Abstract
In numerous insects, including bushcrickets (Tettigoniidae), males are known to transfer substances in the ejaculate that inhibit the receptivity of females to further matings, but it has not yet been established whether these substances reduce the lifetime degree of polyandry of the female. The aim of this study was to test the hypothesis that larger ejaculate volumes should be associated with a lower degree of polyandry across tettigoniid taxa, controlling for male body mass and phylogeny. Data on ejaculate mass, sperm number, nuptial gift mass and male mass were taken primarily from the literature. The degree of polyandry for 14 species of European bushcrickets was estimated by counting the number of spermatodoses within the spermathecae of field-caught females towards the end of their adult lifespans. Data for four further species were obtained from the literature. Data were analysed by using both species regression and independent contrasts to control for phylogeny. Multiple regression analysis revealed that, as predicted, there was a significant negative association between the degree of polyandry and ejaculate mass, relative to male body mass, across bushcricket taxa. Nuptial gift size and sperm number, however, did not contribute further to interspecific variation in the degree of polyandry. A positive relationship was found, across bushcricket taxa, between relative nuptial gift size and relative ejaculate mass, indicating that larger nuptial gifts allow the male to overcome female resistance to accepting large ejaculates. This appears to be the first comparative evidence that males can manipulate the lifetime degree of polyandry of their mates through the transfer of large ejaculates.
Keywords: antagonistic coevolution, polyandry, receptivity, spermatophylax, sexual conflict, spermatodose
1. Introduction
Research on sexual selection has focused increasingly on polyandry (females mating with more than one male) in recent years (reviewed by Hosken & Stockley 2003; Zeh & Zeh 2003; Bretman & Tregenza 2005; Garcia-Gonzalez & Simmons 2005; Ivy & Sakaluk 2005; Simmons 2005). Polyandry is known to occur in many insect taxa (reviewed by Ridley 1988; Eberhard 1996; Arnqvist & Nilsson 2000; Simmons 2001), yet there have been relatively few studies on the degree of polyandry in natural insect populations (reviewed by Eberhard 1996; Bretman & Tregenza 2005) or on factors that are associated with differences in the degree of polyandry between species.
It is becoming increasingly clear that there is often sexual conflict over the degree of polyandry (reviewed by Arnqvist & Nilsson 2000; Arnqvist & Rowe 2005; Parker 2006). While polyandrous matings may yield both direct and indirect benefits to females (reviewed by Hosken & Stockley 2003; Zeh & Zeh 2003; Garcia-Gonzalez & Simmons 2005; Simmons 2005), males will be selected to delay or prevent their mates from mating with other males (reviewed by Simmons 2001). In many insect taxa, males are known to transfer substances in the ejaculate that directly influence the receptivity of the female to further matings, and their effects are often dose-dependent (reviewed by Simmons 2001; Gillott 2003; Arnqvist & Rowe 2005; Colonello & Hartfelder 2005). In bushcrickets (Orthoptera: Tettigoniidae), for example, a dose-dependent response by females to refractory-inducing substances in the ejaculate has been confirmed both by comparisons between species (Heller & Helversen 1991; Wedell 1993; Heller & Reinhold 1994) and by laboratory manipulations (Gwynne 1986; Wedell & Arak 1989; Simmons & Gwynne 1991; Bateman 2001; see also Lehmann & Lehmann 2000).
The transfer of large ejaculates in bushcrickets is facilitated through the nuptial gift (spermatophylax). This is a gelatinous secretion of the male's accessory glands that is eaten by the female while the ejaculate is transferred from the ampulla of the spermatophore (reviewed by Vahed 1998; Gwynne 2001). Comparative studies have revealed that relatively larger nuptial gifts are associated with relatively larger spermatophore ampullae and relatively higher sperm counts in bushcrickets (Wedell 1993; Vahed & Gilbert 1996; see also Heller et al. 2004). It is possible that the spermatophylax itself may also contain refractory-inducing substances (Sakaluk et al. 2006).
