Abstract
Rhodococcus equi can cause severe or fatal pneumonia in foals as well as in immunocompromised animals and humans. Its ability to persist in macrophages is fundamental to how it causes disease, but the basis of this is poorly understood. To examine further the general application of a recently developed system of targeted gene mutation and to assess the importance of different genes in resistance to innate immune defenses, we disrupted the genes encoding high-temperature requirement A (htrA), nitrate reductase (narG), peptidase D (pepD), phosphoribosylaminoimidazole-succinocarboxamide synthase (purC), and superoxide dismutase (sodC) in strain 103 of R. equi using a double-crossover homologous recombination approach. Virulence testing by clearance after intravenous injection in mice showed that the htrA and narG mutants were fully attenuated, the purC and sodC mutants were unchanged, and the pepD mutant was slightly attenuated. Complementation with the pREM shuttle plasmid restored the virulence of the htrA and pepD mutants but not that of the narG mutant. A single-crossover mutation approach was simpler and faster than the double-crossover homologous recombination technique and was used to obtain mutations in 6 other genes potentially involved in virulence (clpB, fadD8, fbpB, glnA1, regX3, and sigF). These mutants were not attenuated in the mouse clearance assay. We were not able to obtain mutants for genes furA, galE, and sigE using the single-crossover mutation approach. In summary, the targeted-mutation system had general applicability but was not always completely successful, perhaps because some genes are essential under the growth conditions used or because the success of mutation depends on the target genes.
Résumé
Rhodococcus equi peut causer une pneumonie sévère ou fatale chez les poulains aussi bien que chez les animaux ou humains immunocompromis. Sa capacité à persister dans les macrophages est fondamentale à sa pathogénie, mais la base de ce phénomène est mal connue. Afin d’examiner plus en détails l’application générale d’un nouveau système de mutation génétique dirigée et d’évaluer l’importance de différents gènes dans la résistance à la réponse immunitaire innée, un dérèglement des gènes codant pour la protéase htrA (htrA), la nitrate réductase (narG), la peptidase D (pepD), la phosphoribosylaminoimidazole-succinocarboxamide synthétase (purC) et la superoxide dismutase (sodC) de la souche 103 de R. equi a été produit à l’aide d’une approche utilisant une recombinaison homologue à double croisement. Une évaluation de la virulence par évaluation de la clairance après injection intraveineuse chez la souris a permis de démontrer que les mutants htrA et narG étaient complètement atténués, les mutants purC et sodC étaient inchangés et le mutant pepD était légèrement atténué. Une complémentation avec le plasmide vecteur pREM a rétabli la virulence des mutants htrA et pepD mais pas celle du mutant narG. Une approche de mutation par croisement unique était plus simple et rapide que la technique de recombinaison homologue à double croisement et a été utilisée afin d’obtenir des mutations dans six autres gènes potentiellement impliqués dans la virulence (clpB, fadD8, fbpB, glnA1, regX3 et sigF). Ces mutants n’étaient pas atténués dans l’épreuve de clairance chez la souris. Il a été impossible d’obtenir des mutants pour les gènes furA, galE et sigE en utilisant l’approche de mutation par croisement unique. En résumé, le système à mutation dirigée a une application globale mais le résultat désiré n’est pas toujours obtenu, peut-être parce que certains gènes sont essentiels dans les conditions de culture utilisées ou parce que le succès de la mutation dépend des gènes ciblés.
(Traduit par Docteur Serge Messier)
Introduction
Rhodococcus equi is a gram-positive bacterium that infects alveolar macrophages and can cause severe or fatal pneumonia in foals. The bacterium has also been increasingly identified as an opportunistic pathogen in immunocompromised humans, particularly AIDS patients (1). As a facultative intracellular organism, R. equi is also of comparative medical interest since understanding how it survives and replicates in macrophages has application to similar infections, particularly those caused by Mycobacterium tuberculosis. Although studies have shown that the virulence of R. equi depends on plasmids of 80 to 90 kb, the understanding of most aspects of the basis of this virulence is rudimentary (2).
Recently, chromosomal genes have been shown to play a role in the resistance of R. equi to oxidative stress (with H2O2) and low pH (3,4). A limited number of chromosomal genes have been disrupted by allelic exchange or transposon mutagenesis (5–9). Such an approach, combined with gene complementation, identified vapA as a virulence gene (8). However, the precise survival strategies used by R. equi and the genes required for intracellular survival are largely unknown (2). A number of putative virulence and virulence-related chromosomal genes were identified in a partial (one-quarter) genome sequence of R. equi ATCC (American Type Culture Collection) 33701 (10), including homologs of genes for M. tuberculosis heat-shock proteins (clpB and htrA), 2-component regulatory systems (senX3-regX3), iron regulation (furA), sigma factors or sigma-factor regulation (pepD, sigE, and sigF), oxidative-stress detoxifying enzymes (sodC), and purine biosynthesis (purC), as well as genes involved in anaerobic growth (narG), nitrogen metabolism (glnA1), lipid metabolism (fadD8), and cell wall synthesis (fbpB). Many of these genes have been shown to be important in the survival of M. tuberculosis in macrophages.
