sodium sarcosinate (Sigma, Oakville, Ontario) in phosphate-buffered saline, pH 7.4, and DNAse I (Sigma), 100 μg/mL. After centrifugation, the supernatant was treated with proteinease K (PK; Sigma) at a final concentration of 50 μg/mL, and the PK activity was stopped by the addition of Pefablock SC (Roche Diagnostics, Laval, Quebec). The supernatant was incubated with PTA and then centrifuged at 16 249 × g for 30 min. The pellet was resuspended in water. For the sample used as the PK-positive control, a 10% homogenate adjusted in lysis buffer was treated with PK and the PK activity stopped by Pefablock. For the sample used as the PK-negative control, a 10% homogenate adjusted in lysis buffer was further diluted with an equal volume of sterile water.
Each sample underwent sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) on NuPAGE precast 12% Bis-Tris-buffered gels with relevant reagents (Invitrogen, Burlington, Ontario). The gels were then electroblotted with a semidry transfer apparatus (Bio-Rad Laboratories, Mississauga, Ontario) for 1.5 h at 15 V onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). After blocking with blocking buffer for 1 h at room temperature or overnight at 4°C, the membrane was incubated for 1 h at room temperature with 3.5 μg/mL of monoclonal antibody F99/97.6.1 (10) (VMRD, Pullman, Washington, USA). Next, the membrane was incubated with goat polyclonal serum against mouse immunoglobulin conjugated with horseradish peroxidase (1:3000) (Bio-Rad) diluted in blocking buffer. The membrane was then incubated with the chemiluminescent substrate ECL Plus (Amersham Biosciences, Uppsala, Sweden) for 5 min. Chemiluminescent signals were visualized by exposing the membrane to radiographic film (Eastman Kodak Company, Rochester, New York, USA).
The intensities of the WB bands could not be accurately analyzed quantitatively by an imaging program owing to highly saturated PrPSc bands in the PTA-concentrated samples on day 0. Therefore, the bands were analyzed qualitatively, the intensity being rated as ++++ (bands very intense; difficult to clearly differentiate their boundaries), +++ (bands very intense, with identifiable boundaries), ++ (clear, typical bands), + (only 1 or 2 bands visible), ± (suspect: atypical band pattern), or − (no bands visible).
Microbial DNA extraction and genetic profiling
We extracted DNA from the lysed samples with the FastDNA kit for soil (Q-Biogene) according to the manufacturer’s instructions. Nested primer pairs (Table II) were used to amplify 16S ribosomal DNA (rDNA) genes from the total DNA of the remnants or the stained surrounding sawdust after composting. Primary and nested polymerase chain reaction (PCR) assays were performed as described previously (11,12). Denaturing gradient gel electrophoresis (DGGE) was performed with a DCode universal mutation detection system and the DCode control reagent kit (Bio-Rad). The nested PCR products were analyzed with the use of 6% (w/v) acrylamide gels (acrylamide-N,N′-methylenebisarylamide, 37.5:1) with a denaturing gradient from 20% to 80%, created according to the manufacturer’s instructions. The electrophoresis was performed in 1 × TAE (40 mM Tris [Sigma-Aldrich, St. Louis, Missouri, USA], 20 mM acetic acid [Fisher Scientific, Fair Lawn, New Jersey, USA], and 1 mM ethylene diamine tetraacetic acid, pH 8.3 [EM Science, Darmstadt, Germany]), pH 8.0, at 150 V and 60°C for 5 h. After electrophoresis, the gels were stained with ethidium bromide (50 μg/mL) and photographed with ultraviolet transillumination by means of a Chemi Doc system (Bio-Rad).
Table II.
Primers used for microbial DNA analysis
| Primer (and reference) | Nucleotide sequence (5′–3′)a | Positionb | Target region of 16S ribosomal DNA |
|---|---|---|---|
| 8f (11) | CAC GGA TCC AGA GTT TGA T (C/T) (A/C) TGG CTC AG | 8 | Entire 16S rDNA |
| 1510r (11) | GTG AAG CTT ACG G(C/T)T ACC TTG TTA CGA CTT | 1510 | Entire 16S rDNA |
| Com1 (12) | CAG CAG CCG CGG TAA TAC | 519 | V4–V5 |
| Com2-GC (12) | CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGG CCG TCA ATT CCT TTG AGT TT | 907 | V4–V5 |
The underlined portion indicates GC-clamp, a DNA sequence rich in guanine and cytosine that is attached to the primer to avoid complete strand separation for detection of all possible base substitutions in the target fragment using denaturing-gradient gel.