Whether or not large ejaculates and associated male-induced sexual refractory periods actually lead to a reduction in the degree of polyandry in bushcrickets and other insect taxa has not yet been established. Comparative studies of fungus-gardening ants (Hymenoptera: Formicidae; Attini), however, indicate that relatively large accessory glands (which may produce ejaculate substances that inhibit female sexual receptivity) are associated with a lower degree of polyandry (Baer & Boomsma 2004; Mikheyev 2004). Accessory gland substances or large ejaculates have also been implicated in the induction of monandry in several insect taxa (reviewed by Simmons 2001), including dipteran species such as Musca domestica (Arnqvist & Andres 2006) and in the cockroach Nauphoeta cinerea (Blattaria; Harris & Moore 2005).
In predicting the relationship between ejaculate characteristics and polyandry across species, however, it is difficult to determine the cause and the effect. On the one hand, ejaculate volume (reviewed in Vahed 1998; Simmons 2001; Gillott 2003; Arnqvist & Rowe 2005; Colonello & Hartfelder 2005) or even the number of sperm (see Cook & Wedell 1999; Simmons & Achmann 2000) could influence the degree of polyandry directly in some cases. On the other hand, both the risk of sperm competition (i.e. the probability, between 0 and 1, that the female will engage in promiscuous mating activity that will result in the temporal or spatial overlap of the ejaculates of two or more males; Simmons 2001) and the intensity of sperm competition (i.e. the absolute number of different males engaged in competition for the ova of a single female; Simmons 2001) are predicted to influence male ejaculate allocation strategies (reviewed in Parker 1998; Simmons 2001; Wedell et al. 2002; Parker & Ball 2005). A recent model, for example, predicts that while relative testes mass is expected to increase with sperm competition intensity across species, sperm number (or ejaculate units) should increase as the degree of polyandry increases from 1 (no sperm competition) to 2 and should decrease for all intensities greater than 2 (Parker & Ball 2005). Although few comparative studies have actually measured ejaculate volume or numbers of ejaculated sperm (Parker & Ball 2005), comparative studies of a range of vertebrate taxa have found that testes mass does increase with measures of sperm competition risk and/or intensity (reviewed in Birkhead & Moller 1998; see also Byrne et al. 2002; Pitcher et al. 2005). In insects, however, a simple association between sperm competition intensity and testes size or ejaculate characteristics might not be expected because of the diversity of sperm competition mechanisms (Simmons & Siva-Jothy 1998; but see also Svärd & Wiklund 1989; Gage 1994; Bissoondath & Wiklund 1995; Karlsson 1995; Hosken & Ward 2001 in which a positive relationship between sperm competition intensity and testes mass or ejaculate mass has been found). Models of ejaculate expenditure (Parker 1998; Parker & Ball 2005) have not taken into account the possibility that ejaculate size might directly affect the degree of polyandry. Therefore, the extent to which such models are applicable to many insect taxa is questionable.
The main aim of this study was to test the hypothesis that relatively larger ejaculates should be associated with a reduction in the degree of polyandry, across species in bushcrickets (see Wedell 1993). The possibility that the degree of polyandry might additionally be influenced by relative sperm number (see Cook & Wedell 1999; Simmons & Achmann 2000) and relative spermatophylax mass (Sakaluk et al. 2006; see also Kondoh 2001) was also examined.