A genome sequence of R. equi 103 is now complete (www.sanger.ac.uk/Projects/R_equi/), so that it may be possible to mutate any R. equi gene to better understand the basis of virulence. The purpose of the work described here was to examine further the application of a recently developed system for targeted gene mutation and complementation (9) using genes with a wide range of functions as described above. Mouse clearance studies were also used to assess whether these mutants were attenuated and whether complemented mutants regained virulence (9).
Materials and methods
Bacterial strains and growth conditions
Escherichia coli DH5α was the host for all plasmid constructions. We used R. equi 103+, originally isolated from a pneumonic foal. All bacteria were grown on Luria Bertani (LB) agar or in LB broth. When required, antibiotics were used at the following concentrations: apramycin (Sigma Chemical Company, St. Louis, Missouri, USA), 50 μg/mL for both E. coli and R. equi; kanamycin (Sigma), 50 μg/mL for E. coli but 200 μg/mL for R. equi; and ampicillin (Sigma), 40 μg/mL. For blue–white selection, X-gal (Sigma), 50 μg/mL, was added to antibiotic-containing solid medium. All bacteria were grown at 37°C in broth with shaking at about 200 rpm. Electrocompetent E. coli and R. equi cells were prepared as previously described (10,11). All DNA electroporations were done with the use of a Pulser Electroporater (Bio-Rad Laboratories, Hercules, California, USA). To improve the efficiency of homologous recombination, we performed alkaline denaturation of the suicide plasmid DNA as previously described (6,9).
DNA manipulation
All restriction enzymes and T4 DNA ligase were from New England Biolabs (NEB, Beverly, Massachusetts, USA), and the reactions used followed the manufacturer’s instructions. The procedure for R. equi genomic DNA isolation is described below, under “Preparation of chromosomal DNA for Southern blotting”. Plasmid DNA was extracted from E. coli by means of the Qiagen plasmid purification kit (Qiagen, Mississauga, Ontario). All polymerase chain reactions (PCRs) were conducted in a TGradient96 thermocycler (Biometra, Göttingen, Germany) with a touchdown program and annealing temperatures varying from 60°C to 45°C. The preparation of reaction mixtures followed the instruction manual of the Taq polymerase supplier (Applied Biosystems, Branchburg, New Jersey, USA), with the addition of acetamide (Sigma) at a final concentration (w/v) of 5%. The primers are described in Tables I and II. The PCR products were purified from agarose gels with the Qiaquick purification system (Qiagen).
Table I.
Oligonucleotide primers used in double-crossover homologous genetic recombination to construct mutants and for gene complementation
| Gene | Primer for suicide plasmid construction by PCRa | Primer for gene complementationa | |
|---|---|---|---|
| htrA | H1 up-F | cagttggccttccatgttcac | H9 gene 424F gtcgaccggtggcctctc |
| H2 up-R | cgttgaattcgtccgacggactgatcatgacc | H10 gene 2873R cagatcggtgtcgggtag | |
| H3 down-F | cctcaagcttgacgctcggcacgaccttg | ||
| H4 down-R | taccgccggaccgagcagat | ||
| narG | N1 up-F | cttgccacgtgcgcgctggtag | N12 narG-R ctcgtagtagtcgcggatgttg |
| N2 up-R | cgttgaattcgtggagtcacgacaaggtg | N13 narG-F gaaccaccggccgacgaaagac | |
| N3 down-F | cctcaagcttgactttcgatcggtgcgggtg | ||
| N4 down-R | gacacgatgcccgtcgacatc | ||
| pepD | P1 up-F | gacgtacgccaccagttgcttg | P10 pepD-R cgctcgggctcctgtgtc |
| P2 up-R | cgttgaattcgttcaccgaggcgtacgtgttc | P11 pepD-F gagcgcacccatgtagtcg | |
| P3 down-F | cctcaagcttctccatcgcaccgaggttg | ||
| P4 down-R | gatccggtcgacgaagcgac | ||
| purC | PU1 up-F | tcgaggttcttgaaccgcagc | ND |
| PU2 up-R | cgttgaattcggcctgtcgttcgaggactgg | ||
| PU3 down-F | cctcaagcttgctcgtacgcctcgatgtag | ||
| PU4 down-R | gatacaagttcgaatcggtc | ||
| sodC | S1 up-F | ccaccgttcttcaggtcctc | S7 sodC379F gtatccctcgaactgcgtcc |
| S2 up-R | cgttgaattctcgctcaaggatgccaag | S8 sodC2063R gaccggcaaatacagttacg | |
| S3 down-F | cctcaagcttggctcctcgttgttcgag | ||
| S4 down-R | gttacggggcagatccaag | ||
PCR — polymerase chain reaction; F — forward; R — reverse; ND — not done.
