Abstract
One of the more vexing issues in ecology is how historical processes affect contemporary patterns of biodiversity. Accordingly, few models have been presented. Two corollary models (centre of origin, time-for-speciation) can be used to make quantitative predictions characterizing the tropical niche conservatism hypothesis and describe diversification as diffusion and subsequent cladogenesis of species away from the place of origin of a higher taxon in the tropics. Predictions derived from such models are: (i) species richness declines toward the periphery of the range of a higher taxon; (ii) taxa are more derived toward the periphery than the centre; (iii) ages of taxa are lower toward the periphery than the centre; and (iv) ages and measures of derivedness are less variable toward the periphery of the range of a higher taxon. I tested these predictions to better understand the formation of one of the most ubiquitous patterns of biodiversity—the latitudinal gradient in species richness. Results indicate well-supported predictions for New World leaf-nosed bats and that diversification has had strong influences on latitudinal gradients of species richness. A better understanding of how evolutionary diversification of taxa contributes to formation of patterns of species richness along environmental gradients is necessary to fully understand spatial variation in biodiversity.
Keywords: bats, biodiversity, diversification, history, historical processes, latitudinal gradient
1. Introduction
Although much of the work attempting to understand large-scale patterns of diversity has focused on the differential responses of species to contemporary environmental gradients (Wright et al. 1993; Currie et al. 1999; Francis & Currie 2003), the current level and geographical distribution of biodiversity has ultimately resulted from concerted ecological and evolutionary mechanisms that have operated throughout geologic time (Dobzhansky 1950; Fischer 1960; Rohde 1992; Ricklefs 2004; Weins 2004). Accordingly, the notion that contemporary patterns of biodiversity can be understood by focusing on contemporary ecological mechanisms has left the importance of historical phenomena under-appreciated (however, see Haffer 1969; Fjeldsa 1994; Fraser & Currie 1996; Kerr & Currie 1999; Schemske 2002; Hawkins & Porter 2003; Hawkins et al. 2003a,b; Diniz-Filho et al. 2004). Undeniably, historical influences contribute to contemporary patterns of biodiversity to a similar or greater extent as contemporary climatic regimes, even at the local level.
The latitudinal gradient in biodiversity offers an excellent example. Latitudinal gradients in species richness are general across the current biota as well as across space and time (Willig et al. 2003; Hillebrand 2004). Moreover, it has been solidly demonstrated that species richness can be predicted by the underlying correlates of latitude such as productivity, precipitation, temperature and seasonality to name only a few (Currie 1991; Badgley & Fox 2000; Hawkins et al. 2003a). Nonetheless, these environmental variables do not produce species per se (Weins & Donoghue 2004), but may only enhance underlying differences in diversification that contribute to contemporary latitudinal gradients in species richness. At the least, species originate in particular areas and this strongly influences the location of their geographical distributions. Moreover, if diversification rates are spatially variable along a latitudinal gradient, this could contribute substantially to contemporary patterns of biodiversity (Cardillo 1999; Cardillo et al. 2005).
Two similar concepts (centre of origin hypothesis (Hennig 1979; Ricklefs & Schluter 1993), time-for-speciation hypothesis (Stephens & Wiens 2003)) propose a historical mechanism that can produce gradients of species richness. These hypotheses propose that the area occupied by the ancestor of a monophyletic group represents its centre of origin and diversification results from the production of new taxa, some of which disperse and diversify away from the centre. After sufficient time, a diversity gradient develops in which species richness is greatest toward the centre of the range of the higher taxon and decreases toward the periphery. Moreover, dispersal from a productive (in terms of the origination of new taxa) origin accompanied by added speciation as species colonize and adapt to new regions results in gradients of both species richness and the phylogenetic characteristics of taxa. Historically, testing for such centres of origin has been problematic. For example, the actual location of a centre may be obscure and different from taxon to taxon (Moritz et al. 2000). Nonetheless, the measurement of the actual expansion does not require precise identification of the centre of origin because the ‘genetic footprint’ of such expansion is often detectable with modern molecular techniques (Lessa et al. 2003).
These two similar hypotheses (e.g. centre of origin hypothesis, time-for-speciation hypothesis) provide five testable predictions: (i) species richness declines with proximity to the edge of the range of the higher taxon; (ii) toward the edge, taxa continuously enter new habitats and evolve to new selective regimes, thereby enhancing rates of molecular evolution (Bromham 2003) and ultimately speciation. To this end, relative rates of evolution measured as average sequence divergence between taxa and the putative ancestor of the entire clade should be higher toward the edge than toward the centre of the higher taxon's geographical distribution; (iii) as a result, the average age of taxa on the edge should be lower than toward the centre. Finally, because the duration of diversification is longer toward the centre than toward the edge, both primitive and derived taxa should co-occur toward the centre and primarily derived taxa should co-occur toward the periphery. Accordingly, (iv) the variance of ages; and (v) the variance of sequence divergences should decrease toward the periphery of the range of the higher taxon. Confirmation of these predictions could provide strong evidence for a historical component to variation in species richness along a particular environmental gradient. Moreover, niche conservatism will only enhance such a process (Weins & Graham 2005) if the environmental gradient is sufficiently long so as to span different selection regimes.
