Abstract
We investigated the relative contribution of COX-1 and/or COX-2 to oxidative damage, prostaglandin E2 (PGE2) production and hippocampal CA1 neuronal loss in a model of 5 min transient global cerebral ischemia in gerbils. Our results revealed a biphasic and significant increase in PGE2 levels after 2 and 24-48 h of reperfusion. The late increase in PGE2 levels (24 h) was more potently reduced by the highly selective COX-2 inhibitor Rofecoxib (20 mg/kg) relative to the COX-1 inhibitor Valeryl Salicylate (20 mg/kg). The delayed rise in COX catalytic activity preceded the onset of histopathological changes in the CA1 subfield of the hippocampus. Post-ischemia treatment with Rofecoxib (starting 6 h after restoration of blood flow) significantly reduced measures of oxidative damage (glutathione depletion and lipid peroxidation) seen at 48 h after the initial ischemic episode, indicating that the late increase in COX-2 activity is involved in the delayed occurrence of oxidative damage in the hippocampus after global ischemia. Interestingly, selective inhibition of COX-2 with Rofecoxib or inhibition of COX-1 with Valeryl Salicylate significantly increased the number of healthy neurons in the hippocampal CA1 sector even when the treatment began 6 h after ischemia. These results provide the first evidence that both COX isoforms are involved in the progression of neuronal damage following global cerebral ischemia, and have important implications for the potential therapeutic use of COX inhibitors in cerebral ischemia.
Keywords: COX-1, COX-2, global cerebral ischemia, hippocampal neuronal loss, neuroprotection, prostaglandin E2, oxidative stress, Rofecoxib, Valeryl Salicylate
Introduction
In humans and in animals subjected to transient global cerebral ischemia, specific neurons degenerate following the ischemic episode (Kirino, 1982, 2000; Pulsinelli et al., 1982; Petito et al., 1987). It is now known that much of the brain damage produced by transient global cerebral ischemia is not the result of the initial ischemic episode but, rather, develops over a period of hours to days after the primary event. Indeed, it is the complex secondary molecular mechanisms initiated at the time of ischemia that play an important role in the delayed progression of neuronal cell loss. The cornu Ammonis 1 (CA1) neurons of the hippocampus are widely regarded as among the most vulnerable in the mammalian brain to ischemia (Pulsinelli et al., 1982; Schmidt-Kastner and Freund, 1991). Although several pathophysiological mechanisms have been proposed to explain delayed neuronal death of hippocampal CA1 pyramidal neurons (Jain, 2000), post-ischemic inflammation and the formation of oxygen-derived free radicals are thought to play pivotal roles in reperfusion-induced delayed neurodegeneration (Kitagawa et al., 1990; Yamamoto et al., 1997; Urabe et al., 2000).
Previous studies have shown that one of the primary sources of reactive oxygen species in the ischemic brain is through the metabolism of arachidonic acid by cyclooxygenase or COX (Nelson et al., 1992; Busija et al., 1998; Chan, 1996, 2001). There is mounting evidence that induction of the COX-2 isoform contributes to ischemic brain damage (Ohtsuki et al., 1996; Nogawa et al., 1997; Nakayama et al., 1998). Induction of COX-2 mRNA and protein through activation of AMPA receptors in global ischemia (Koistinaho et al., 1999) suggests that COX-2 is a mediator of glutamate excitotoxicity. COX-2 is one of a select few proteins that still remains upregulated in CA1 hippocampal cells even at 3 days after ischemia (Nakayama et al., 1998; Koistinaho et al., 1999), thus preceding the death of these neurons. Administration of inhibitors of COX, but not lipooxygenase inhibitors, ameliorates delayed hippocampal CA1 neuronal death in rodents after transient global cerebral ischemia (Sasaki et al., 1988; Nakagomi et al., 1989; Hall et al., 1993). Recently, it has been reported that COX-2 selective inhibitors prevent both post-ischemic prostaglandin accumulation and ischemic neuronal damage (Nogawa et al., 1997; Nakayama et al., 1998; Iadecola et al., 2001a), suggesting that the beneficial effects observed with non-selective COX inhibitors are probably associated with inhibition of COX-2 rather than COX-1. Given the observation that increased COX activity and enhanced release of prostanoids are associated with the generation of large amounts of free radicals (Marnett et al., 1999; Niwa et al., 2001), the goal of this study was to determine the relative contribution of COX-1 and/or COX-2 to ischemia-induced oxidative injury in the gerbil hippocampus, and to test the hypothesis that the neuroprotective effects observed with COX-2 selective inhibitors are partly mediated through reduction of oxidative damage in the reperfused brain.
Methods
Animals and surgical procedures
Studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by National Institutes of Health (Bethesda, MD, USA). Our institutional animal care and use committee approved the experimental protocol (No. 02/99). Adult male Mongolian gerbils (Meriones unguiculatus, 12-15 weeks, 55-65 g) were subjected to transient global cerebral ischemia under diethyl ether anesthesia by occluding both common carotid arteries for 5 min with microaneurysmal clips (Sugita, Japan) exactly as in our previous reports (Candelario-Jalil et al., 2001, 2002a; Martínez et al., 2001), which consistently resulted in delayed neuronal death in the CA1 region of the hippocampus (Kirino, 1982; Hong et al., 2001; Candelario-Jalil et al., 2002c). Blood flow during the occlusion and reperfusion after removal of the clips was visually confirmed and the incision was closed with 4-0 silk sutures. The onset of cerebral ischemia was associated with a brief period of panting breathing and body movements followed by quiescence. Successful occlusion of both carotid arteries was evident with the rapid onset of complete bilateral ptosis and the adoption of a ‘hunched’ posture. Only animals showing this behavior were considered in this study (Martí et al., 2001; Martínez et al., 2001). In sham- operated animals, the arteries were freed from connective tissue but were not occluded. The rectal temperature was carefully monitored and maintained at 37 ± 0.5°C using an incandescent lamp and the animals were allowed to recover on an electrical heated blanket. In addition, rectal temperature was monitored at 6-h intervals for 3 days of reperfusion in all experimental groups.
Treatments with selective inhibitors of COX-1 and COX-2
The selective COX-1 inhibitor Valeryl Salicylate (Bhattacharyya et al., 1995; Davidson and Lang, 2000; IC50 for ovine COX-1: 0.8 mM, IC50 for ovine COX-2: 15 mM; Cayman Chemical, Ann Arbor, MI, USA) and the highly selective COX-2 inhibitor Rofecoxib (Chan et al., 1999; IC50 for purified human COX-2: 0.34 μM, IC50 for purified human COX-1: 26.3μM, Merck Sharp & Dohme, Whitehouse Station, NJ, USA) were employed as pharmacological tools to investigate the relative contribution of COX isoforms to delayed neuronal death following global cerebral ischemia. Both COX inhibitors readily cross the blood-brain barrier (Bhattacharyya et al., 1995; Halpin et al., 2000). The drug treatment paradigms used for each measure are described below.
Effect of Selective COX Inhibitors on Hippocampal CA1 Neuronal Cell Numbers
In an initial set of studies, gerbils received a 5 min transient global cerebral ischemia episode and were sacrificed at different reperfusion periods (1, 2, 3, 4 and 7 days; n=5 at each time point) and the brains were used to determine neuronal cell counts in the hippocampal CA1 region (see counting procedure below). To determine the effects of COX-1 and COX-2 inhibition on cell numbers, an additional set of animals received Valeryl Salicylate (20 and 80 mg/kg; i.p.), Rofecoxib (5, 10 and 20 mg/kg; i.p.) or vehicle (polyvinylpyrrolidone; i.p.) administered in two different treatment schedules: 1) 30 min before ischemia and 2) 6 h after the onset of ischemia. In both paradigms, additional doses were administered at 6, 12, 24, 48 and 72 h of reperfusion based on the pharmacokinetic properties of Rofecoxib (Halpin et al., 2000) and evidence showing persistent COX-2 induction after global ischemia (Nakayama et al., 1998; Koistinaho et al., 1999). These animals were sacrificed at 7 days following surgery and neuronal cell counts obtained from the hippocampal CA1 region.