The nucleotide sequences are shown 5′ to 3′. Non-Rhodococcus equi-linkers are italicized, and restriction-enzyme sites are bolded (HindIII and EcoRI).
Table II.
Oligonucleotide primers used in single-crossover homologous genetic recombination and to amplify the accC4 and vapA genes
| Gene | Forward primersa | Reverse primersa | ||
|---|---|---|---|---|
| clpB | clpB-F | ccgtctagaggccaccgaactcgacgacga | clpB-R | ccgtggtaccggccaccgaactcgacgacg |
| fadD8 | fadD-F | cgtttctagactcgacgacgtgatcgtccgtg | fadD-R | gcctcggtacccagcgaggcaccggagtactc |
| fbpA | fbpA-F | cgtttctagagtcgagcagcgtgggcagctc | fbpA-R | gcctcggtaccgatccgagacgcgcggcgagac |
| furA | furA-F | ccgttctagacgcgttgcggttctgaacac | furA-R | gcctggtaccgtggtggttgtccgcggtgc |
| galE | galE-F | ccgttctagaggatccggcaacagcacacc | galE-R | cggtggtaccgaagtagcgcaggctggtgg |
| glnA1 | gln-F | cgtttctagagatcgcgtcacgcaggtcgac | gln-R | gcctggtaccctgacgaacgcgggcttcgag |
| regX3 | regX-F | ccgttctagagaagtcgtcggtgccggtc | regX-R | gcctggtaccgcgcacggaagctcactcct |
| sigF | sigF-F | ccgttctagagatcgcctccatgaccgagt | sigF-R | ccgtggtacctcgctcgacgcttctccag |
| accC4 | APF | cgttgaattccttcatgtgcagctccatcagc | APR | cctcaagcttgcatatcatcagcgagctgaa |
| vapA | F | taatgcgaccgttcttgattccb | R | tgtagagacgctgaaggtcatttgc |
The restriction-enzyme sites bolded include HindIII, EcoRI, XbaI, and KpnI.
Positions 12632 to 12653 in the primer sequence of the virulence plasmid.
Positions 12937 to 12960 in the primer sequence of the virulence plasmid.
Plasmids
The plasmids used were pBluescript II SK+ (Stratagene, La Jolla, California, USA), pUC18 (MBI Fermentas, Hamilton, Ontario), pAPvlacZ, used as the suicide plasmid backbone for R. equi gene mutation (9), and pREM, used for gene complementation in R. equi (9).
Plasmid construction and mutant selection
Targeted gene mutation with double-crossover homologous recombination
Construction of the suicide plasmid and mutant selection were performed as previously described (9), with the following modifications. Briefly, the PCR products were generated separately for the upstream and downstream homologous regions of the targeted gene and for the apramycin-resistance gene (aacC4). The oligonucleotide primers for each construction are listed in Table I. The primers incorporated HindIII or EcoRI sites. The digested PCR products for the upstream and downstream homologous regions, along with aacC4, were ligated into SspI-digested pBluescript II SK+. The ligation mixture was electroporated into E. coli DH5α, and apramycin-resistant transformants were selected. Apramycin-resistant colonies were tested by PCR with the use of forward and reverse primers related to the upstream and downstream homologous regions. The amplicon was ligated into ScaI/NruI-digested pAPvlacZ, the suicide plasmid backbone used for subsequent gene mutation in R. equi (9). The resulting suicide plasmid construct was sequenced to confirm the fidelity of the cassette. First-stage and second-stage selection were as previously described (9).
Targeted gene mutation with single-crossover homologous recombination
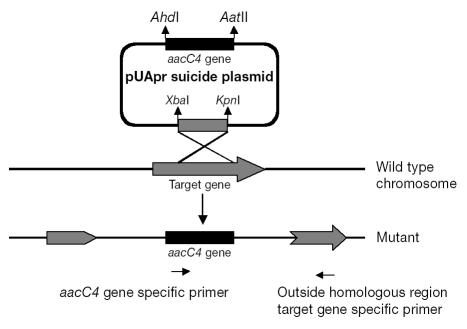
For construction of the suicide vector pUApr, the E. coli plasmid pUC18 was chosen as the backbone of the suicide plasmid for a single-crossover strategy (Figure 1). The aacC4 apramycin-resistance gene was PCR-amplified and cloned into AatII (2617)- and AhdI (1694)-digested sites of pUC18 to replace the ampicillin gene (bla), which resulted in the new suicide vector. This new suicide plasmid was used in targeted-mutation procedures, and single-crossover mutants were obtained. The genes clpB, fadD8, fbpB, furA, galE, glnA1, regX3, sigE, and sigF were amplified by PCR from purified chromosomal R. equi DNA with the oligonucleotide primers described in Table II. The PCR products were subcloned into the XbaI and KpnI sites (positions 423 and 428, respectively) of the suicide vector. For electroporation of R. equi 103+, nonreplicating vector (2 μg) was alkali-denatured in order to stimulate homologous recombination. Single-crossover recombinants were selected with the use of apramycin. Plates were incubated at 37°C for 48 h.