Although attempts have been made to evaluate the influence of historical phenomena on contemporary gradients of diversity, few (Brown 1988; Rohde 1992; Ricklefs & Schluter 1993; Brown & Lomolino 1998; Weins 2004; Weins & Donoghue 2004; Weins & Graham 2005) have proposed a mechanism whereby the diversification of taxa through time could lead to commonly described latitudinal gradients in species richness (i.e. tropical conservatism hypothesis). If particular higher taxa are monophyletic and of tropical origin and diversify along latitudinal gradients according to a historical process such as the one presented, then the five aforementioned predictions should hold with respect to distance from the equator and should at least in part account for latitudinal gradients in the species richness of taxa of tropical origin.
Phylogenetic approaches can enlighten understanding of historical diversification that has resulted in the contemporary biota (Moritz et al. 2000; Lessa et al. 2003). Moreover, phylogenies combined with spatial variation in species composition can be used to infer historical processes and ultimately the formation of contemporary patterns of biodiversity. Indeed, biodiversity is not distributed randomly across the tree of life. Similarly, ecological diversity is not uniformly distributed in terms of phylogeny. For bats, a few clades represent disproportionate amounts of the ecological diversity of the entire order (Simmons & Conway 2003). The best example is of the New World bat family Phyllostomidae. The oldest known fossils from this family are from the Miocene of Colombia suggesting a tropical origin (Savage 1951; Czaplewski 1997; Jones et al. 2005). Moreover, this family represents a highly diverse group that comprises approximately 53 genera and 141 species (Wetterer et al. 2000); more than half of all bats found in the continental New World. Members of Phyllostomidae exhibit dietary specializations for insectivory, frugivory, carnivory, nectarivory and sanguinivory (Wilson 1975). Consequently, this family exhibits more morphological diversity than any other family level group of mammals (Baker et al. 2003). Indeed, understanding this diversity, in particular its mechanistic basis, has been an area of active research.
Although phyllostomids are by far the most-studied family of bats from a phylogenetic perspective (Jones et al. 2002), such elevated diversity has made the determination of systematic relationships problematic for at least the last century (Baker et al. 2003). Two recent phylogenies (Baker et al. 2003) based on independently evolving genomes, the RAG2 nuclear gene and combined 12S RNA, tRNAVAL and 16S rRNA mitochondrial genes, provide a digenomically congruent reconstruction of the evolution of this species-rich and ecologically diverse family. Such congruence provides powerful inference regarding systematic relationships of taxa and presents a promising baseline from which to explore the geographical diversification of taxa and resultant formation of latitudinal gradients in species richness. Herein, I evaluate a quantitative model integrating predictions of both the centre of origin hypothesis and the time-for-speciation hypothesis and apply this model to better understand the diversification of New World leaf-nosed bats along latitudinal gradients.
2. Material and methods
(a) Assemblages, phylogenies and phylogenetic diversity
The species composition of 30 well-sampled assemblages comes from the literature (see electronic supplementary material). I assigned species to the New World leaf-nosed bat family Phyllostomidae following Koopman (1993). I obtained a working hypothesis of the evolutionary relationships of phyllostomid taxa from a genus level phylogeny based on (i) the RAG2 nuclear gene and (ii) mitochondrial genes (12S rRNA, tRNAVAL and 16S RNA) from Baker et al. (2003, fig. 5b). Scleronycteris ega was removed from one assemblage and Lichonycteris obscura was removed from five assemblages because these two monotypic genera were not represented in cladograms. I used the distance in terms of per cent sequence divergence (Baker et al. 2003, fig. 5b) of each taxon to its sister taxon as a relative measure of the age of that taxon. Taxa with short distances are newly derived relative to those with long distances. I also measured the distance of a taxon to the root of the phyllostomid tree and used this as a measure of the rate of sequence divergence of that taxon. Taxa with long root distances are the product of more diversification than taxa with short root distances (Kerr & Currie 1999). From these two measures of per cent sequence divergence, the mean and variance was used to determine the average and variability of rates of sequence divergence and age of species in a particular assemblage, respectively. Since, this is a genus level cladogram, generic values were weighted relative to the number of species per genus present at a site when averages and variances for assemblages were calculated. Estimates of phylogenetic characteristics and richness of species as well as latitude and longitude of each site can be obtained by contacting the author.