Hippocampal CA1 Neuronal Cell Counting
At the appropriate time following surgery, animals were deeply anesthetized with diethyl ether and perfused transcardially with cold saline followed by 4% formalin in 0.1 M phosphate-buffered saline (pH 7.4). The brains were removed from the skull and placed in the same fixative for 24 h. Brains were embedded in paraffin wax and representative coronal sections (5 μm thick) obtained using a rotary microtome (Leica, Model RM2135, Meyer Instruments, Houston, TX, USA). Tissue sections were stained with hematoxylin and eosin. Cell counts of CA1 neurons were performed at three levels of the dorsal hippocampus. Specifically, alternate sections were obtained at 1.0, 1.5 and 2.2 mm posterior to bregma, and two sections from each level (n=6 sections for each animal) were used to count cells in the CA1 region. The number of intact neurons within the CA1 layer were counted using a light microscope (Olympus, Model BH-2, Tokyo, Japan) at a magnification of 40x and expressed as the number of CA1 neurons per mm linear length as described earlier (Kirino et al., 1991; Satoh et al., 1996; Lee et al., 2000). To maintain consistency across animals, a rectangular box (1 mm X 0.25 mm) was centered over the CA1 cell layer beginning 1.0mm lateral to the midline. Only neurons with normal visible nuclei were counted. The mean number of CA1 neurons per mm linear length for both hemispheres was calculated for each treatment group. An observer who was unaware of the drug treatment for each gerbil made all assessments of histological sections.
Effect of Selective COX Inhibitors on Prostaglandin E2 (PGE2) Levels
In a different set of studies, hippocampal PGE2 levels were measured at different reperfusion times (2, 6, 12, 24, 48 and 72 h) after transient global cerebral ischemia (n=5 per time point). Only a shamoperated group, sacrificed at 24 h, was included (n=5), because our pilot studies showed that levels of PGE2 in shamoperated animals sacrificed at 2, 6, 12, 24, 48 and 72 h after ischemia (n=5, per time point) showed no statistically significant differences among groups (data not shown). Once the time course of PGE2 levels was determined, the effect of COX-1 and COX-2 selective inhibitors on PGE2 concentrations in normal (shamoperated animals) and ischemic hippocampus was examined. Each inhibitor was administered intraperitoneally (20 mg/kg) 30 min before surgery and again at 6, 14 and 22 h and animals were sacrificed at 24 h following surgery. In an additional group of animals, drugs were administered 30 min before surgery and animals sacrificed 2 h after ischemia. These time points were based on our observation indicating maximal PGE2 production at 2 and 24 h after global ischemia (see Results section).
At the appropriate time following surgery, the hippocampus from both hemispheres were rapidly dissected, weighed and frozen in liquid N2. Tissue concentration of PGE2, one of the major COX reaction products in the brain (Nogawa et al., 1997), was determined using a commercial enzyme immunoassay kit (RPN 222, Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) according to the instructions of the manufacturer. The tissue was homogenized in 50 mM Tris-HCl (pH 7.4) and extracted with 100 % methanol (Powell, 1982). After centrifugation, the supernatant was diluted with acidified 0.1 M phosphate buffer (pH 4.0; final methanol concentration, 15%) and applied to activated octadecylsilyl (ODS)-silica reverse-phase columns (Sep-Pak C18, Waters Associates, Milford, MA, USA). The columns were rinsed with 5 ml of distilled water followed by 5 ml of n-hexane, and PGE2 was eluted twice with 2 ml of ethyl acetate containing 1% methanol. The ethyl acetate fraction was evaporated and resuspended in 1 ml of the buffer provided with the kit. The assay is based on competition between unlabeled PGE2 and a fixed amount of peroxidase-labeled PGE2 for a limited number of binding sites on a PGE2-specific antibody. Briefly, samples or PGE2 standard were incubated, shaken at room temperature for 60 min with specific anti-PGE2 antibody and peroxidase-conjugated PGE2 antibody in a goat anti-mouse IgG-precoated 96-well plate. After washing, 3,3′,5,5′-tetramethylbenzidine substrate was added to the wells and after 30 min the reaction was stopped by adding 1 mM sulphuric acid. Subsequently, absorbance was measured at 450 nm. The detection limit of this assay is 16 pg/ml.
Effect of Selective COX Inhibitors on Measures of Oxidative Damage
The following experimental groups were examined to study the effect of COX-1 and COX-2 selective inhibitors on several measures of oxidative injury in hippocampus after global ischemia: a shamoperated group (n=6), an ischemic group treated with the vehicle (n=8) and two groups of ischemic gerbils treated with either Valeryl Salicylate (20 mg/kg; i.p., n=9) or Rofecoxib (20 mg/kg; i.p., n=8) at 6, 12 and 24 h of reperfusion. Two groups of sham-operated animals (n=6 each) treated with Valeryl Salicylate or Rofecoxib, using the exact dose and treatment schedule of those ischemic animals treated with the inhibitors, were also included. Animals were sacrificed at 48 h after ischemia, because according to our previous results, oxidative damage is maximal at this time point in gerbil global cerebral ischemia (Candelario-Jalil et al., 2001). Since there is temporal difference in PGE2 production by COX-1 (2 h) and COX-2 (24-48 h, see Results section), we also studied the effects of COX inhibitors on measures of oxidative stress at 2 h after ischemia. The following experimental groups were prepared: a sham-operated group (n=5), a vehicle-treated ischemic group (n=7) and two groups of ischemic gerbils (n=7 each) treated with either Valeryl Salicylate (20 mg/kg; i.p.) or Rofecoxib (20 mg/kg; i.p.) given 30 min prior to ischemia.
At the end of the experiment (2 or 48 h of reperfusion), gerbils were deeply anesthetized with diethyl ether and perfused transcardially with ice-cold saline to flush all blood components from the vasculature. Brains were quickly removed, kept in ice-cold saline and hippocampi were immediately dissected out on a chilled plate, exactly as in our previous reports (Candelario-Jalil et al., 2000, 2001; Martínez et al., 2001). Hippocampi were weighed and homogenized in ice-cold 20 mM Tris-HCl buffer (pH 7.4) and centrifuged for 10 min at 12 000 g. The supernatant was collected, frozen at -70°C and employed for biochemical analyses.
The following assays were used as biochemical measures of oxidative damage. Reduced and oxidized glutathione (GSH and GSSG, respectively) were measured enzymatically in 5-sulphosalicylic acid-deproteinized hippocampal samples by using a modification (Anderson, 1985) of the procedure of Tietze (1969) as described for brain homogenates (Floreani et al., 1997). Samples were assayed rapidly to minimize GSH oxidation. Specificity of this method for glutathione quantification is ensured by highly specific glutathione reductase. Lipid peroxidation was assessed by measuring the concentration of malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA) using the LPO-586 kit obtained from Calbiochem (La Jolla, CA, USA). For standards, freshly prepared solutions of malondialdehyde bis [dimethyl acetal] (Sigma, St. Louis, MO, USA) and 4-hydroxynonenal diethylacetal (Cayman Chemical, Ann Arbor, MI, USA) were employed and assayed under identical conditions. Concentrations of MDA and 4-HDA in brain samples were calculated using the corresponding standard curve and values were expressed as nmol MDA+4-HDA per mg protein. This kit has been used widely for the measurement of products of lipid peroxidation in homogenates obtained from ischemic brain (Kondo et al., 1997; Hong et al., 2001; Candelario-Jalil et al., 2001; Martínez et al., 2001).