Figure 1.
Schematic diagram of the 1-step strategy for single-crossover mutation. The homologous region is located in the middle of the target gene, so that the entire recombinant vector is integrated in the middle of the gene after a single crossover event. An aacC4-specific primer (right-pointing arrow at bottom) and a primer specific for part of the gene outside the homologous target region (left-pointing arrow at bottom) were used to confirm the single-crossover mutation.
Preparation of chromosomal DNA for Southern blotting
Briefly, 1.5 mL of cell culture was centrifuged at 12 000 × g for 1 min. The pellet was resuspended in 400 μL of TES buffer (50 mM Tris [pH 8.0], 1 mM ethylene diamine tetraacetic acid [pH 8.0], and 6.7% sucrose) to which was added 100 μL of lysozyme (10 mg/mL in TE), and the suspension was incubated for 30 min at 37°C. Next, 100 μL of 6% sodium dodecyl sulfate (SDS) in TE was added, and the suspension was vortexed for 10 s. An aliquot of 67 μL of 5 M NaCl was added and the tube placed on ice for 1 h. The mixture was then centrifuged at 12 000 × g for 10 min, after which the supernatant was mixed with an equal volume of phenol–chloroform– isoamyl alcohol (25:24:1 v/v) and then centrifuged at 13 000 × g for 3 min. The aqueous layer was added to 0.1 volume of 3 M sodium acetate (pH 5.2). After the addition of 2 volumes of 95% ethanol to precipitate the DNA on ice for 1 h, the mixture was centrifuged at 13 000 × g for 10 min. The DNA pellet was air-dried for 10 min and then resuspended in 50 μL of TE buffer.
Southern blotting
Genomic DNA of the R. equi mutants was digested with SalI, electrophoresed in a 0.8% agarose gel, and transferred to nylon membrane (Roche, Laval, Quebec) by capillary transfer, as previously described (12). The conditions for hybridization were as described by the manufacturer. The nylon membranes were prehybridized for at least 4 h at 42°C in hybridization solution without labeled probe, then hybridized separately at 42°C with the apramycin-specific DNA-didoxygenin (DIG) probes for 16 h. The membranes were washed at 68°C under high-stringency conditions.
Complementation studies
The entire htrA, narG, pepD, and sodC genes, including the potential regulatory and terminator regions flanking the structural genes, were PCR-amplified and cloned individually into the SmaI site of pREM (9) with the primers shown in Table I. The DNA inserted into the resulting plasmids was sequenced to verify the fidelity of the target-gene sequence, and the plasmids were then electroporated into the corresponding R. equi mutants, resulting in complemented strains of the mutants.
Assessment of R. equi virulence in mice
To assess the virulence of the mutants using clearance from mouse organs after intravenous injection, we diluted R. equi strains grown in log phase in saline to 5 × 106 colony-forming units (CFUs)/mL, then injected 100 μL of bacterial suspension intravenously into each of 7 adult female CD1 mice. The mice were euthanized after 4 d, and their livers were removed, homogenized individually in saline, and spread on trypticase soy agar (Difco, Detroit, Michigan, USA), with and without apramycin, in serial 10-fold dilutions. After 2 days’ incubation at 37°C, the CFUs were counted, and the clearance of the mutant test strains was compared with that of virulent (103+) and plasmid-cured nonvirulent (103−) controls (13). Statistical evaluation of differences in the CFU counts between groups of mice was performed by Student’s t-test.
To confirm the presence of the virulence plasmid, of the targeted gene mutations, and, when relevant, of the corresponding complementation plasmids, we conducted PCR on randomly picked colonies recovered from the mice, using primers to vapA (Table II).
Permission for the experiments in mice was obtained from the Animal Care Committee, University of Guelph, Guelph, Ontario, and care of the mice was in accord with the guidelines of the Canadian Council on Animal Care.
Comparison of growth of R. equi mutants with that of the parent 103+ strain
Overnight cultures of strains were diluted to an optical density at 540 nm (OD540) of 0.2. Then 2.5 mL of each strain was inoculated into 50 mL of trypticase soy broth (Difco). For mutants, 50 μg/mL of apramycin was added. Samples were withdrawn at 8-h intervals to determine the OD540 values.