Based on the sequence in Escherichia coli.
Results
Physical characteristics of compost piles and specimens
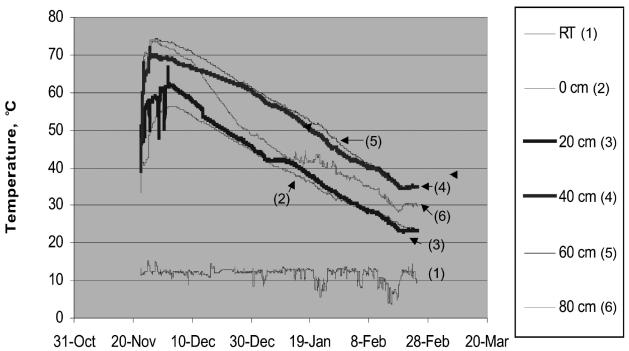
In both experiments, the temperature in the compost piles increased rapidly and was between 60°C and 70°C for about 2 wk initially; it then gradually declined to room temperature, about 20°C to 30°C (Figure 2).
Figure 2.
Temperature 0 to 80 cm from the bottom of the compost pile in experiment 1; the pattern in experiment 2 was similar. RT — room temperature inside the facility.
After storage in plastic bags at −20°C, the mesh bags removed from the compost piles were examined. The sawdust in close contact with the tissue remnants held together as a frozen mass, whereas the sawdust further from the remnants contained less moisture and readily crumbled away from the mass. In experiment 1, after 108 d of composting, the tissue remnants were reduced in size and, although moist, were much drier than fresh tissues. The sawdust that formed the frozen mass was brown; the remaining sawdust in the bags had undergone less color change. The average weight of tissue per bag on day 0 had been 147.70 g in experiment 1 and 9.98 g in experiment 2. The reduction in weight after composting was 76.3% to 97.2% per bag in experiment 1 and 50.3% to 83.7% in experiment 2. (Table I).
Microbial DNA analysis
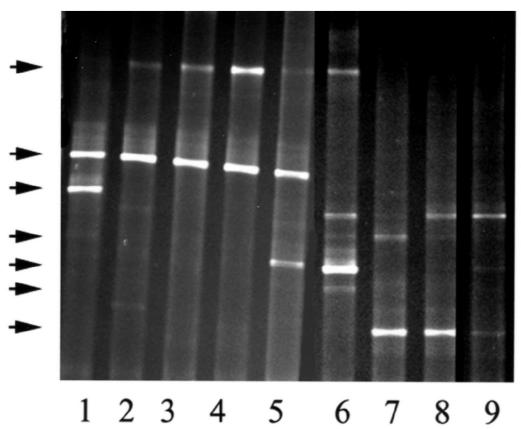
Figure 3 shows the patterns of microbial 16S rDNA generated by PCR from tissue remnants and surrounding stained sawdust after composting. Compared with the bands from experiment 2, the bands from experiment 1 were lower, and their patterns more diverse, in the gel where the acrylamide concentration or the denaturing gradient was higher. This indicated that the samples from experiment 1 contained microbes with higher guanine and cytosine content in their 16S rDNA genes than those from experiment 2.
Figure 3.
Detection of thermophilic microbial DNA in specimens from bags 1 to 5 of sheep 3 in experiment 2 (lanes 1 to 5) and from bags 1 to 4 of sheep 1 in experiment 1 (lanes 6 to 9). Arrows indicate individual DNA bands.
Detection of PrPSc
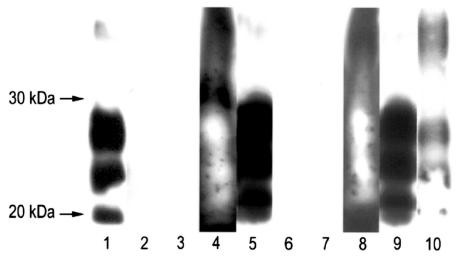
In both experiments, on day 0 all specimens tested positive for PrPSc by WB, although the specimens of experiment 1 contained less PrPSc than those of experiment 2 (Table I and Figure 4). After composting, PrPSc was not detected in the tissue remnants in any of the bags from experiment 1 or in 1 of the bags from experiment 2, but it was detected in the other 4 bags of experiment 2, though in much lower amounts than before composting. In both experiments, PrPSc was not detected in the sawdust immediately surrounding the tissue remnants.
Figure 4.