Glutathione peroxidase (GPx) activity was assayed using a commercial kit obtained from Randox Laboratories (Antrim, UK), which is based on the procedure described by Flohé and Gunzler (1984) using cumene hydroperoxide as substrate. The reaction was followed for 3 min at 340 nm in a Pharmacia LKB Ultraspec Plus spectrophotometer. The contribution of spontaneous NADPH oxidation was always subtracted from the overall reaction rate. GPx activity was expressed as nmol NADPH oxidized per minute per mg protein. Glutathione reductase (GR) activity was measured according to Carlberg and Mannervik (1985) in a mixture containing 0.1 M potassium phosphate buffer (pH 7.0), 0.5 mM EDTA, 1 mM GSSG, 0.1 mM NADPH and the sample. The oxidation of NADPH was followed for 3 min at 340 nm and the activity of GR was calculated using a molar extinction coefficient of 6.3 mM cm-1. Non-enzymatic NADPH oxidation was subtracted from the overall rate. GR activity was expressed as nmol NADPH oxidized per minute on the basis of total protein content. Total protein concentrations were analyzed using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Analytical grade bovine serum albumin was used to establish a standard curve.
In vitro antioxidant properties of Rofecoxib and Valeryl Salicylate
Phospholipid peroxidation:
The ability of Rofecoxib and Valeryl Salicylate to inhibit both spontaneous autooxidation and iron-catalyzed lipid peroxidation of membrane lipids at pH 7.4 was tested using rat brain phospholipids as described in details by Aruoma et al. (1992).
Scavenging effect on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical:
This assay was conducted as described in previous reports (Chen and Ho, 1997; Hung et al., 2002).
Statistics
Data are expressed as mean ± standard deviation (S.D.). Statistical analysis was performed with one-way ANOVA followed by a Student-Newman-Keuls post-hoc test. The value of P less than 0.05 was considered to be statistically significant.
Results
Time course of histopathological changes in hippocampal CA1 sector: Effects of COX-1 and COX-2 inhibitors
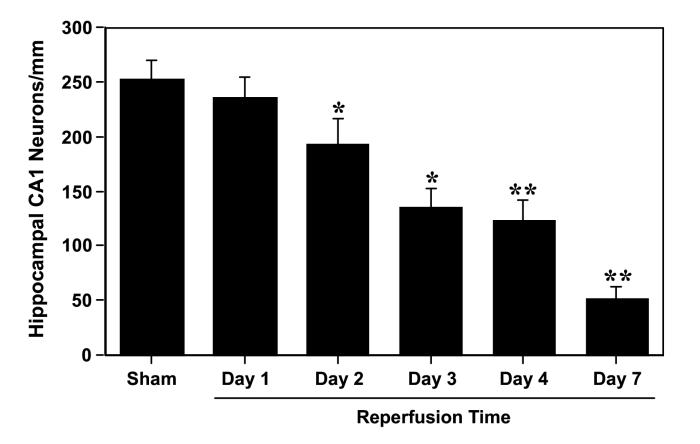
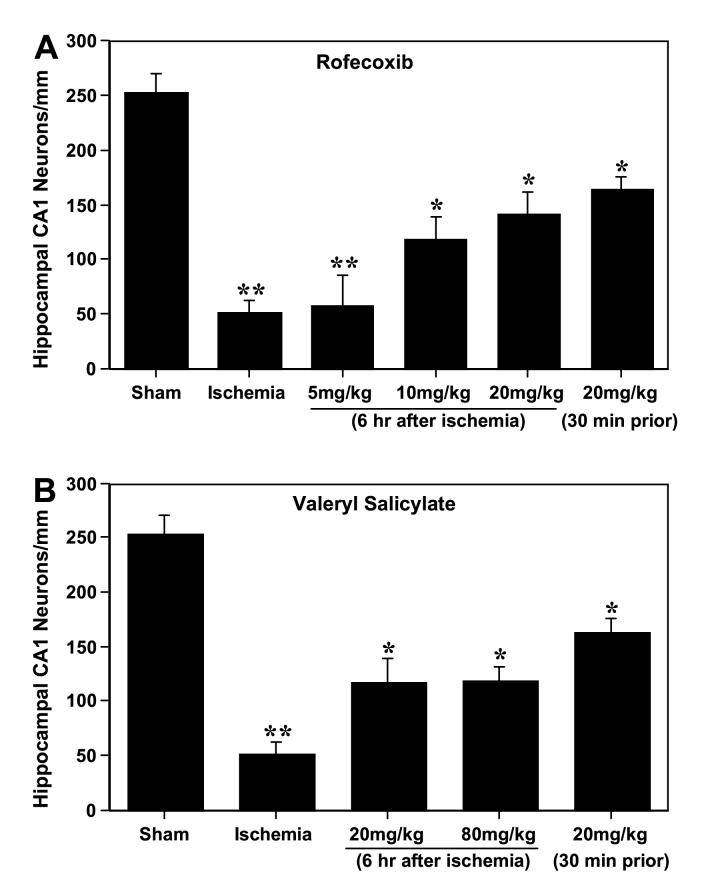
Microscopic evaluation of the hematoxylin & eosin-stained brain sections showed no evidence of neuronal damage in the hippocampus following transient global cerebral ischemia and reperfusion up to 1 day compared to the sham-operated control (Fig. 1). There was a progressive and significant decrease in neuronal density in the hippocampal CA1 region at 2 days (by 24 %, p<0.05), 3 days (by 46 %, p<0.05), 4 days (by 51 %, p<0.01) and 7 days (by 78 %, p<0.01) of recirculation compared to the sham group (Fig. 1). Delayed neuronal death in the CA1 hippocampal region was significantly reduced (p<0.05) by administration of Valeryl Salicylate (COX-1 inhibitor) at the two doses examined when treatment started 6 h after ischemia (Figs. 2B and 3C). In a similar way, delayed treatment with Rofecoxib led to a dose-dependent, significant reduction (p<0.05) in delayed neuronal loss in the CA1 subfield (Figs. 2A and 3D). For both COX inhibitors, when treatment is delayed until 6 h after the ischemic insult, the neuroprotection is similar to that seen in the groups in which the treatment started 30 min before ischemia (Fig. 2). The neuroprotective effects conferred by Rofecoxib and Valeryl Salicylate are not attributable to effects on body temperature as this variable was monitored up to 72 hr following surgery and did not differ between the treated and untreated groups (data not shown).
Figure 1.
Hippocampal CA1 neuronal counts as a function of reperfusion time following 5 min transient global cerebral ischemia in the gerbil. Values are mean counts of normal-appearing CA1 neurons per mm linear length ± S.D. *p<0.05 and **p<0.01 compared with the sham-operated control group.
Figure 2.
Effect of selective inhibition of COX-2 with Rofecoxib (A) and COX-1 with Valeryl Salicylate (B) on the number of surviving neurons in the CA1 hippocampal subfield 7 days after 5 min transient global cerebral ischemia in Mongolian gerbils. Values are mean counts of normal-appearing CA1 neurons per mm linear length ± S.D. (*p<0.05 between ischemia+vehicle and ischemia+drug treatments, **p<0.01 between sham and ischemia).