Results
Nucleotide sequence analysis
The complete DNA sequence of htrA, narG, pepD, purC, and sodC was obtained using an inverse PCR approach based on the available partial sequences of these genes (10) (details not shown). The sequences reported in this work have been deposited in the GenBank database under accession numbers AY772449, AY922322, AY830683, AY854634, and AY762535, respectively.
Construction of suicide plasmids
Double-crossover recombinants
Table I summarizes the individual targeted-gene suicide-plasmid construction and organization. Double-crossover mutants were more frequent at the first-selection stage after electroporation of single-stranded DNA than at the second stage (6,9). For some genes, at the first stage white colonies were found to be single-crossover mutants. Therefore, we selected blue colonies at the first stage, confirmed these as single-crossover recombinants by PCR, and then carried out the second-stage selection to obtain double-crossover white colonies. These were confirmed by PCR as the desired mutants, and Southern blotting was used to confirm that only a single aacC4 gene was present in the chromosome (data not shown).
Single-crossover recombinants
Using a 1-step strategy of single crossover was a rapid way to generate recombinants. Single- crossover recombinants of genes clpB, fadD8, fbpB, glnA1, regX3, and sigF were developed with the use of a design strategy to ensure that no gene duplication occurred, and recombinants were selected with the use of apramycin. By means of PCR amplification with vector-specific primers, in combination with gene-specific primers (Figure 1), we confirmed that the entire suicide-plasmid pUApr had been inserted through a single-crossover event of homologous recombination and that the recombination had occurred in the central region of each specific gene (data not shown).
Virulence assessment in mice of R. equi mutants and their complementary strains
Double-crossover recombinants
Using liver clearance of intravenously injected bacteria as an indicator of virulence, we found the htrA and narG mutants to be fully attenuated, the pepD mutant to be slightly attenuated, and the purC and sodC mutants to be unaffected (Table III) in comparison with the parental R. equi virulent strain 103+. Complementation restored the virulence of the htrA and pepD mutants but not of the narG mutant, and the sodC mutant became slightly more virulent than the parent strain. The PCR reactions showed that all colonies of R. equi mutants and their complementary strains recovered from mice possessed the correct genotype.
Table III.
Virulence assessment of R. equi mutants
| Liver clearance in mice; mean log10 colony-forming units (and standard deviation)
|
|||
|---|---|---|---|
| Gene | Wild type, 103+a | Mutant | Complemented mutant |
| clpB | 4.98 (0.56) | 4.73 (0.54) | ND |
| fadD8 | 5.25 (0.51) | 5.32 (0.21) | ND |
| fbpB | 5.25 (0.51) | 5.29 (0.22) | ND |
| gln | 5.25 (0.51) | 5.38 (0.29) | ND |
| htrA | 4.86 (0.14) | 0 | 3.33 (1.49) |
| narG | 5.74 (0.57) | 0 | 0 |
| pepD | 5.25 (0.29) | 4.06 (0.40), P < 0.0002b | 4.85 (0.30), P < 0.03c |
| purC | 5.83 (0.38) | 5.53 (0.43) | ND |
| regX | 4.98 (0.31) | 5.0 (0.47) | ND |
| sigF | 4.98 (0.31) | 4.70 (0.64) | ND |
| sodC | 5.31 (0.55) | 5.53 (0.48), P = 0.44b | 5.87 (0.39), P < 0.05b |
The clearance differs because it was assessed in different studies at different times. Plasmid-cured strains (R. equi 103−) were totally cleared by day 4 in different experiments.
Compared with the value for the wild type.
Compared with the value for the mutant.
Single-crossover recombinants
None of the single-crossover mutants (clpB, fadD8, fbpB, glnA1, regX3, and sigF) was attenuated, as assessed by liver clearance in mice (Table III).
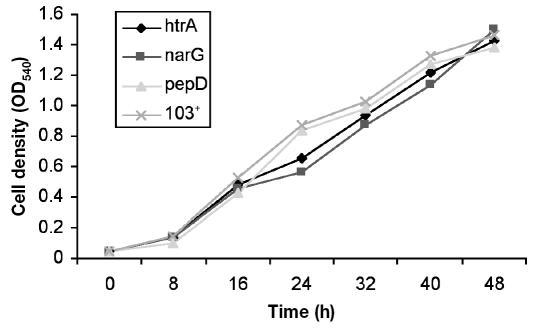
Comparison of growth of R. equi mutants with that of the parent 103+ strain
The in vitro growth phenotype of the 3 attenuated mutants (htrA, narG, and pepD) was similar to that of the wild type 103+ (Figure 2).
Figure 2.
Growth curves for the htrA, narG, and pepD mutant strains and the parent strain (103+) of Rhodococcus equi at 37°C. Results are presented as optical density at 540 nm (OD540) at intervals after inoculation into growth medium.