(b) Inferential analyses
Latitudinal gradients in phylogenetic characteristics need not be linear to support predictions. Accordingly, I used orthogonal polynomial regression analysis (Dutka & Ewens 1971; Sokal & Rohlf 1995) to quantify latitudinal relationships of species richness and each of the phylogenetic characteristics. Orthogonal polynomials produce uncorrelated parameter estimates required for meaningful simulation analyses described below. I also examined longitudinal diversification. No significant longitudinal gradients exist for any of the variables examined (see electronic supplementary material). I used a function written in Matlab (Math Works 1995) to conduct these analyses. Because latitudinal patterns of diversity probably exhibit spatial autocorrelation, traditional samples sizes used in hypothesis tests inflate type 1 error rates (Legendre 1993). In regression analyses I used geographically effective samples sizes (Griffith 2003) to determine degrees of freedom for F-tests. Autoregressive parameters were determined using the program Spatial Analysis in Macroecology (Rangel et al. in press).
Distributional attributes of phylogenetic characteristics could affect a latitudinal gradient in the absence of a historical effect. For example, greater numbers of younger than old taxa predisposes the empirical distribution of ages to be skewed. Accordingly, the a priori expectation would be that as you increase the species richness of a sample, you enhance the likelihood of obtaining an old taxon and this might affect a systematic change in phylogenetic characteristics that is due to variation among sites in terms of their species richness as you move along a latitudinal gradient. Accordingly, phylogenetic characteristics could exhibit a latitudinal gradient without the operation of a natural process (i.e. the selection probability effect; Huston (1997)). Latitudinal gradients in phylogenetic characteristics were deemed non-trivial only if they exhibited a parameterization that was different from those that could result from a latitudinal gradient in species richness. To determine if observed gradients in phylogenetic diversity could be generated by latitudinal increases in species richness, I conducted simulation analyses. Species were drawn randomly from a pool comprising all phyllostomids occurring in the continental New World (minus S. ega and L. obscura). More specifically, for each real assemblage, a simulated assemblage was generated by randomly selecting without replacement the same number of species as occurred in the actual assemblage from the new world pool. Measures of phylogenetic diversity were calculated and then each measure was regressed separately on latitude using orthogonal polynomial regression. This process was iterated 1000 times to create a distribution of gradients in a particular phylogenetic characteristic that could be produced by a latitudinal gradient in species richness. Parameter estimates (R2, b1 and b2) characterizing the empirical latitudinal gradient in phylogenetic diversity from actual assemblage were then compared to the distributions of such values from the simulated gradients to determine p-values. The position of the parameter estimate for real assemblage relative to the distribution of simulated values describes the probability that the observed value is a random variate from the simulated distribution, and that the observed latitudinal gradient in phylogenetic diversity is a product of the latitudinal gradient in species richness. Parameter estimates for the actual relationship were deemed to be significant when they were not encompassed by the middle 95% of the distribution of simulated values.
3. Results
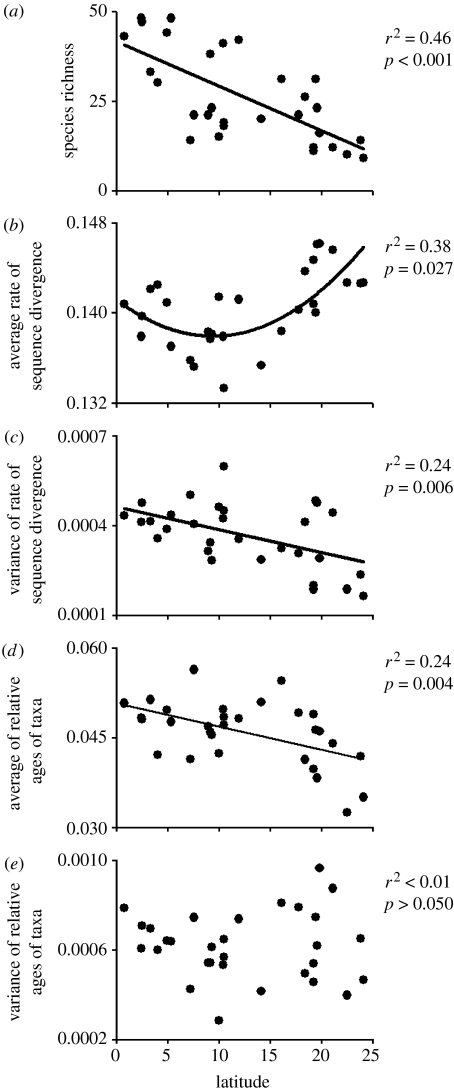
As with a wide variety of plant and animal taxa occurring in both aquatic and terrestrial domains, a significant latitudinal gradient in species richness exists for New World leaf-nosed bats (figure 1a; r2=0.46, p≪0.001). Average rate of sequence divergence exhibits a latitudinal gradient, but its relationship was quantitatively and qualitatively different than that for species richness (figure 1b; R2=0.38, p=0.027). Although the relationship was not linear, on average, species have a higher rate of sequence divergence toward the edge of the latitudinal range of New World leaf-nosed bats than at the equator. The average relative age of taxa also decreased with increases in latitude (figure 1d; r2=0.24, p=0.006). These observation combined with the way in which the variance of rates of sequence divergence (figure 1c; r2=0.24, p=0.004) decreased away from the equator suggested that primarily derived taxa exist toward the edge of the range of the Phyllostomidae. The variance of ages failed to exhibit significant latitudinal variation (figure 1e; r2<0.01, p<0.05).