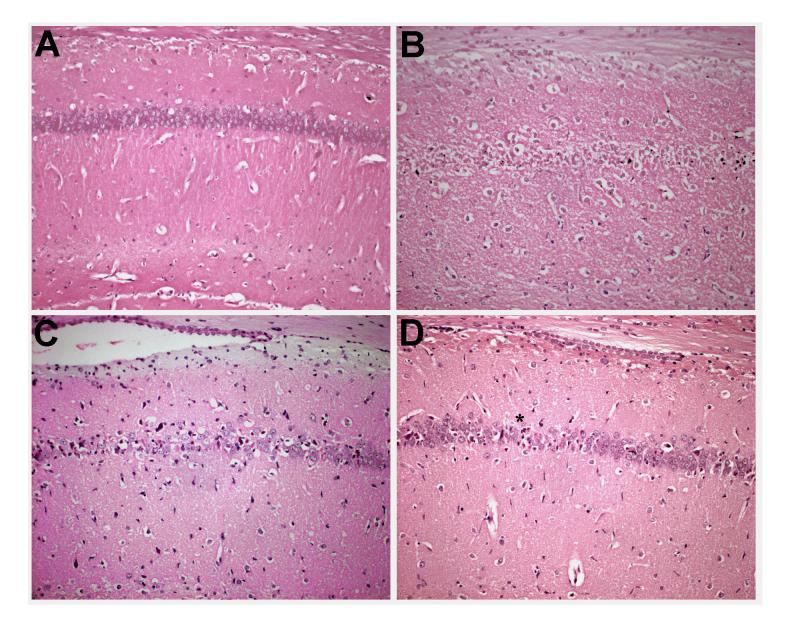
Figure 3.
Representative photomicrographs depicting neuronal cell loss in the hippocampal CA1 region at 7 days following (A) sham surgery, (B) ischemia + vehicle, (C) ischemia + Valeryl Salicylate (20 mg/kg, starting 6 h after ischemia), and (D) ischemia + Rofecoxib (20 mg/kg, starting 6 h after ischemia). Magnification bar equals 100 microns.
Temporal profile of PGE2 production following global cerebral ischemia: Effects of COX-1 and COX-2 inhibitors
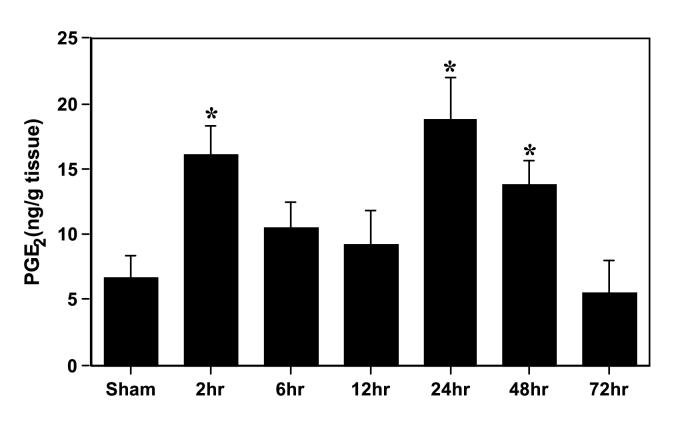
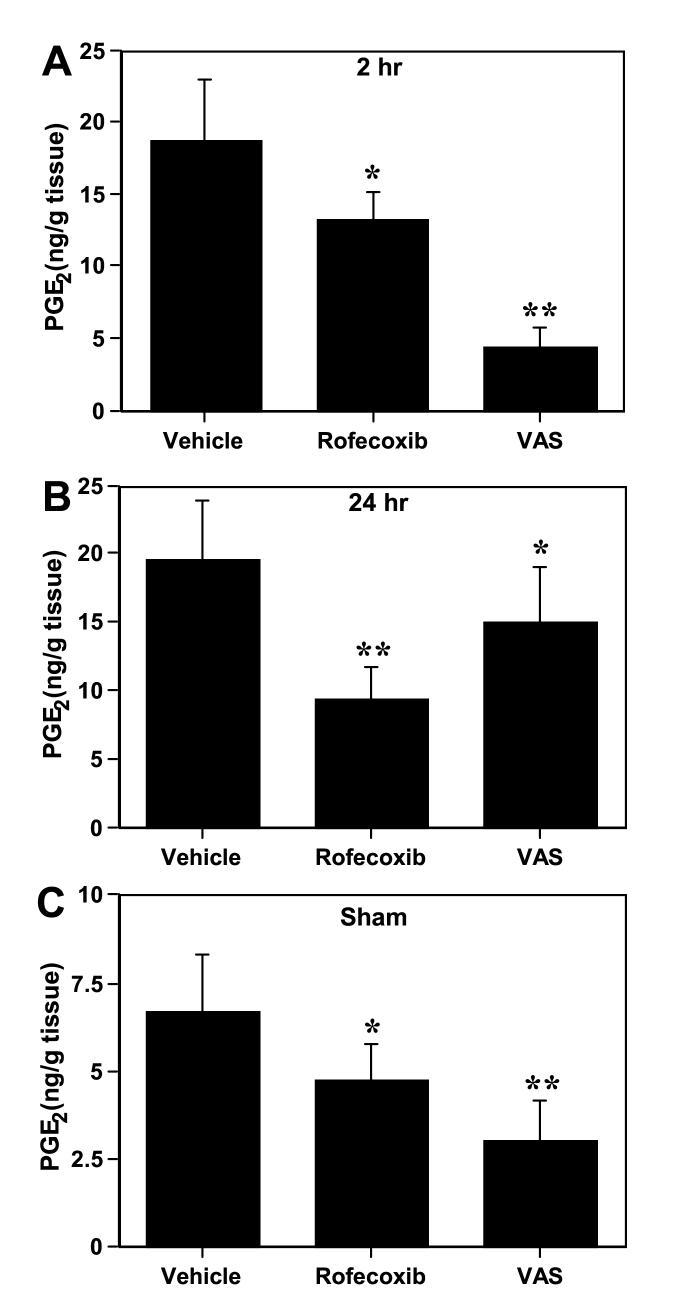
Bilateral carotid artery occlusion for 5 min resulted in a biphasic and significant increase (p<0.05) in hippocampal PGE2 concentrations (2 and 24-48 h) when compared with sham-operated animals (Fig. 4). The late increase in PGE2 levels (24-48 h) preceded the onset of morphological changes in the CA1 subfield of the hippocampus (Figs. 1 and 4). The selective COX-1 and COX-2 inhibitors were used to investigate the relative contribution of each COX isoform to the significant increase in hippocampal PGE2 production seen after a brief episode of global ischemia. After 2 h of reperfusion, Valeryl Salicylate was more potent in reducing PGE2 than Rofecoxib (Fig. 5A). In contrast, the increase in hippocampal PGE2 seen at 24 h after ischemia (Fig. 5B) was more significantly reduced by Rofecoxib treatment (52 %) than by the COX-1 inhibitor Valeryl Salicylate (23 %). These results indicate that most of the ischemia-induced increase in PGE2 at late stages after global ischemia originates from COX-2 activity.
Figure 4.
Time course of hippocampal PGE2 production following 5 min of transient global cerebral ischemia in the gerbil. *p<0.05 with respect to sham-operated animals.
Figure 5.
Effect of Rofecoxib (COX-2 inhibitor) and Valeryl Salicylate (COX-1 inhibitor) on the increase in hippocampal PGE2 seen at 2 h (A) and 24 h (B) after transient global cerebral ischemia in gerbils. In panel (C) is shown the effect of selective inhibition of COX-1 and COX-2 on the basal concentrations of PGE2 in the normal gerbil hippocampus. *p<0.05 and **p<0.01 with respect to vehicle-treated animals. In panel (C), *p<0.05 and **p<0.01 with respect to sham-vehicle group.