Discussion
The overall objective of this study was to apply a recently developed system for targeted gene mutation and complementation (9) to genes with a wide range of functions identified in a partial genome sequence of R. equi ATCC 33701 (10). Southern blotting confirmed the lack of illegitimate recombination in strain 103+, since there was shown to be only a single insertion in the chromosome, and the insertions were shown by PCR to be in the target gene (9). This contrasts with strain ATCC 33701, which has a high frequency of illegitimate recombination (9). The study also confirmed the value of the gene complementation approach using pREM. Mutants were generated by both the double-crossover homologous allelic exchange approach developed by Ren and Prescott (9) and a single-crossover approach. After making a number of double homologous recombination mutants, we adopted the single-crossover approach because it was a far faster way to obtain gene mutations, although, unlike the double-crossover approach, there is the potential for mutated genes to revert to wild type in the absence of antibiotic selection; for example, in chronic infections. No significant reversion should occur in the short term, such as in mouse-clearance or macrophage-survival studies, and none was observed in this study.
This study showed that most, but not all, attempts at targeted gene mutation were successful. The reason for the lack of success for individual genes may relate either to their essential nature or to variation between genes in their ability to be mutated. Differences between genes in ease of mutation have previously been noted (9). Clearance of the mutants in mice was compared with that of the virulent parent strain because mouse clearance data appear to correlate well with survival and growth in macrophages (8), and clearance studies are easier to perform.
The high-temperature requirement A protein encoded by htrA is a stress-induced serine protease involved in the folding and maturation of secreted proteins known to be involved in the virulence of many gram-negative bacteria, including Salmonella Typhimurium (14). This protease probably provides resistance to oxidative stress in vivo (15). The gene narG encodes nitrate reductase G, which plays a major role in respiration in the absence of oxygen. Anaerobic nitrate reduction has been shown to be essential for metabolism of M. bovis bacille Calmette–Guérin in immunocompetent but not immunodeficient mice (16). The complete attenuation of the narG mutant of R. equi in mice indicates that narG is important for full expression of virulence in R. equi and suggests that anaerobic or microaerophilic conditions may be important for R. equi growth during infection. In M. tuberculosis, genes encoding the subunits of nitrate reductase and permitting anaerobic growth in the presence of nitrate are found in the narGHJI gene cluster (17). Complementation of the narG mutant in R. equi did not restore virulence, likely because narG and narGHJI are cotranscribed in R. equi. Our work shows that htrA and narG are apparently required for virulence in R. equi since these were fully attenuated in mice.
In M. tuberculosis, the products of the sodA and sodC genes are important for providing resistance against various oxidative stresses and for virulence (18). In a study in guinea pigs, mycobacterial sodC was found not to be essential for intracellular growth within macrophages and did not detectably contribute to the pathogenicity of M. tuberculosis (19). However, a copper or zinc-cofactored sodC mutant of M. tuberculosis had enhanced susceptibility to killing by gamma interferon (IFN-γ)-activated murine peritoneal macrophages producing an oxidative burst but was unaffected by macrophages not activated by IFN-γ and macrophages from respiratory-burst- deficient mice (20). Our mouse clearance studies showed no virulence attenuation in the sodC mutant of R. equi. The use of healthy adult CD1 mice to assess the virulence of mutant strains will, however, fail to identify any attenuation that may be observed only in activated macrophages and is a limitation of the model. The hypervirulence of the complemented sodC mutant compared with the wild type suggests that sodC activity contributes to survival in macrophages. Further studies using activated macrophages are required to determine whether this gene is involved in the survival of R. equi under these circumstances.
The product of the gene purC is a 1-phosphoribosylaminoimidazole- succinocarboxamide synthase involved in purine biosynthesis. Mutants of M. bovis and M. tuberculosis attenuated in mice conferred some level of protection in a challenge against aerosolized virulent M. tuberculosis in the guinea pig model (21). However, unlike the M. tuberculosis complex, the purC mutant of R. equi showed no attenuation in mice.
In M. tuberculosis, pepD (Rv0983), which encodes peptidase D, appears to be part of the sigE regulon; a sigE mutant showed defective growth in macrophages (22). The pepD gene is also controlled in M. tuberculosis by mprA–mprB, a 2-component regulatory system involved in establishing and maintaining persistent infection (23). Our study showed that pepD is involved in the survival of R. equi in mice, since the pepD mutant was slightly attenuated, and virulence was restored by complementation.
A heat-shock protein required for virulence in a murine model in Listeria monocytogenes (24), ClpB is regulated in M. tuberculosis by the alternative sigma factor SigH (25), which regulates the response to heat and oxidative stress. The lack of effect of mutation of clpB on mouse clearance suggests that this protein is not essential for short-term survival of R. equi in macrophages. In M. tuberculosis, the alternative sigma factor SigF is expressed under stationary-phase growth and stress conditions and provides protection against oxidative stress (26,27). Our results suggest that, as with M. tuberculosis, SigF is not essential for virulence in R. equi (18,28).