2. Material and methods
The degree of polyandry was estimated for 14 species of European bushcrickets, belonging to the sub-family Tettigoniinae (table 1), by counting the number of spermatodoses contained within the spermathecae of field-caught females. Spermatodoses are spermatophore-like capsules that envelop individual ejaculates within the spermatheca (Cholodkovsky 1913; Gwynne 1984; Viscuso et al. 2002; Vahed 2003). Because a separate spermatodose is formed after each mating (Boldyrev 1915; Gwynne 1993) and they appear to persist within the female's spermatheca for her entire adult lifespan (Vahed 2003), spermatodose counts can be used to determine how many times females have mated (Gwynne 1984, 1993). One assumption of this study is that spermatodose counts correspond to the degree of polyandry. Without genotyping the stored sperm, it is not possible to determine whether or not the spermatodose count of a female represents repeated matings with the same male or truly polyandrous matings. Repeated matings with the same male are unlikely in tettigoniine bushcrickets, however, due to factors such as the lack of mate-guarding, the long duration of the male sexual refractory periods and the high level of mobility of individuals between matings (see Kindvall et al. 1998; Diekötter et al. 2005). It should be noted that the degree of polyandry based on spermatodose counts should only be treated as a rough estimate because numerous factors such as population density and climate could influence the degree of polyandry and this could vary between years and between populations (Wiklund & Fosberg 1991). Furthermore, some of the sample sizes for the estimation of the degree of polyandry were small (table 1), decreasing the chance that the estimated mean reflects the true population mean.
Table 1.
Mean estimated lifetime number of matings for females. (Data for the Tettigoniininae were based on counts of spermatodoses within the spermathecae of field-caught females collected towards the end of the season.)
| species and sub-family | estimated lifetime number of matings for females | ||
|---|---|---|---|
| mean±s.e. | n | range | |
| Tettigoniinae | |||
| Tettigonia viridissima | 3.10±0.57 | 10 | 1–7 |
| Decticus verrucivorus | 6.75±1.93 | 8 | 3–12 |
| Poecilimon affinis | 10.45±1.63 | 11 | 5–23 |
| Platycleis albopunctata | 11.44±1.56 | 9 | 5–19 |
| Platycleis tesselata | 7.67±0.33 | 3 | 7–8 |
| Sepiana sepium | 5.33±1.67 | 6 | 2–7 |
| Metrioptera brachyptera | 1.87±0.30 | 14 | 1–3 |
| Metrioptera roeselii | 2.67±0.67 | 6 | 2–4 |
| Yersinella raymondi | 8.33±1.17 | 12 | 5–13 |
| Anonconotus alpinus | 22.33±2.19 | 3 | 18–25 |
| Anonconotus baracunensis | 38.00±3.7 | 6 | 34–44 |
| Antaxius pedestris | 2.00±0.4 | 4 | 1–3 |
| Pholidoptera griseoaptera | 5.48±0.51 | 21 | 1–11 |
| Eupholidoptera chabrieri | 4.00±0.58 | 3 | 3–5 |
| Phaneropterinae | |||
| Poecilimon veluchianus | 5a | — | — |
| Poecilimon affinis | 15a | — | — |
| Bradyporinae | |||
| Ephippiger ephippiger | 1.55±0.16b | 22b | 1–3b |
| Steropleurus stali | 2.10±0.32c | 29c | 1–4c |
Data from Heller & Helversen (1991) calculated for a female of maximum age, from the regression of number of matings against time.
Data from Hockham et al. (2004).
Data from Bateman (1997): combined data for light and heavy females in both years.
Species of tettigoniine bushcricket were collected from the field of a variety of European locations including Devon, UK (Platycleis albopunctata, Pholidoptera griseoaptera, Tettigonia viridissima, Metrioptera brachyptera) in 1998, 1999 and 2004, Central Spain in 2002 (Platycleis affinis and Platycleis tesselata) and the French Alps in 2001 (Decticus verrucivorus, Metrioptera roeselii, Sepiana sepium, Yersinella raymondi, Anonconotus alpinus, Anonconotus baracunensis, Antaxius pedestris and Eupholidoptera chabrieri). Precise collection localities for species collected in the UK and France are the same as those given in Vahed (1994). All specimens were collected in early September, which is towards the end of their adult lifespans. Dr N. Wedell provided additional specimens of T. viridissima and M. brachyptera, collected near Stockholm, Sweden, in September 2004 and late August 2005. Females were preserved either by freezing at −80 °C or by immersion in 80% ethanol. Spermatodose counts were obtained by dissecting out the spermatheca in water and removing its walls using mounted needles, under a Wild M5 stereomicroscope (following Vahed 2003). Spermatodoses were less distinct in A. pedestris than in other species. Sperm counts from the spermatheca (using the method given in Vahed & Gilbert 1996) were therefore used to confirm the estimated number of ejaculates in the spermatheca of this species. The number of different females obtained for each species varied from 3 to 21 (table 1).