The effect of the selective COX-1 inhibitor Valeryl Salicylate on PGE2 concentration in non-ischemic hippocampus was compared with the effect of the selective COX-2 inhibitor Rofecoxib. As shown in Figure 5C, basal PGE2 concentrations were more potently reduced by the COX-1 inhibitor compared to the COX-2 inhibitor. The larger decrease in PGE2 content following Valeryl Salicylate treatment (55%), relative to Rofecoxib treatment (29%), provides additional evidence that COX-1 plays a major role in maintaining physiological hippocampal PGE2 concentrations. However, the significant decrease in PGE2 levels following Rofecoxib treatment indicates that the COX-2 isoform is also involved in regulating physiological COX activity.
Effect of COX-1 and COX-2 selective inhibitors on markers of oxidative damage
Our previous work clearly indicated that accumulation of biomarkers of oxidative damage and depletion of antioxidant reserves occur in hippocampus at late stages after the initial ischemic episode (Candelario-Jalil et al., 2001), and our present results show that global cerebral ischemia produces a biphasic increase in COX catalytic activity (2 and 24-48 h) (Fig. 4). Therefore, we next studied the effect of administration of inhibitors of COX-1 and COX-2 on measures of oxidative injury at 2 and 48 h after ischemia. GSH levels at 2 h were completely restored in animals treated 30 min prior to ischemia with the COX-1 inhibitor Valeryl Salicylate, but not with the highly selective COX-2 inhibitor Rofecoxib. On the contrary, at 48 h of reperfusion, Rofecoxib conferred protection against the significant reduction in levels of GSH and increase in GSSG and lipid peroxidation that result from transient ischemia as shown in Table 1. In contrast, the COX-1 inhibitor Valeryl Salicylate significantly reduced lipid peroxidation, but had no effect on the ischemia-induced modification in glutathione homeostasis seen at 48 h (Table 1).
Table 1.
Effect of selective inhibitors of COX-1 (Valeryl Salicylate) and COX-2 (Rofecoxib) on the measures of oxidative damage in the hippocampus at 2 and 48 h of reperfusion following 5 min transient global cerebral ischemia.
| Groups | GSH (mg/g tissue) | GSSG (ng/g tissue) | MDA + 4-HDA (nmol/mg protein) |
|---|---|---|---|
| 2h | |||
| Sham | 1.57 ± 0.22 | 1.41 ± 0.65 | 2.92 ± 0.49 |
| Ischemia | 0.99 ± 0.13 ‡ | 5.93 ± 1.42 ‡ | 3.43 ± 1.07 |
| Ischemia + Rofecoxib | 1.08 ± 0.15 ‡ | 5.59 ± 1.29 ‡ | 3.14 ± 1.03 |
| Ischemia + Valeryl Salicylate | 1.39 ± 0.18 † | 3.16 ± 1.65 † | 3.02 ± 1.31 |
| Salicylate | |||
| 48 h | |||
| Sham | 1.50 ± 0.16 | 1.48 ± 0.95 | 2.83 ± 2.00 |
| Ischemia | 1.01 ±0.19* | 9.20 ± 3.38* | 7.01 ± 1.82* |
| Ischemia + Rofecoxib | 1.32 ± 0.26** | 4.67 ± 1.76** | 4.16 ± 0.98**, & |
| Ischemia + Valeryl Salicylate | 1.10 ± 0.26* | 8.85 ± 2.20* | 4.74 ± 0.96**, & |
| Salicylate |
Data are mean ± SD.
p<0.05 compared to sham at 2 h.
p<0.05 compared to ischemia at 2 h.
p<0.05 compared to sham at 48 h,
p<0.05 compared to sham at 48 h,
p<0.05 compared to ischemia at 48 h.
We then examined whether the effects observed using Rofecoxib and Valeryl Salicylate on the glutathione homeostasis is mediated through modifications in the activity of the glutathione-related enzymes, glutathione peroxidase (GPx) and glutathione reductase (GR). In the hippocampus of ischemic animals, a significant reduction (p<0.05) in the activities of GPx and GR was found at 48 h after ischemia (Table 2) as compared to those found in sham-operated control group, confirming our earlier observation (Candelario-Jalil et al., 2001). There were no differences in either GPx or GR activities between ischemic and control animals at 2 h after ischemia (data not shown). Selective inhibition of COX-1 and COX-2 failed to prevent the profound decrease in hippocampal GPx and GR activities seen at 48 h as shown in Table 2. Administration of Rofecoxib or Valeryl Salicylate to sham-operated animals failed to significantly modify any of the evaluated oxidative stress parameters (data not shown).
Table 2.
Effect of Rofecoxib and Valeryl Salicylate on ischemia-induced reduction in the activity of glutathione peroxidase and glutathione reductase after 48 h of recirculation following 5 min of transient cerebral ischemia in gerbils.
| Groups | Glutathione Reductase (nmol NADPH oxidized/mg protein/min) | Glutathione Peroxidase (nmol NADPH oxidized/mg protein/min) |
|---|---|---|
| Sham | 12.10 ± 1.82 | 21.63 ±3.81 |
| Ischemia | 6.91 ± 1.89* | 13.40 ± 3.14* |
| Ischemia + Rofecoxib | 8.07 ± 1.69* | 12.42 ± 3.03* |
| Ischemia + Valeryl Salicylate | 7.18 ± 2.00* | 14.36 ± 2.51* |
Data are mean ± SD.
p<0.05 compared to sham.
To further rule out that the effect observed with Valeryl Salicylate and Rofecoxib on markers of oxidative damage are mediated through direct free radical scavenging activity of these compounds and not to COX inhibition, we performed in vitro experiments to assess antioxidant properties of both inhibitors. Results showed that neither Rofecoxib nor Valeryl Salicylate are able to scavenge reactive free radicals, even at the highest concentrations, which are unlikely to be reached in the in vivo situation (Tables 3 and 4).
Table 3.
Effect of Rofecoxib and Valeryl salicylate on the spontaneous and iron-catalyzed peroxidation of rat brain phospholipids.
| Spontaneous Peroxidation |
Iron-catalyzed Peroxidation |
||||
|---|---|---|---|---|---|
| Sample | λ=532 nm | Inhibition (%) | Sample | λ=532 nm | Inhibition (%) |
| Blank | 0.362 | - | Blank | 0.559 | - |
| Rofecoxib 100 μM | 0.291 | 19 | Rofecoxib 100 mM | 0.545 | 2 |
| Rofecoxib 1mM | 0.407 | 0 | Rofecoxib 1mM | 0.556 | 0 |
| Rofecoxib 2mM | 0.383 | 0 | Rofecoxib 2mM | 0.573 | 0 |
| VAS 100 μM | 0.304 | 16 | VAS 100 μM | 0.614 | 0 |
| VAS 1 mM | 0.361 | 0 | VAS 1 mM | 0.628 | 0 |
| VAS 2 mM | 0.137 | 62 | VAS 2 mM | 0.526 | 6 |
Results represent the extent of lipid peroxidation as measured by thiobarbituric acid reactive substances (TBARS) at a wavelength of 532 nm. See Aruoma et al., 1992 for details.
Table 4.