Figure 1.
Latitudinal gradients of diversity and phylogenetic characteristics of New World leaf-nosed bat assemblages.
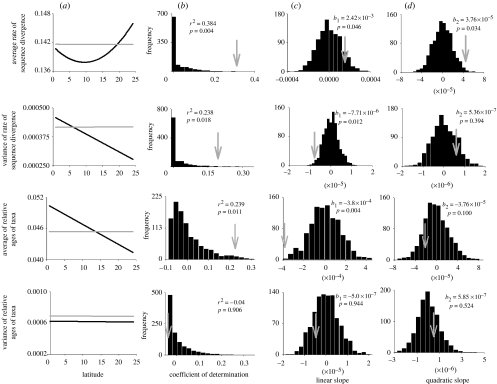
Changes in species richness associated with latitude cannot account for most gradients in phylogenetic characteristics of species (figure 2). In particular, changes in species richness produce no expected latitudinal gradient in phylogenetic characteristics. Highly significant differences between empirical and simulated latitudinal gradients exist for the average and variance of rates of sequence divergence as well as the average age of taxa and this was true for coefficients of determination as well as linear slopes. Thus, latitude accounts for significantly more of the variation in these three phylogenetic characteristics across the empirical assemblage than the simulated assemblage and the linear rate in which these characteristics changed with latitude was also greater than expected from simulation. The quadratic slope was also significantly different between simulated and empirical gradients for the average rate of sequence divergence. No significant difference between simulated and empirical gradients existed for the variance of age of taxa and this simply may be a reflection of no consistent latitudinal variation. Indeed, the geographical structure to the diversification of taxa along latitudinal gradients probably is not the result of a confounded relationship between species richness and phylogenetic diversity.
Figure 2.
Results from simulation analyses evaluating the degree to which latitudinal gradients in phylogenetic diversity can result from a latitudinal gradient in species richness. (a) Illustrates differences between expected values for empirical (black line) and simulated (grey line) assemblages. (b–d) Illustrate comparisons of the empirical parameter estimates (grey arrows) with the distributions of simulated parameter estimates (black histograms) regarding the coefficient of variation, linear slope and quadratic slope, respectively.
4. Discussion
(a) The historical effect
Minimally, these results strongly suggest that significant historical effects influence contemporary latitudinal gradients in biodiversity of New World leaf-nosed bats. The mean ages and rates of sequence divergence of taxa as well as the variance of rate of sequence divergence can predict the latitudinal position of assemblages. Moreover, such strong latitudinal gradients in these phylogenetic characteristics, in which the most derived and least variable taxa occur at the periphery of the geographical range of the family, suggests niche conservatism (Weins & Donoghue 2004; Weins & Graham 2005) and that the Phyllostomidae is still expanding its geographical range and yet has to reach an equilibrium.
The poleward expansion of the Phyllostomidae is probably constrained by at least two other factors and not solely by the physical ability to disperse to areas of higher latitude. First, most phyllostomids, the nectarivores and frugivores in particular, are dependent on the types of flowers and fleshy fruits that are found in tropical and subtropical forests and the poleward expansion of these bat groups likely depends on the poleward expansion of these forests. Second, and not necessarily independent of the first, phyllostomids are precise thermoregulators (McNab 1969) and the ability to colonize the temperate zone probably depends either on the evolution of more labile thermoregulation or the existence of warmer global temperatures. While not rapid, such scenarios of environmental change are possible, especially in light of the wide distribution of tropical vegetation in the Tertiary in general (Behrensmeyer et al. 1992) and the extensive Eocene expansion as far as the Arctic Circle in particular (Janis 1993).
The high tropical diversity of this taxon probably has resulted from a tropical origin giving more time to accumulate greater species richness in the face of elevated speciation at the latitudinal extents of the family. Higher species richness should be expected at lower latitudes in taxa of tropical origin because more time has allowed for the accumulation of more species (Pianka 1966; Whittaker 1969). If the rate of diversification is the same across a latitudinal gradient, the time differential will mean that more species will accumulate at lower latitudes. This is because equal amounts of speciation occur at both higher and lower latitudes. Owing to the time differential, per capita speciation would be the same but total speciation and hence diversity would be greater at lower latitudes. Moreover, if the time differential were substantive, such that many more species accumulate toward the equator, this would counteract relatively faster speciation at higher latitudes.