Data on the degree of polyandry for a further four species (Poecilimon veluchianus, Poecilimon affinis, Ephippiger ephippiger and Steropleurus stali) were taken from the literature. These species belong to the sub-families Phaneropterinae and Bradyporinae (Ephippigerinae), respectively, in which spermatodoses are not present. Studies of these species have used a range of other methods to determine the degree of polyandry. Heller & Helversen (1991) recorded the degree of polyandry directly by marking individual females of P. veluchianus and P. affinis in the field and recording whether or not females were carrying spermatophores twice per night over the entire season. Hockham et al. (2004) determined the degree of polyandry in E. ephippiger by genotyping the eggs laid by field-collected females. Bateman (1997) recorded the degree of polyandry in S. stali by monitoring marked females in semi-natural mixed-sex caged populations (two cages each containing about 15 males and 15 females were used) over the reproductive season. Because Bateman (1997) used caged populations, there is the danger that the degree of polyandry could have been higher than in the field due to the ready availability of mates. This does not appear to have been the case, however. The mean degree of polyandry recorded by Bateman (1997) (2.1 different matings, see table 1) is very low compared to other bushcricket species and is comparable with that recorded by Hockham et al. (2004) for the closely related E. ephippiger.
Data for most species on ampulla mass, spermatophylax mass, male body mass and sperm number (table 2) were taken from Vahed & Gilbert (1996). New data for all these variables were collected for P. tesselata, M. brachyptera and A. baracunensis, while additional sperm count data were also obtained for A. pedestris and S. stali using methods given in Vahed & Gilbert (1996). Sperm count data for E. ephippiger were taken from Wedell & Ritchie (2004). The data used were for males that had not been mated for three weeks, to be comparable with sperm count data from Vahed & Gilbert (1996), in which only males that had not mated for 2–4 weeks were used. An assumption of this study is that differences in ampulla mass between species reflect differences in ejaculate volume. While this is likely to be the case, it is conceivable that some of the variation in ampulla mass across species is due to differences in the thickness of the ampulla wall, rather than ejaculate volume.
Table 2.
Mean sperm number, ampulla mass, spermatophylax (sp'lax) mass and male mass for the different tettigoniid species. (Data are from Vahed & Gilbert (1996), unless otherwise indicated.)
| species and sub-family | sperm number×104 | n | ampulla mass (mg) | n | sp'lax mass (mg) | n | male mass (mg) | n |
|---|---|---|---|---|---|---|---|---|
| Tettigoniinae | ||||||||
| Tettigonia viridissima | 454.0 | 1 | 78.63 | 1 | 250.0 | 1 | 1450 | 1 |
| Decticus verrucivorus | 169.56 | 3 | 56.09 | 3 | 123.42 | 3 | 1618 | 3 |
| Platycleis affinis | 75.14 | 5 | 13.78 | 5 | 23.05 | 5 | 576 | 5 |
| Platycleis albopunctata | 71.7 | 2 | 12.20 | 3 | 14.37 | 3 | 479 | 3 |
| Platycleis tesselata | 45.3a | 1 | 7.0a | 1 | 8.3a | 1 | 302a | 1 |
| Sepiana sepium | 39.55 | 2 | 15.98 | 2 | 23.87 | 2 | 529 | 2 |
| Metrioptera brachyptera | 140.5a | 1 | 10.2a | 1 | 20.8a | 1 | 279a | 1 |
| Metrioptera roeselii | 40.24 | 3 | 15.73 | 3 | 20.23 | 3 | 345 | 3 |
| Yersinella raymondi | 20.83 | 2 | 2.26 | 3 | 11.00 | 1 | 200 | 2 |
| Anonconotus alpinus | 59.02 | 5 | 4.94 | 6 | 7.71 | 6 | 604 | 6 |
| Anonconotus baracunensis | 40.1a | 1 | 4.0a | 1 | 6.4a | 1 | 500a | 1 |
| Antaxius pedestris | 520.75a | 2 | 25.69 | 1 | 89.83 | 1 | 716 | 1 |
| Pholidoptera griseoaptera | 84.6 | 2 | 16.34 | 2 | 37.09 | 2 | 498 | 2 |
| Eupholidoptera chabrieri | 197.0 | 1 | 56.40 | 1 | 103.6 | 1 | 1233 | 1 |
| Phaneropterinae | ||||||||
| Poecilimon veluchianus | 630.0b | 34 | 37.0 | 1 | 145.0 | 1 | 660c | 107 |
| Poecilimon affinis | 438.0 | 3 | 30.89 | 3 | 170.27 | 4 | 1328 | 4 |
| Bradyporinae | ||||||||
| Ephippiger ephippiger | 300.0d | 8 | 148.97 | 6 | 468.76 | 5 | 2313 | 5 |
| Steropleurus stali | 129.2a | 3 | 90.9 | 1 | 362.5 | 1 | 1296 | 1 |
Novel data.