Scavenging effect of Rofecoxib and Valeryl Salicylate on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical.
| Absorbance (λ=517 nm)Mean ± SD | Inhibition (%) | |
|---|---|---|
| Control | 0.910 ± 0.011 | - |
| Rofecoxib 20 μM | 0.921± 0.009 | 0 |
| Rofecoxib 100 μM | 0.903 ± 0.01 | 0 |
| VAS 20 μM | 0.918 ± 0.002 | 0 |
| VAS 100 μM | 0.898 ± 0.004 | 1 |
Results represent the mean of three determinations ± S.D.
Discussion
The main findings of this study are: 1) transient global cerebral ischemia in gerbils results in a biphasic increase in the catalytic activity of COX involving an early COX-1-dependent production of PGE2 and a delayed persistent increase in COX-2 activity, 2) the increase in COX-2 activity and delayed production of PGE2 precedes the onset of morphological changes in the vulnerable hippocampal CA1 sector, and 3) the late increase in COX-2 activity is involved in the delayed impairment of glutathione homeostasis and oxidative damage in hippocampus, linking the inducible COX-2 enzyme to neurodegeneration following global cerebral ischemia. Results from the present study also suggest that both COX-1 and COX-2 contribute to maintain basal hippocampal COX activity. This observation is consistent with other studies reporting that a variety of neuronal populations contain detectable levels of COX-2 mRNA and protein under normal conditions (Yamagata et al., 1993; Breder et al., 1995).
Several lines of evidence indicate that oxidative damage in hippocampus is maximal at late stages after ischemia (Hall et al., 1997; Oostveen et al., 1998; Yamaguchi et al., 1998; Urabe et al., 2000; Candelario-Jalil et al., 2001). The delayed occurrence of oxidative stress correlates well with delayed neuronal loss of hippocampal CA1 pyramidal neurons (Hall et al., 1993, 1997; Oostveen et al., 1998), suggesting that reactive oxygen species formation may cooperate in a series of molecular events that link ischemic injury to neuronal cell death. Nevertheless, the mechanisms underlying the increase in oxidative damage at late stages after the initial ischemic insult are not completely understood.
Results obtained in the present investigation demonstrate for the first time that COX-2 is involved in the late increase in measures of oxidative injury in hippocampus. These data support the hypothesis that the neuroprotective effects observed with COX-2 selective inhibitors (Nakayama et al., 1998; Candelario-Jalil et al., 2002a,c) are partly mediated through reduction of oxidative damage in the ischemic brain. In support of this, we have recently found that a COX-2 inhibitor is able to reduce hippocampal oxidative damage following excitotoxic brain injury (Candelario-Jalil et al., 2000), which is a key pathological event of cerebral ischemia. In addition, COX inhibitors have also been shown to reduce free radical production in global cerebral ischemia (Hall et al., 1993) and in traumatic brain injury (Tyurin et al., 2000). These observations are particularly relevant in view of evidence showing that oxidative stress is implicated in the development and progression of apoptotic cell death in the central nervous system (Sastry and Rao, 2000; Callaway et al., 2001) and that activation of COX-2 is required for execution of oxidative neuronal death (Lee et al., 2001).
One mechanism by which oxidative stress events may contribute to delayed neuronal cell death following ischemia is the loss of glutamate transporter function. It was recently found that glial (GLT-1 and GLAST) and neuronal (EAAC1) high-affinity glutamate reuptake mechanisms are downregulated at late stages after ischemia, which precedes delayed neuronal death in gerbil hippocampus (Rao et al., 2000). In addition, several studies have demonstrated that glutamate uptake is compromised by pathophysiological events associated with the generation of free radicals (Volterra et al., 1994; Keller et al., 1997; Springer et al., 1997). Dysfunction of the glutamate transporters can lead to neuronal damage by allowing glutamate to remain in the synaptic cleft for a longer duration, contributing to excitotoxicity-induced oxidative injury in the ischemic brain.
A second interrelated mechanism that might account for the late increase in oxidative damage is the delayed expression of inducible nitric oxide synthase (iNOS) (Yrjänheikki et al., 1998). Nogawa et al. (1998) have shown that COX-2 selective inhibitors decrease ischemic injury in wild-type mice but not in iNOS null mice. These results suggest that these two inflammation-related enzymes (COX-2 and iNOS) may work synergistically to exacerbate damage in brain, perhaps through the formation of peroxynitrite. Interestingly, neuronal and glial glutamate transporters possess a SH-based redox regulatory mechanism that is critical for their activity (Trotti et al., 1997). Previously, these authors had found that glutamate transporters are sensitive to a number of biological oxidants, including H2O2, superoxide anion and, especially, peroxynitrite (Trotti et al., 1996). Further work is needed to determine if inflammatory-related enzymes (iNOS and COX-2) are involved in the late glutamate transporters dysfunction in the ischemic brain.
COX-2 enzymatic activity can also mediate tissue damage by producing pro-inflammatory prostanoids (Marnett et al., 1999). Interestingly, prostaglandins have been shown to stimulate calcium-dependent glutamate release in astrocytes (Bezzi et al., 1998), thus contributing to excitotoxicity. However, the precise role of prostaglandins in neurotoxicity is controversial because PGE2 has also been reported to limit the cytotoxic effects of glutamate (Akaike et al., 1994; Cazevieille et al., 1994). Alternatively, pharmacological inhibition of COX-2 has been shown to reduce N-methyl-D-aspartate-mediated neuronal cell death both in vitro (Hewett et al., 2000) and in vivo (Iadecola et al., 2001a). In addition, recent investigations have found a potentiation of excitotoxicity in transgenic mice overexpressing neuronal COX-2 (Kelley et al., 1999) and a significant reduction in ischemic brain injury in COX-2-deficient mice (Iadecola et al., 2001a).
Delayed treatment with Rofecoxib (COX-2 selective), but not with the COX-1 inhibitor Valeryl Salicylate, protected against hippocampal GSH depletion seen at late stages after ischemia. It is important to note that GSH is employed as a reducing agent in the peroxidase step of the COX reaction (Hamberg et al., 1974). As our present results have shown, at late stages following the ischemic episode, COX-2 accounts for the delayed increase in COX activity in hippocampus. This might explain how treatment with Rofecoxib, but not the COX-1 selective inhibitor Valeryl Salicylate, completely prevented GSH depletion at 48 h. In support of this, Valeryl Salicylate prevented the early depletion of GSH seen in hippocampus at 2 h, a time point at which PGE2 production is much more dependent on COX-1 activity (Fig. 5). These findings indicate that enhanced COX activity is able to deplete GSH in the ischemic brain, thus contributing to oxidative damage. It is noteworthy that a delayed treatment (starting after 6 h of reperfusion) with the COX-2 inhibitor significantly reduced late-onset oxidative injury and neuronal loss. This suggests that only the late increase in COX activity and oxidative damage is involved in neurodegeneration, since no further protection was afforded by an additional drug treatment 30 min prior to ischemia. These observations indicate that COX-2 activity is involved in the late disruption of the oxidant-antioxidant balance in the ischemic hippocampus. Intriguingly, even when Rofecoxib protected against the delayed impairment in GSH homeostasis, it failed to restore GPx and GR activities. The mechanisms responsible for the inhibition of these key antioxidant enzymes at late stages of reperfusion following global ischemia in gerbils remain currently unknown. Nevertheless, an overproduction of nitric oxide and/or an extensive depletion in NAD(P)H in the ischemic hippocampus might be involved, based on evidences showing that under these conditions the activities of GPx and GR are significantly impaired (Kosenko et al., 1998; Wong et al., 2001; Maciel et al., 2001).