Glutamine synthase, encoded by the gene glnA1, is an enzyme of central importance in nitrogen metabolism that also catalyzes the extracellular synthesis of l-glutamine, an important component of the mycobacterial cell wall. This enzyme is important for the survival of M. tuberculosis in macrophages and essential for virulence in guinea pigs (29,30). Our study indicates that it is not essential for virulence in R. equi.
In M. tuberculosis, fbpB encodes the protein Ag85B, an immunodominant component of the mycobacterial antigen 85 complex (Ag85), which is a secreted protein with mycolyl transferase activity that is involved in the biogenesis of the mycobacterial cell wall (31). Of the 3 mycolyl transferase genes in M. tuberculosis, only fbpA when mutated will attenuate the growth of this organism in macrophages (32). Our study indicates that fbpB is not essential for virulence in R. equi. The senX3–regX3 2-component regulatory system may be a sensor of oxidative stress (33) and is required for virulence in M. tuberculosis (34). In R. equi, the fbpB mutant had unimpaired survival in mice.
The enzyme FadD8 is the homolog of an acyl-CoA synthase of M. tuberculosis involved in lipid degradation (10). We chose fadD8 as a target acyl-CoA synthase gene because M. tuberculosis genes such as fadD26 and fadD28 that are involved in lipid metabolism are essential for virulence (18). The ability to metabolize fatty acids appears to be essential for the virulence of R. equi (35). In addition, a recent study of gene expression in R. equi showed that fadD13, a related-chain fatty acid CoA ligase gene, was upregulated by the organism inside macrophages (36).
We mutated galE since galactose is thought to be predominant in the outer lipid layer of the cell wall of R. equi as arabino-d-galactan linked to mycolic acids (37).
In summary, the described study results confirm the value of a system of targeted gene mutation and complementation for studies of R. equi infection. Because of speed, the single-crossover recombination approach is preferred, at least for screening for relevant attributes. There is still a need to improve targeted-mutation systems for double-crossover homologous recombination, particularly by use of a positive selection system equivalent to that of the sacB system used in targeted mutation in M. tuberculosis, although this approach is ineffective in R. equi.
Acknowledgments
This work was supported by the Ontario Ministry of Agriculture, Food, and Rural Affairs, the Ontario Horse Racing Industry Association, and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijer WG, Prescott JF. Rhodococcus equi. Vet Res. 2004;35:383–396. doi: 10.1051/vetres:2004024. [DOI] [PubMed] [Google Scholar]
- 3.Benoit S, Benachour A, Taouji S, et al. Induction of vap genes encoded by the virulence plasmid of Rhodococcus equi during acid tolerance response. Res Microbiol. 2001;152:439–449. doi: 10.1016/s0923-2508(01)01217-7. [DOI] [PubMed] [Google Scholar]
- 4.Benoit S, Benachour A, Taouji S, et al. H2O2, which causes macrophage-related stress, triggers induction of expression of virulence-associated plasmid determinants in Rhodococcus equi. Infect Immun. 2002;70:3768–3776. doi: 10.1128/IAI.70.7.3768-3776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangan MW, Meijer WG. Random insertion mutagenesis of the intracellular pathogen Rhodococcus equi using transposomes. FEMS Microbiol Lett. 2001;205:243–246. doi: 10.1111/j.1574-6968.2001.tb10955.x. [DOI] [PubMed] [Google Scholar]
- 6.Navas J, Gonzalez-Zorn B, Ladron N, et al. Identification and mutagenesis by allelic exchange of choE, encoding a cholesterol oxidase from the intracellular pathogen Rhodococcus equi. J Bacteriol. 2001;183:4796–4805. doi: 10.1128/JB.183.16.4796-4805.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashour J, Hondalus MK. Phenotypic mutants of the intracellular actinomycete Rhodococcus equi created by in vivo Himar1 transposon mutagenesis. J Bacteriol. 2003;185:2644–2652. doi: 10.1128/JB.185.8.2644-2652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain S, Bloom BR, Hondalus MK. Deletion of vapA encoding virulence associated protein A attenuates the intracellular actinomycete Rhodococcus equi. Mol Microbiol. 2003;50:115–128. doi: 10.1046/j.1365-2958.2003.03689.x. [DOI] [PubMed] [Google Scholar]
- 9.Ren J, Prescott JF. The effect of mutation on Rhodococcus equi virulence plasmid gene expression and mouse virulence. Vet Microbiol. 2004;103:219–230. doi: 10.1016/j.vetmic.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MT, Herron LL, Kapur V, et al. Partial genome sequencing of Rhodococcus equi ATCC 33701. Vet Microbiol. 2003;94:143–158. doi: 10.1016/s0378-1135(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Tkachuk-Saad O, Prescott JF. Development of a Rhodococcus equi–Escherichia coli plasmid shuttle vector. Plasmid. 1997;38:180–187. doi: 10.1006/plas.1997.1311. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory, 1989.