Data from Wedell & Ritchie (2004), see text for further details.
For each species, the values obtained for male body mass, ampulla mass, spermatophylax mass, sperm number and degree of polyandry were log transformed to meet the assumptions of parametric linear regression. Comparative analysis by independent contrasts was used to control for phylogeny (see Harvey & Pagel 1991; Purvis & Rambaut 1995 for details). The phylogeny used in the analysis was taken from Vahed & Gilbert (1996). This, in turn, was based on Rentz & Coless (1990) for relationships between the different genera within the sub-family Tettigoniinae and Gorochov (1988) for possible phylogenetic relationships between the different sub-families (in this study, the Tettigoniinae, Bradyporinae and Phaneropterinae). Since information on branch lengths was not available, the contrasts were not scaled. To control for allometry, analyses were based on the residuals from the linear regressions of contrasts in log ampulla mass, log spermatophylax mass and log sperm number against contrasts in log male body mass. Stepwise regression analysis (backward deletion method) was performed to determine the effects of residual contrasts in ampulla mass, spermatophylax mass and sperm number on contrasts in log degree of polyandry. In all cases, regressions were forced through the origin as recommended by Harvey & Pagel (1991) and Purvis & Rambaut (1995). Simple species regression was also used to analyse the data, following previous comparative studies (e.g. Byrne et al. 2002; Pitcher et al. 2005). It should be noted, however, that comparisons across species can be confounded by common ancestry (Harvey & Pagel 1991). In order to control for allometry, analyses were based on residuals from the linear regressions of the male traits against log male body mass. Stepwise regression (backward deletion method), weighted for polyandry sample size, was used to determine the effects of log ampulla mass, log spermatophylax mass and log sperm number on log degree of polyandry. All analyses were performed using SPSS.
3. Results
The mean degree of polyandry based on spermatodose counts of field-caught females varied across species from 1.87 to 38 (table 1). The distributions of spermatodose counts within species were not significantly different from normal for all the 14 species (Kolmogorov–Smirnov test, all p values were greater than 0.05), although it should be noted that the relatively small sample sizes for some species would make significant deviations from normality hard to detect.
(a) Comparative analysis by independent contrasts
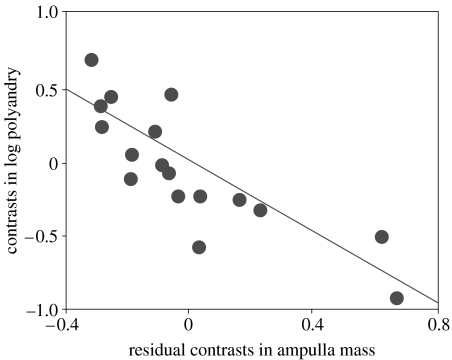
Contrasts in log ampulla mass were positively related to contrasts in log male body mass (slope=1.46±0.23, F1,17=41.99, p<0.001), as were contrasts in log spermatophylax mass (slope=1.38±0.29, F1,17=22.80, p<0.001) and log sperm number (slope=0.98±0.33, F1,17=8.76, p<0.01). Stepwise regression (backward deletion method) of contrasts in log polyandry against residual contrasts in ampulla mass, spermatophylax mass and sperm number revealed that residual contrasts in ampulla mass was the best predictor of log polyandry contrasts. Removal of (i) residual contrasts in sperm number and (ii) residual contrasts in spermatophylax mass caused no significant change in the fit of the model (F change=0.085, p=0.78; F change=1.30, p=0.27, respectively). As predicted, there was a significant negative relationship between contrasts in log polyandry and residual contrasts in ampulla mass (slope=−1.20±0.21; F1,17=32.35, p<0.001; figure 1).