Although previous studies have shown that COX-1 is not upregulated after ischemic brain injury (Ohtsuki et al., 1996; Nogawa et al., 1997; Nakayama et al., 1998; Koistinaho et al., 1999), results obtained in the present study suggest that COX-1 is also involved in delayed neuronal death of hippocampal CA1 neurons following global ischemia. It is interesting to note that a recent study reported that COX-1 protein levels are elevated in several non-neuronal cell populations following traumatic brain injury in humans (Schwab et al., 2002). To our knowledge, the effect of selective inhibition of COX-1 on neuronal damage induced by ischemia has not been previously investigated. Unexpectedly, Valeryl Salicylate reduced lipid peroxidation, but failed to protect against GSH depletion. These data suggest that attenuation of lipid peroxidation could account for the neuroprotective effect seen with Valeryl Salicylate. In addition, Wüllner et al. (1999) found that GSH depletion does not necessarily result in increased generation of reactive oxygen species and neurodegeneration, which might explain the findings obtained with Valeryl Salicylate in the present study. In addition, the possibility that Valeryl Salicylate reduced delayed neuronal death by a blockade of transcription factors, as already reported for acetylsalicylic acid and sodium salicylate on the nuclear factor kappa B (Grilli et al., 1996), cannot be excluded. Interestingly, Iadecola et al. (2001b) have found that mice lacking COX-1 are more susceptible to focal cerebral ischemia. Nevertheless, the effects of global ischemia in COX-1 deficient animals have not been yet investigated.
We have recently found that COX-2 inhibition with nimesulide, but not COX-1 inhibition with Valeryl Salicylate is able to confer protection against ischemia-induced blood-brain barrier breakdown and leukocyte infiltration in a model of transient focal cerebral ischemia in rats (Candelario-Jalil et al., 2002b). These previous findings and those obtained in the present study suggest that both COX isoforms are involved in neuronal damage following transient global cerebral ischemia and that only COX-2 contributes to brain injury in focal cerebral ischemia. Taken together, these results have important implications for the therapeutic potential of using COX inhibitors in the treatment of cerebral ischemia.
Acknowledgements:
The authors are greatly indebted to Dr. M. Kerry O’Banion (University of Rochester Medical Center, Rochester, USA) and Dr. Bernd L. Fiebich (Department of Psychiatry, University of Freiburg, Germany) for their critical comments on the manuscript. Supported in part by PHS grant NS40015 (JES).
Footnotes
- COX
- cyclooxygenase
- DPPH
- 2,2-diphenyl-1-pycrylhydrazyl radical
- GSH
- reduced glutathione
- GSSG
- oxidized glutathione
- GPx
- glutathione peroxidase
- GR
- glutathione reductase
- 4-HDA
- 4-hydroxyalkenals
- iNOS
- inducible nitric oxide synthase
- MDA
- malondialdehyde
- PGE2
- prostaglandin E2
References
- Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Prostaglandin E2 protects cultured neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 1994;663:237–243. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Aeschbach R, Löliger J. Antioxidant and prooxidant properties of active rosemary constituents: carnosol and carnosoic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya DK, Lecomte M, Dunn J, Morgans DJ, Smith WL. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Arch. Biochem. Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Bari F, Degi R, Domoki F, Veltkamp R, Louis TM, Thrikawala N, Robins G. Pathophysiology of COX-2 and NOS-derived metabolites and free radicals during brain ischemia. In: Krieglstein J, editor. Pharmacology of Cerebral Ischemia. Wissenschaftliche Verlagsgesellschaft mbH.; Stuttgart: 1998. pp. 237–241. [Google Scholar]
- Callaway JK, Beart PM, Jarrott B, Giardina SF. Incorporation of sodium channel blocking and free radical scavenging activities into a single drug, AM-36, results in profound inhibition of neuronal apoptosis. Br. J. Pharmacol. 2001;132:1691–1698. doi: 10.1038/sj.bjp.0704018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Ajamieh HH, Sam S, Martínez G, León OS. Nimesulide limits kainate-induced oxidative damage in the rat hippocampus. Eur. J. Pharmacol. 2000;390:295–298. doi: 10.1016/s0014-2999(99)00908-5. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martínez G, León OS. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci. Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Alvarez D, Castañeda JM, Al-Dalain SM, Martínez G, Merino N, León OS. The highly selective cyclooxygenase-2 inhibitor DFU is neuroprotective when given several hours after transient cerebral ischemia in gerbils. Brain Res. 2002a;927:212–215. doi: 10.1016/s0006-8993(01)03358-3. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, González A, García M, León OS, Springer JE. Involvement of cyclooxygenase-2 in blood-brain barrier damage and leukocyte infiltration following transient focal cerebral ischemia in rats. Soc. Neurosci. Abstr. 2002b;28:580–10. [Google Scholar]
- Candelario-Jalil E, Alvarez D, González-Falcón A, García-Cabrera M, Martínez-Sánchez G, Merino N, Giuliani A, León OS. Neuroprotective efficacy of nimesulide against hippocampal neuronal damage following transient forebrain ischemia. Eur. J. Pharmacol. 2002c;453:189–195. doi: 10.1016/s0014-2999(02)02422-6. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. Vol. 113. Academic Press; Orlando: 1985. pp. 484–490. [DOI] [PubMed] [Google Scholar]
- Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C. Protection by prostaglandins from glutamate toxicity in cortical neurons. Neurochem. Int. 1994;24:395–398. doi: 10.1016/0197-0186(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Chan C, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson A, Forrest M, Gauthier J, Gordon R, Gresser M, Guay J, Patrick D, Percival M, Perrier H, Prasit P, Rodger I, Riendeau D. Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J. Pharmacol. Exp. Ther. 1999;290:551–60. [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen JH, Ho CT. Antioxidant Activities of Caffeic Acid and its related Hydroxycinnamic Acid Compouds. J. Agric. Food Chem. 1997;45:2374–2378. [Google Scholar]
- Davidson ME, Lang RJ. Effects of selective inhibitors of cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2) on the spontaneous myogenic contractions in the upper urinary tract of the guinea-pig and rat. Br. J. Pharmacol. 2000;129:661–670. doi: 10.1038/sj.bjp.0703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohé L, Gunzler WA. Assays of glutathione peroxidase. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. Vol. 105. Academic Press; Orlando: 1984. pp. 114–121. [DOI] [PubMed] [Google Scholar]
- Floreani M, Skaper SD, Facci L, Lipartiti M, Giusti P. Melatonin maintains glutathione homeostasis in kainic acid-exposed rat brain tissues. FASEB J. 1997;11:1309–1315. doi: 10.1096/fasebj.11.14.9409550. [DOI] [PubMed] [Google Scholar]
- Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Hall ED, Andrus PK, Althaus JS, VonVoigtlander PF. Hydroxyl radical production and lipid peroxidation parallels selective post-ischemic vulnerability in gerbil brain. J. Neurosci. Res. 1993;34:107–112. doi: 10.1002/jnr.490340111. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Andrus PK, Anderson DK, Thomas CE. Immunocytochemical method for investigating in vivo neuronal oxygen radical-induced lipid peroxidation. J. Neurosci. Meth. 1997;76:115–122. doi: 10.1016/s0165-0270(97)00089-7. [DOI] [PubMed] [Google Scholar]
- Halpin R, Geer L, Zhang K, Marks T, Dean D, Jones A, Melillo D, Doss G, Vyas K. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab. Dispos. 2000;28:1244–54. [PubMed] [Google Scholar]
- Hamberg M, Svensson J, Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc. Natl. Acad. Sci. USA. 1974;71:3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett SJ, Uliasz TF, Vidwans AS, Hewett JA. Cyclooxygenase-2 contributes to N-methyl-D-aspartate-mediated neuronal cell death in primary cortical cell culture. J. Pharmacol. Exp. Ther. 2000;293:417–425. [PubMed] [Google Scholar]