A second process that could combine with niche conservatism and contribute to the historical signal of latitudinal gradients of diversity is reduced extinction at lower latitudes. It has long been hypothesized that the tropics offer more benign environments in terms of harshness, stability, predictability and seasonality (Dobzhansky 1950; Fischer 1960) than their neighbouring temperate zones. Moreover, increased vertical stratification makes the tropics more physically heterogeneous as well as allows tropical forests to support a greater number of species (Rohde 1992). This enhanced physical heterogeneity may provide a greater number of refuges to animal species thereby enhancing their persistence through time. Indeed, beta diversity is higher at lower latitudes in bats and other taxa (Stevens & Willig 2002; Koleff et al. 2003) and this at least suggests that lower latitude tropical sites are perceived as more spatially heterogeneous than their temperate counterparts. Indeed, Hawkins et al. (in press) have demonstrated differences in richness patterns between derived and basal clades of New World birds. This suggests lower extinction rates at lower latitudes and that increased climatic variability at higher latitudes enhances the diversification process (Connell & Orias 1964; McGlone 1996).
(b) Generality of gradients in phylogenetic characteristics
Many higher taxa exhibit tropical peaks in diversity (Willig et al. 2003; Hillebrand 2004), and such a process of geographical diversification may generally at least contribute to latitudinal variation in species richness. Passerine birds provide a good illustration. In MacGillivray's warbler (Oporonis tolmiei), neotropical taxa exhibit much greater intraspecific genetic variability than their nearctic counterparts and this pattern is due to Pleistocene postglacial expansion into higher latitudes (Milá et al. 2000). A variety of mammalian taxa including members of the Insectivora, Rodentia and Carnivora exhibit similar spatial structure to genetic variability and appear to be diversifying in a geographically similar manner as bats presented here in response to climatic change (Lessa et al. 2003). Finally, this same pattern of higher intraspecific genetic divergences at lower latitudes has recently been generalized to many vertebrate taxa (Martin & McKay 2004).
Interspecific examples suggest similar patterns. Ricklefs & Schluter (1993) present an interspecific example contrasting the age of passerine taxa in Panama and Illinois. Temperate sites were assembled by fewer species that were on average younger in age than tropical sites, suggesting a similar historical process as reported here. A corresponding latitudinal gradient exists for the age of tribes of New World birds; age increases toward the equator (Gaston & Blackburn 1996). Additionally, Crame (2000) identified younger bivalve taxa as having steeper latitudinal gradients than their older counterparts suggesting a similar pattern of geographical diversification as reported here.
While patterns described herein suggest that a historical model may aid our understanding of the formation of gradients of diversity, its general applicability to latitudinal gradients needs further evaluation. This is because, while general, the latitudinal gradient in species richness is not ubiquitous (Willig et al. 2003; Hillebrand 2004), not even among families of the order Chiroptera (Stevens 2004), a higher taxon that exhibits a remarkably strong latitudinal gradient (Willig & Selcer 1989; Lyons & Willig 2002; Stevens & Willig 2002; Willig & Bloch 2006). Nonetheless, such exceptions may simply reflect the diversification of particular lower taxa from non-tropical originations yet still resulting from the same type of geographical diversification process. For example, examination of cladograms for the entire family of vesper bats suggests that members of the family Vespertilionidae probably have invaded North America numerous times. Each phylogenetically distinct and fairly species rich group (i.e. Eptesicus, Histiotus, Lasionycteris, Nycticeius (Hoofer & Van Den Bussche 2003); Lasiurus, Corynorhinus (Hoofer & Van Den Bussche 2003); Rhogeesa, Antrozous, Euderma, Idionycteris (Hoofer & Van Den Bussche 2003); Myotis (Ruedi & Mayer 2001)) has its most derived taxa occurring at the most southerly latitudes in either middle or South America. Such a pattern suggests origins in North America followed by southerly spread. Moreover, such diversification probably explains the temperate peak in diversity of this family in North America (Stevens 2004).
Analogous patterns have also been demonstrated along other non-latitudinal gradients for taxa with non-tropical diversity peaks. For example, emydid turtles demonstrate an inverse latitudinal gradient when measured across continental regions (Stephens & Wiens 2003). Their peak in diversity in eastern North America coincides with the greatest average age of taxa, with age of taxa decreasing from this centre with decreasing species richness (termed the time-for-speciation effect by Stephens & Wiens (2003)). Such similar patterns suggest the potential generality of concerted geographical and evolutionary diversification of taxa giving rise to variation in species richness along environmental gradients in general and that along latitudinal gradients representing a particular case. Empirical evidence that diversification in response to differential environmental regimes contributes to diversity gradients is growing. Placing more emphasis on historical mechanisms and how they contribute to contemporary patterns of diversity will be required to fully understand the relative contributions of historical and contemporary processes and how they ultimately determine global patterns of diversity.