Figure 1.
Controlling for phylogeny, the degree of polyandry decreases as the mass of the ejaculate (relative to male body mass) increases across bushcricket taxa (slope=−1.20±0.21; F1,17=32.35, p<0.001).
Positive relationships were found between residual contrasts in spermatophylax mass and residual contrasts in ampulla mass (slope=0.95±0.22, F1,17=19.04, p<0.001) and between residual contrasts in spermatophylax mass and residual contrasts in sperm number (slope=0.55±0.17, F1,17=10.27, p<0.01). The relationship between residual contrasts in ampulla mass and residual contrasts in sperm number, however, was not statistically significant (slope=0.27±0.16, F1,17=3.14, p=0.095).
(b) Species data
Log ampulla mass was positively related to log male body mass across species (log ampulla mass=−2.81(±0.59)+1.45(±0.21) log body mass; r2=0.75, F1,17=48.92, p<0.001), as were log spermatophylax mass (log spermatophylax mass=−3.22(±0.78)+1.73(±0.28) log body mass; r2=0.71, F1,17=39.42, p<0.001) and log sperm number (log sperm number=3.1(±0.78)+1.05(±0.27) log body mass; r2=0.48, F1,17=14.72, p<0.01). Stepwise regression (backward deletion method), weighted for polyandry sample size, of log polyandry against residual ampulla mass, residual spermatophylax mass and residual sperm number revealed that residual ampulla mass was the best predictor of log polyandry. Removal of (i) residual sperm number and (ii) residual spermatophylax mass caused no significant change in the fit of the model (F change=0.79, p=0.39; F change=0.81, p=0.38, respectively). As predicted, there was a significant negative relationship between log polyandry and residual ampulla mass (log polyandry=0.73(±0.05)−1.47(±0.25) residual ampulla mass; r2=0.68, F1,17=33.83, p<0.001).
Positive relationships were found between residual spermatophylax mass and residual ampulla mass (residual spermatophylax mass=1.05(±0.20) residual ampulla mass; r2=0.63, F1,17=27.36, p<0.001) and between residual spermatophylax mass and residual sperm number (residual spermatophylax mass=0.58(±0.21) residual sperm number; r2=0.33, F1,17=7.99, p<0.05). The relationship between residual ampulla mass and residual sperm number, however, was not statistically significant (residual ampulla mass=0.31(±0.17) residual sperm number; r2=0.17, F1,17=3.16, p=0.094).
4. Discussion
Both the species regression (weighted for polyandry sample size) and the comparative analysis by independent contrasts revealed a significant negative relationship between the degree of polyandry and relative ampulla mass across bushcricket taxa. This appears to be the first comparative study to demonstrate a negative relationship between the degree of polyandry and a measure of ejaculate size (but see also Wedell 1993; Baer & Boomsma 2004; Mikheyev 2004) and strongly suggests that males can manipulate the lifetime degree of polyandry of their mates by transferring large ejaculates (although caution should be taken in inferring cause and effect from correlations).