- 13.Prescott JF, Patterson MC, Nicholson VN, et al. Assessment of the immunogenic potential of Rhodococcus equi virulence-associated protein (VapA) in mice. Vet Microbiol. 1997;56:213–225. doi: 10.1016/s0378-1135(97)00090-4. [DOI] [PubMed] [Google Scholar]
- 14.Baumler AJ, Kusters JG, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival in macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutunga M, Graham S, DeHormaeche RD, et al. Attenuated Salmonella typhimurium htrA mutants cause fatal infections in mice deficient in NADPH oxidase and destroy NADPH oxidase-deficient macrophage monolayers. Vaccine. 2004;22:4124–4131. doi: 10.1016/j.vaccine.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Fritz C, Maass S, Kreft A, et al. Dependence of Mycobacterium bovis BCG on anaerobic nitrate reductase for persistence is tissue specific. Infect Immun. 2002;70:286–291. doi: 10.1128/IAI.70.1.286-291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohaskey CD, Wayne LG. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol. 2003;185:7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dussurget O, Stewart G, Neyrolles O, et al. Role of Mycobacterium tuberculosis copper–zinc superoxide dismutase. Infect Immun. 2001;69:529–533. doi: 10.1128/IAI.69.1.529-533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun. 2001;69:4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson M, Phalen SW, Lagranderie M, et al. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manganelli R, Voskuil MI, Schoolnik GK, et al. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol Microbiol. 2001;41:423–437. doi: 10.1046/j.1365-2958.2001.02525.x. [DOI] [PubMed] [Google Scholar]
- 23.He H, Zahrt TC. Identification and characterization of a regulatory sequence recognized by Mycobacterium tuberculosis persistence regulator MprA. J Bacteriol. 2005;187:202–212. doi: 10.1128/JB.187.1.202-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chastanet A, Derre I, Nair S, et al. clpB, a novel member of the Listeria monocytogenes CtsR regulon, is involved in virulence but not in general stress tolerance. J Bacteriol. 2004;186:1165–1174. doi: 10.1128/JB.186.4.1165-1174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raman S, Song T, Puyang X, et al. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J Bacteriol. 2001;183:6119–6125. doi: 10.1128/JB.183.20.6119-6125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMaio J, Zhang Y, Ko C, et al. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiman DE, Kaushal D, Ko C, et al. Attenuation of late-stage disease in mice infected by the Mycobacterium tuberculosis mutant lacking the SigF alternate sigma factor and identification of SigF-dependent genes by microarray analysis. Infect Immun. 2004;72:1733–1745. doi: 10.1128/IAI.72.3.1733-1745.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen P, Ruiz RE, Li Q, et al. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigF. Infect Immun. 2000;68:5575–5580. doi: 10.1128/iai.68.10.5575-5580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harth G, Clemens DL, Horwitz MA. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci U S A. 1994;91:9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tullius MV, Harth G, Horwitz MA. Glutamine synthetase glnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect Immun. 2003;71:3927–3936. doi: 10.1128/IAI.71.7.3927-3936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armitige LY, Jagannath C, Wanger AR, Norris SJ. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect Immun. 2000;68:767–778. doi: 10.1128/iai.68.2.767-778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickman L, Saldanha JW, Hunt DM, et al. A two-component signal transduction system with a PAS domain-containing sensor is required for virulence of Mycobacterium tuberculosis in mice. Biochem Biophys Res Commun. 2004;314:259–267. doi: 10.1016/j.bbrc.2003.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parish T, Smith DA, Roberts G, Betts J, Stoker NG. The senX3–regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology. 2003;149:1423–1435. doi: 10.1099/mic.0.26245-0. [DOI] [PubMed] [Google Scholar]
- 35.Wall DM, Duffy PS, Dupont C, et al. Isocitrate lyase activity is required for virulence of the intracellular pathogen Rhodococcus equi. Infect Immun. 2005;73:6736–6741. doi: 10.1128/IAI.73.10.6736-6741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman MT, Parreira V, Prescott JF. In vitro and intra- macrophage gene expression by Rhodococcus equi strain 103. Vet Microbiol. 2005;110:131–140. doi: 10.1016/j.vetmic.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Sutcliffe I. Macroamphiphilic cell envelope components of Rhodococcus equi and closely related bacteria. Vet Microbiol. 1997;56:287–299. doi: 10.1016/s0378-1135(97)00097-7. [DOI] [PubMed] [Google Scholar]