Acknowledgments
R. Baker, D. Vazquez, K. Jones, E. Lessa, M. Cardillo, D. Allen, F. Hoffmann, S. Floeter, J. Brown, B. Hawkins and an anonymous reviewer kindly contributed to the development of this manuscript. Portions of this manuscript were written while I was funded by the NSF (grant no. DEB 0535939).
Supplementary Material
Characteristics of each of 30 bat communities used in analyses.
Regressions between measures of species richness together with phylogenetic diversity and longitude. In all analyses longitude was the independent variable and measures of diversity the dependent variable. All regressions failed to account for significant amounts of variation at the alpha=0.05 level.
References
- Badgley C, Fox D.L. Ecological biogeography of North American mammals: species density and ecological structure in relation to environmental gradients. J. Biogeogr. 2000;27:1437–1467. doi:10.1046/j.1365-2699.2000.00498.x [Google Scholar]
- Baker R.J, Hoofer S.R, Porter C.A, Van Den Bussche R.A. Diversification among New World leaf-nosed bats: an evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occas. Pap. Mus. Texas Tech Univ. 2003;230:1–32. [Google Scholar]
- Behrensmeyer A.K, Damuth J.D, DiMichele W.A, Potts R, Sues H.D, Wing S.L. University of Chicago Press; Chicago, IL: 1992. Terrestrial ecosystems through time: evolutionary paleoecology of terrestrial plants and animals. [Google Scholar]
- Bromham L. Molecular clocks and explosive radiations. J. Mol. Evol. 2003;57:S13–S20. doi: 10.1007/s00239-003-0002-7. doi:10.1007/s00239-003-0002-7 [DOI] [PubMed] [Google Scholar]
- Brown J.H. Species diversity. In: Myers A.A, Giller P.S, editors. Analytical biogeography. Chapman and Hall; New York, NY: 1988. pp. 57–97. [Google Scholar]
- Brown J.H, Lomolino M.V. Sinauer and Associates; Sunderland, MA: 1998. Biogeography. [Google Scholar]
- Cardillo M. Latitude and rates of diversification in birds and butterflies. Proc. R. Soc. B. 1999;266:1221–1225. doi:10.1098/rspb.1999.0766 [Google Scholar]
- Cardillo M, Orme C.D.L, Owens I.P.F. Testing for latitudinal bias in diversification rates: an example using new world birds. Ecology. 2005;86:2278–2287. [Google Scholar]
- Connell J.H, Orias E. The ecological regulation of species diversity. Am. Nat. 1964;98:399–414. doi:10.1086/282335 [Google Scholar]
- Crame J.A. Evolution of taxonomic diversity gradients in the marine realm: evidence from the composition of recent bivalve faunas. Paleobiology. 2000;26:188–214. [Google Scholar]
- Currie D.J. Energy and large-scale patterns of animal-species and plant-species richness. Am. Nat. 1991;137:27–49. [Google Scholar]
- Currie D.J, Francis A.P, Kerr J.T. Some general propositions about the study of spatial patterns of species richness. EcoScience. 1999;6:392–399. [Google Scholar]
- Czaplewski N.J. Chiroptera. In: Kay R.F, Madden R.H, Cifelli R.L, Flynn J.J, editors. Vertebrate paleontology in the neotropics: the Miocene fauna of La Venta. Smithsonian Press; Washington, DC: 1997. pp. 410–431. [Google Scholar]
- Diniz-Filho J.A.F, Rangel T.F.L.V.B, Hawkins B.A. A test of multiple hypotheses for the species richness gradient of South American owls. Oecologia. 2004;140:633–638. doi: 10.1007/s00442-004-1577-4. doi:10.1007/s00442-004-1577-4 [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Evolution in the tropics. Am. Sci. 1950;38:208–221. [Google Scholar]
- Dutka A.F, Ewens F.J. A method for improving the accuracy of polynomial regression analysis. J. Qual. Technol. 1971;3:149–155. [Google Scholar]
- Fjeldsa J. Geographical patterns for relict and young species of birds in Africa and South America and implications for biodiversity. Biodiv. and Cons. 1994;3:207–226. [Google Scholar]
- Fischer A.G. Latitudinal variations in organic diversity. Evolution. 1960;14:64–81. doi:10.2307/2405923 [Google Scholar]
- Francis A.P, Currie D.J. A globally consistent richness–climate relationship for angiosperms. Am. Nat. 2003;161:523–536. doi: 10.1086/368223. doi:10.1086/368223 [DOI] [PubMed] [Google Scholar]
- Fraser R.H, Currie D.J. The species richness-energy hypothesis in a system where historical factors are thought to prevail: coral reefs. Am. Nat. 1996;148:138–159. doi:10.1086/285915 [Google Scholar]
- Gaston K.J, Blackburn T.M. The tropics as a museum of biological diversity: an analysis of the new world avifauna. Proc. R. Soc. B. 1996;263:63–68. [Google Scholar]
- Griffith D.A. Springer; Berlin, Germany: 2003. Spatial autocorrelation and spatial filtering: gaining understanding through theory and scientific visualization. [Google Scholar]