Detection of abnormal prion protein (PrPSc) in animals with scrapie by a sensitive Western blot procedure involving enrichment by precipitation with sodium phosphotungstic acid (PTA) before and after composting. Lanes 1 to 3 are the results for bag 3 from sheep 1 in experiment 1: PrPSc was detected on day 0 (lane 1) but not after composting in tissue remnants (lane 2) or immediately surrounding sawdust (lane 3). The results for the other bags in experiment 1 were similar. Lanes 4 to 7 are the results for bag 5 of experiment 2: PrPSc was detected on day 0 in both the PTA-enriched sample, which had an excessive amount of PrPSc loaded onto the gel (lane 4), and the crude extract (lane 5) but not after composting in tissue remnants (lane 6) or immediately surrounding sawdust (lane 7), both PTA-enriched. Lanes 8 to 10 are the results for bag 2 of experiment 2: PrPSc was detected on day 0 in both the PTA-enriched sample (lane 8) and the crude extract (lane 9), as well as after composting in PTA-enriched tissue remnants (lane 10).
Discussion
Abnormal prions have been shown to be resistant to destruction by conventional means of inactivating pathogens, and this has led to the use of incineration and deep burial as the preferred methods for disposing of carcasses of animals with scrapie, BSE, or CWD (2,3). However, these processes are costly and undesirable from an environmental standpoint and are impractical for large-scale disposal of animal wastes such as feces. In addition, the infectivity of hamster-adapted scrapie agent was shown to persist for at least 3 y after deep burial (13). However, there is a lack of information on survival of the agent when deeply buried. Furthermore, there is no information on how long the agent would survive in a burial site. Although composting has been widely used as a means for disposing of animal carcasses (7), it had not been investigated as a safe method for disposing of TSE-contaminated carcasses and wastes. The microbial activity that occurs in properly managed compost has been shown to degrade animal carcasses to earth-like material (14), and it has been suggested that enzymes of microbial origin could degrade PrPSc (4–6). The current study has provided evidence that microbial activity during composting does degrade PrPSc.
Although the techniques we used did not allow us to accurately quantify the degradation of PrPSc, the results indicated that the degradation was more complete in experiment 1 than in experiment 2. The loss in tissue weight during composting was also greater in experiment 1 than in experiment 2. The tests conducted on the specimens before composting indicated that the level of abnormal prions was somewhat lower in the mixture of tissues used for experiment 1 than in the CNS tissues used for experiment 2. This difference could have been a factor in the less complete breakdown of PrPSc in the 2nd experiment than in the 1st experiment. However, PrPSc was not detected in 1 of the 5 bags from experiment 2 after composting; thus, the initial amount of PrPSc in the specimens may not have been the main factor influencing the rate of PrPSc degradation. Since the tissues for the 2 experiments were different, there may have been differences in the microbial content of the specimens, and this could have influenced the rate of PrPSc degradation. The results of the DNA analysis for microbial activity in the tissue remnants supports this suggestion: the guanine and cytosine content of the extracted microbial 16S rDNA was higher for the 1st than for the 2nd experiment, and the higher content suggests greater dominance of thermophilic microbes (15). The results also showed greater microbial diversity in the tissues of experiment 1 than in those of experiment 2. In addition, many factors, such as moisture, nutrient composition, aeration, and temperature, could have influenced the microbial activity in the tissue specimens during the period of composting (7). The effects of these factors on the degradation of PrPSc need to be determined in future studies.
The highly sensitive WB procedure used in this study involved PTA precipitation to enrich PrPSc. This enrichment process has been shown to be effective for WB and immunoassays in various tissues, including brain, tonsil, and muscle tissues from humans, mice, sheep, deer, and elk (9,16–22). The monoclonal antibody used (F99/97.6.1) binds to residues 220 to 225 of the C-terminus of prion protein (10), a region that is relatively resistant to PK cleavage (1). Therefore, the negative WB results for PrPSc in some of the composted samples appear to indicate that abnormal prion protein had been degraded.
This study provides the first evidence that PrPSc can be degraded during composting, but bioassays are required to determine the extent to which infectivity was reduced. If infectivity could be destroyed during composting, then this process could be used as a practical and safe method for disposing of carcasses and wastes of animals affected by TSEs.
Acknowledgments
We thank Drs. Jim Algire and Dan A. Stevenson for help in sample collection or diagnostic results, Steven Foster, Pekka Maattanen, Nashandan Yogasingam, and Kavitha Srighanthan for technical help in sample preparation and WB testing, Chris Grenier for technical help in bacterial DNA testing, John Cole for help in composting, and Jean-Marc Lapointe for imaging.