- Hong JT, Ryu SR, Kim HJ, Lee JW, Lee SH, Yun YP, Lee BM, Kim PY. Protective effects of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbils. Brain Res. 2001;888:11–18. doi: 10.1016/s0006-8993(00)02935-8. [DOI] [PubMed] [Google Scholar]
- Hung LM, Su MJ, Chu WK, Chiao CW, Chan WF, Chen JK. The protective effect of resveratrols on ischemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br. J. Pharmacol. 2002;135:1627–1633. doi: 10.1038/sj.bjp.0704637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross M. Reduced susceptibility to ischemic brain injury and NMDA-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl. Acad. Sci. USA. 2001a;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Sugimoto K, Niwa K, Kazama K, Ross ME. Increased susceptibility to ischemic brain injury in cyclooxygenase-1-deficient mice. J. Cereb. Blood Flow Metab. 2001b;21:1436–1441. doi: 10.1097/00004647-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Jain KK. Neuroprotection in cerebrovascular disease. Expert Opin. Investig. Drugs. 2000;9:695–711. doi: 10.1517/13543784.9.4.695. [DOI] [PubMed] [Google Scholar]
- Keller J, Mark R, Bruce A, Blanc E, Rothstein J, Uchida K, Mattson M. 4-hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death. Neuropathology. 2000;20:S95–S97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J. Cereb. Blood Flow Metab. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Oda T, Niinobe M, Hata R, Handa N, Fukunaga R, Isaka Y, Kimura K, Maeda H. Free radical generation during brief period of cerebral ischemia may trigger delayed neuronal death. Neuroscience. 1990;35:551–558. doi: 10.1016/0306-4522(90)90328-2. [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Koponen S, Chan PH. Expression of cyclooxygenase-2 mRNA after global ischemia is regulated by AMPA receptors and glucocorticoids. Stroke. 1999;30:1900–1906. doi: 10.1161/01.str.30.9.1900. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Asanuma M, Nishibayashi S, Iwata E, Ogawa N. Late-onset lipid peroxidation and neuronal cell death following transient forebrain ischemia in rat brain. Brain Res. 1997;772:37–44. doi: 10.1016/s0006-8993(97)00836-6. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Kaminsky Y, Lopata O, Muravyov N, Kaminsky A, Hermenegildo C, Felipo V. Nitroarginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab. Brain Dis. 1998;13:29–41. doi: 10.1023/a:1020626928259. [DOI] [PubMed] [Google Scholar]
- Lee YA, Ryu BR, Noh JS, Gwag BJ. Activation of cyclooxygenase-2 during excitotoxicity, oxidative stress and apoptosis. Soc. Neurosci. Abstr. 2001;27:97.9. [Google Scholar]
- Lee S, Suh S, Kim S. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci. Lett. 2000;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca2+-induced membrane permeability transition in brain mitochondria. J. Neurochem. 2001;79,:1237–45. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- Marnett L, Rowlinson S, Goodwin D, Kalgutkar A, Lanzo C. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- Martí E, Ferrer I, Blasi J. Differential regulation of chromogranin A, chromogranin B and secretoneurin protein expression after transient forebrain ischemia in the gerbil. Acta Neuropathol. (Berl) 2001;101:159–166. doi: 10.1007/s004010000280. [DOI] [PubMed] [Google Scholar]
- Martínez G, Candelario-Jalil E, Giuliani A, León OS, Sam S, Delgado R, Núñez-Sellés AJ. Mangifera indica L. extract (QF808) reduces ischemia-induced neuronal loss and oxidative damage in the gerbil brain. Free Radic. Res. 2001;35:465–473. doi: 10.1080/10715760100301481. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Sasaki T, Kirino T, Tamura A, Noguchi M, Saito I, Takakura K. Effect of cyclooxygenase and lipoxygenase inhibitors on delayed neuronal death in the gerbil hippocampus. Stroke. 1989;20:925–929. doi: 10.1161/01.str.20.7.925. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu R, Nagayama T, Rose M, Stetler R, Isakson P, Chen J, Graham S. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. USA. 1998;95:10954–59. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CW, Wei EP, Povlishock JT, Kontos KA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am. J. Physiol. 1992;263:H1356–H1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ. Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc. Natl. Acad. Sci. USA. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki T, Kitagawa K, Yamagata K, Mandai K, Mabuchi T, Matsushita K, Yanagihara T, Matsumoto M. Induction of cyclooxygenase-2 mRNA in gerbil hippocampal neurons after transient forebrain ischemia. Brain Res., 1996;736:353–356. doi: 10.1016/0006-8993(96)00948-1. [DOI] [PubMed] [Google Scholar]
- Oostveen JA, Dunn E, Carter DB, Hall ED. Neuroprotective efficacy and mechanisms of novel pyrrolopyrimidine lipid peroxidation inhibitors in the gerbil forebrain ischemia model. J. Cereb. Blood Flow Metab. 1998;18:539–547. doi: 10.1097/00004647-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Powell WS. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. In: Lands WEM, Smith WL, editors. Methods in Enzymology, Academic Press; Orlando: 1982. pp. 466–477. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Rao AM, Dogan A, Bowen KK, Hatcher JF, Rothstein JD, Dempsey RJ. Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem. Int. 2000;36:531–7. doi: 10.1016/s0197-0186(99)00153-9. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Nakagomi T, Kirino T, Tamura A, Noguchi M, Saito I, Takakura K. Indomethacin ameliorates ischemic neuronal damage in the gerbil hippocampal CA1 sector. Stroke. 1988;19:1399–403. doi: 10.1161/01.str.19.11.1399. [DOI] [PubMed] [Google Scholar]
- Sastry PS, Rao KS. Apoptosis and the nervous system. J. Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- Satoh S, Ikegaki I, Suzuki Y, Asano T, Shibuya M, Hidaka H. Neuroprotective properties of a protein kinase inhibitor against ischemia-induced neuronal damage in rats and gerbils. Br. J. Pharmacol. 1996;118:1592–1596. doi: 10.1111/j.1476-5381.1996.tb15579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schwab J, Beschorner R, Meyermann R, Gozalan F, Schluesener H. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J. Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson M. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J. Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione:applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur. J. Neurosci. 1997;9:1236–1243. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J. Biol. Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- Urabe T, Yamasaki Y, Hattori N, Yoshikawa M, Uchida K, Mizuno Y. Accumulation of 4-hydroxynonenal-modified proteins in hippocampal CA1 pyramidal neurons precedes delayed neuronal damage in the gerbil brain. Neuroscience. 2000;100:241–250. doi: 10.1016/s0306-4522(00)00264-5. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J. Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B, Brown D, Pan T, Whiteman M, Liu T, Bu X, Li R, Gambetti P, Olesik J, Rubenstein R, Sy M. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem. 2001;79:689–698. doi: 10.1046/j.1471-4159.2001.00625.x. [DOI] [PubMed] [Google Scholar]
- Wüllner U, Seyfried J, Groscurth P, Beinroth S, Winter S, Gleichmann M, Heneka M, Löschmann PA, Schulz JB, Weller M, Klockgether T. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res. 1999;826:53–62. doi: 10.1016/s0006-8993(99)01228-7. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons, regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Ogata H, Hamaguchi S, Kitajima T. Superoxide radical generation and histopathological changes in hippocampal CA1 after ischemia/reperfusion in gerbils. Can. J. Anaesth. 1998;45:226–232. doi: 10.1007/BF03012907. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito K, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–242. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]