- Haffer J. Speciation in Amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Hawkins B.A, Porter E.E. Relative influence of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Global Ecol. Biogeogr. 2003;12:475–481. doi:10.1046/j.1466-822X.2003.00060.x [Google Scholar]
- Hawkins B.A, et al. Energy, water and broad-scale patterns of species richness. Ecology. 2003a;84:3105–3117. [Google Scholar]
- Hawkins B.A, Porter E.E, Felizola Diniz-Filho J.A. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology. 2003b;84:1608–1623. [Google Scholar]
- Hawkins B.A, Diniz J.A.F, Jaramillo C.A, Soeller S.A. Post-Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. J. Biogeog. 2006;33:770–780. [Google Scholar]
- Hennig W. University of Illinois Press; Urbana, IL: 1979. Phylogenetic systematics. [Google Scholar]
- Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004;163:192–211. doi: 10.1086/381004. doi:10.1086/381004 [DOI] [PubMed] [Google Scholar]
- Hoofer S.R, Van Den Bussche R.A. Molecular phylogenetics of the chiropteran family Vespertilionidae. Acta Chirop. 2003;5(suppl):1–63. [Google Scholar]
- Huston M.A. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. doi:10.1007/s004420050180 [DOI] [PubMed] [Google Scholar]
- Janis C. Victors by default: the mammalian succession. In: Gould S.J, editor. The book of life. W. W. Norton & Co; New York, NY: 1993. pp. 169–218. [Google Scholar]
- Jones K.E, Purvis A, MacLarnon A, Bininda-Emmonds O.R.P, Simmons N.B. A phylogenetic supertree of the bats (Mammalia: Chiroptera) Biol. Rev. Camb. Soc. 2002;77:223–259. doi: 10.1017/s1464793101005899. doi:10.1017/S1464793101005899 [DOI] [PubMed] [Google Scholar]
- Jones K.E, Beninda-Emonds O.R.P, Gittleman J.L. Bats, clocks, and rocks: diversification patterns in Chiroptera. Evolution. 2005;59:2243–2255. [PubMed] [Google Scholar]
- Kerr J.T, Currie D.J. The relative importance of evolutionary and environmental controls on broad scale patterns of species richness in North America. Ecoscience. 1999;6:329–337. [Google Scholar]
- Koleff P, Lennon J.J, Gaston K.J. Are there latitudinal gradients in species turnover. Global Ecol. Biogeogr. 2003;12:483–498. doi:10.1046/j.1466-822X.2003.00056.x [Google Scholar]
- Koopman K.F. Order Chiroptera. In: Wilson D.E, Reeder D.M, editors. Mammal species of the world: a taxonomic and geographic reference. 2nd edn. Smithsonian Institution Press; Washington, DC: 1993. pp. 137–241. [Google Scholar]
- Legendre P. Spatial autocorrelation: trouble or new paradigm. Ecology. 1993;74:1659–1673. doi:10.2307/1939924 [Google Scholar]
- Lessa E.P, Cook J.A, Patton J.L. Genetic footprints of demographic expansion in North America, but not Amazonia, during the late Quarternary. Proc. Natl Acad. Sci. USA. 2003;100:10 331–10 334. doi: 10.1073/pnas.1730921100. doi:10.1073/pnas.1730921100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S.K, Willig M.R. Species richness, latitude, and scale-sensitivity. Ecology. 2002;83:47–58. [Google Scholar]
- Martin P.R, McKay J.K. Latitudinal variation in genetic divergence of populations and the potential for future speciation. Evolution. 2004;58:938–945. doi: 10.1111/j.0014-3820.2004.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Math Works. Math Works; Natick, MA: 1995. Matlab reference guide. [Google Scholar]
- McGlone M.S. When history matters: scale, time, climate and tree diversity. Global Ecol. Biogeogr. Letts. 1996;5:309–314. [Google Scholar]
- McNab B.K. The economics of temperature regulation in neotropical bats. Comp. Biochem. Phys. 1969;31:227–268. doi: 10.1016/0010-406x(69)91651-x. doi:10.1016/0010-406X(69)91651-X [DOI] [PubMed] [Google Scholar]
- Milá B, German D.J, Kimura M, Smith T.B. Genetic evidence for the effect of postglacial expansion on the phylogeography of a North American songbird. Proc. R. Soc. B. 2000;267:1033–1044. doi: 10.1098/rspb.2000.1107. doi:10.1098/rspb.2000.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz C, Patton J.L, Schneider C.J, Smith T.B. Diversification of rainforest faunas: an integrated molecular approach. Annu. Rev. Ecol. Syst. 2000;31:533–536. doi:10.1146/annurev.ecolsys.31.1.533 [Google Scholar]
- Pianka E.R. Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 1966;100:33–46. doi:10.1086/282398 [Google Scholar]
- Rangel, T. F. L. V. B., Felizola Diniz-Filho, J. A. & Bini, L. B. In press. Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Global Ecol. Biogeogr.