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: a review. Vet J. 2000;159:10–17. doi: 10.1053/tvjl.1999.0406. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Food Inspection Agency (CFIA). Scrapie — Manual of Procedure. 3.4 Infected premises. 3.4.4. Eradication Activities. Disposal (date modified Aug 14, 2006), available at www.inspection.gc.ca/english/anima/heasan/man/scrtre/scrtre-3e.shtml (accessed 2006 Sept 25).
- 4.McLeod AH, Murdoch H, Dickinson J, et al. Proteolytic inactivation of the bovine spongiform encephalopathy agent. Biochem Biophys Res Commun. 2004;317:1165–1170. doi: 10.1016/j.bbrc.2004.03.168. [DOI] [PubMed] [Google Scholar]
- 5.Langeveld LP, Wang JJ, Van de Wiel DF, et al. Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis. 2003;88:1782–1789. doi: 10.1086/379664. [DOI] [PubMed] [Google Scholar]
- 6.Tsiroulnikov K, Rezai H, Bonch-Osmolovskaya E, et al. Hydrolysis of the amyloid prion protein and nonpathogenic meat and bone meal by anaerobic thermophilic prokaryotes and Streptomyces subspecies. J Agric Food Chem. 2004;52:6353–6360. doi: 10.1021/jf0493324. [DOI] [PubMed] [Google Scholar]
- 7.Glanville TD, Trampel DW. Composting alternative for animal carcass disposal. J Am Vet Med Assoc. 1997;210:1116–1120. [PubMed] [Google Scholar]
- 8.Huang H, Rendulich J, Stevenson D, O’Rourke K, Balachandran A. Evaluation of Western blotting methods using samples with or without sodium phosphotungstic acid precipitation for diagnosis of scrapie and chronic wasting disease in Canada. Can J Vet Res. 2005;69:193–199. [PMC free article] [PubMed] [Google Scholar]
- 9.Guan J, Spencer JL, Ma BL. The fate of the recombinant DNA in corn during composting. J Environ Sci Health B. 2005;40:463–473. doi: 10.1081/PFC-200047595. [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke KI, Baszler TV, Besser TE, et al. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol. 2000;38:3254–3259. doi: 10.1128/jcm.38.9.3254-3259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoetendal EG, Akkermans AD, de Vos WE. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwieger F, Tebbe CC. A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol. 1998;64:4870–4876. doi: 10.1128/aem.64.12.4870-4876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown P, Gajdusek DC. Survival of scrapie virus after 3 years’ interment. Lancet. 1991;337:269–270. doi: 10.1016/0140-6736(91)90873-n. [DOI] [PubMed] [Google Scholar]
- 14.Stanford K, Larney FJ, Olson AF, Yanke LJ, McKenzie RH. Composting as a means of disposal of sheep mortalities. Compost Sci Utilization. 2000;8:135–146. [Google Scholar]
- 15.Olsen GJ, Woese CR. Ribosomal RNA: a key to phylogeny. FASEB J. 1993;7:113–123. doi: 10.1096/fasebj.7.1.8422957. [DOI] [PubMed] [Google Scholar]
- 16.Safar J, Wille H, Itri V, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 17.Wadsworth JDF, Joiner S, Hill AF, et al. Tissue distribution of protease resistant protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 18.Safar JR, Scott M, Monaghan J, et al. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat Biotechnol. 2002;20:1147–1150. doi: 10.1038/nbt748. [DOI] [PubMed] [Google Scholar]
- 19.Spraker TR, Balachandran A, Zhuang D, O’Rourke KI. Variable patterns of PrPCWD distribution in obex and cranial lymphoid tissues of Rocky Mountain elk with nonclinical chronic wasting disease. Vet Rec. 2004;155:295–302. doi: 10.1136/vr.155.10.295. [DOI] [PubMed] [Google Scholar]
- 20.Bosque PJ, Ryou C, Telling G, et al. Prions in skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:3812–3817. doi: 10.1073/pnas.052707499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt–Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- 22.Bellon A, Seyfert-Brandt W, Lang W, Baron H, Groner A, Vey M. Improved conformation-dependent immunoassay: suitability for human prion detection with enhanced sensitivity. J Gen Virol. 2003;84:1921–1925. doi: 10.1099/vir.0.18996-0. [DOI] [PubMed] [Google Scholar]