- Ricklefs R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Letts. 2004;7:1–15. doi:10.1046/j.1461-0248.2003.00554.x [Google Scholar]
- Ricklefs R.E, Schluter D. Species diversity: regional and historical influences. In: Ricklefs R.E, Schluter D, editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press; Chicago, IL: 1993. pp. 350–363. [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- Ruedi M, Mayer F. Molecular systematics of bats of the genus Myotis (Vespertilionidae) suggests deterministic ecomorphological convergences. Mol. Phylogenet. Evol. 2001;21:436–448. doi: 10.1006/mpev.2001.1017. [DOI] [PubMed] [Google Scholar]
- Savage D.E. A Miocene phyllostomatid bat from Colombia, South America. Univ. Calif. Pub. Geol. Sci. 1951;28:357–365. [Google Scholar]
- Schemske D.W. Ecological and evolutionary perspectives on the origins of tropical diversity. In: Chazdon R, Whitmore T.C, editors. Foundations of tropical forest biology. University of Chicago Press; Chicago, IL: 2002. pp. 163–173. [Google Scholar]
- Simmons N.B, Conway T.M. Evolution of ecological diversity. In: Kunz T.H, Fenton M.B, editors. Bat ecology. University of Chicago Press; Chicago, IL: 2003. pp. 493–535. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W. H. Freeman and Co; New York, NY: 1995. Biometry. [Google Scholar]
- Stephens P.R, Wiens J.J. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am. Nat. 2003;161:112–128. doi: 10.1086/345091. doi:10.1086/345091 [DOI] [PubMed] [Google Scholar]
- Stevens R.D. Untangling latitudinal richness gradients at higher taxonomic levels: familial perspectives on the diversity of new world bat communities. J. Biogeogr. 2004;31:665–674. doi:10.1111/j.1365-2699.2003.01042.x [Google Scholar]
- Stevens R.D, Willig M.R. Geographical ecology at the community level: perspectives on the diversity of new world bats. Ecology. 2002;83:545–560. [Google Scholar]
- Weins J.J. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution. 2004;58:193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Weins J.J, Donoghue M.J. Historical biogeography, ecology, and species richness. Trends Ecol. Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. doi:10.1016/j.tree.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Weins J.J, Graham C.H. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005;36:519–539. doi:10.1146/annurev.ecolsys.36.102803.095431 [Google Scholar]
- Wetterer A.L, Rockman M.V, Simmons N.B. Phylogeny of phyllostomid bats (Mammalia: Chiroptera): data from diverse morphological systems, sex chromosomes, and restriction sites. Bull. Am. Mus. Nat. Hist. 2000;248:1–200. doi:10.1206/0003-0090(2000)248<0001:POPBMC>2.0.CO;2 [Google Scholar]
- Whittaker R.H. Evolution of diversity in plant communities. Brookhaven Symp. Biol. 1969;22:178–196. [PubMed] [Google Scholar]
- Willig M.R, Selcer K.W. Bat species density gradients in the new world: a statistical assessment. J. Biogeogr. 1989;16:189–195. [Google Scholar]
- Willig M.R, Bloch P.C. Latitudinal gradients of species richness: a test of the geographic area hypothesis at two ecological scales. Oikos. 2006;112:163–173. doi:10.1111/j.0030-1299.2006.14009.x [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients in biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]
- Wilson D.E. Bat faunas: a trophic comparison. Syst. Zool. 1975;22:14–29. doi:10.2307/2412374 [Google Scholar]
- Wright D.H, Currie D.J, Maurer B.A. Energy supply and patterns of species richness on local and regional scales. In: Ricklefs R.E, Schluter D, editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago; Chicago, IL: 1993. pp. 66–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of each of 30 bat communities used in analyses.
Regressions between measures of species richness together with phylogenetic diversity and longitude. In all analyses longitude was the independent variable and measures of diversity the dependent variable. All regressions failed to account for significant amounts of variation at the alpha=0.05 level.