Previous studies on the relationship between ejaculate mass and the degree of polyandry across species in insects appear to be limited to studies on the Lepidoptera (Svärd & Wiklund 1989; Bissoondath & Wiklund 1995; Karlsson 1995; but see also Baer & Boomsma 2004; Mikheyev 2004). These studies have used counts of spermatophores within the bursa of the female to assess the number of times females have mated. Strangely, the relationship between relative ejaculate mass and the degree of polyandry in bushcrickets found in the present study is exactly opposite to that found in butterflies. Across butterfly species, there is a significant positive relationship between relative ejaculate mass and polyandry (Svärd & Wiklund 1989; Bissoondath & Wiklund 1995; Karlsson 1995). The difference between bushcrickets and butterflies in this context could be interpreted as being consistent with Parker & Ball's (2005) model of the effect of the degree of polyandry on male ejaculate allocation strategies. This model predicts that sperm number (or ejaculate units) should increase across species as the degree of polyandry rises from 1 to 2 (the so-called ‘risk’ range of sperm competition) and should decrease steadily for all intensities greater than 2. The bulk of the species means for the degree of polyandry in comparative studies of butterflies have been within this ‘risk’ range of 1–2 matings (Svärd & Wiklund 1989; Bissoondath & Wiklund 1995; Karlsson 1995), while the majority of species means for the degree of polyandry in bushcrickets in the present study were well above this range and there were no monandrous species. It might therefore be expected that a genuinely monandrous (i.e. where monandry is not male induced) species of tettigoniid should have a relatively smaller ejaculate mass than any of the species in the present study. Parker & Ball's (2005) model, however, was not designed to take into account the possibility that ejaculate size could have a direct effect on the degree of polyandry by switching off female receptivity; therefore, it is not clear if the model accounts for the relationship between ejaculate mass and polyandry observed in the Lepidoptera or the Tettigoniidae. The difference between the Lepidoptera and Tettigoniidae in the response of females to large ejaculates could also reflect differences in the ability of females of these taxa to resist manipulation by males (see Arnqvist & Rowe 2005; Sakaluk et al. 2006).
That relative sperm number and spermatophylax mass had no significant effect on the degree of polyandry (in the stepwise multiple regression analysis) in the present study suggests that it is primarily non-sperm ejaculate substances that induce a lower degree of polyandry in bushcricket females. The exact mechanism by which this occurs, however, is currently unknown. In other insect groups, male-induced sexual refractory periods may be triggered either by the transfer of specialized accessory gland proteins in the ejaculate or by mechanical stimuli such as the filling of either the female's bursa or spermatheca with ejaculate (reviewed by Cordero 1995; Eberhard 1996; Simmons 2001; Gillott 2003; see also Wigby & Chapman 2005). It is possible that spermathecal filling could be involved in decreasing the receptivity of females to further matings in bushcrickets (Vahed 1994). If the differences that are known to occur between species in relative ejaculate volume (Wedell 1993; Vahed & Gilbert 1996; present study) are not matched by differences in the relative capacity of the spermatheca, then it stands to reason that even though the spermathecal walls may be flexible (Vahed 2003), in species with very large ejaculates, the spermatheca may be filled to capacity by sperm and possibly by other substances in the ejaculate (Vahed 1994). There is some evidence to support this hypothesis: in Requena verticalis, a species with a relatively large sperm load (Vahed & Gilbert 1996), Simmons & Achmann (2000) found that sperm from the ampulla did not appear to be transferred into the spermatheca in previously mated females, unless the females had received a small spermatophore (and therefore less ejaculate) at their first mating. Bushcricket ejaculate contains large amounts of granular material, of unknown composition, in addition to sperm (Viscuso et al. 2002; Vahed 2003). It could be that this material functions to fill the spermatheca in order to inhibit the receptivity of the female to further matings, a function that is implicated for non-fertilizing (apyrene) sperm in the Lepidoptera (Cook & Wedell 1999).
Because there are potentially both direct and indirect benefits to be gained by females from polyandrous matings in crickets and bushcrickets (Orthoptera: Ensifera; Vahed 1998; Tregenza & Wedell 2002; Ivy & Sakaluk 2005; Voigt et al. 2005), females might have been selected to counter the effects of such refractory-inducing substances in the ejaculate by, for example, interrupting insemination or digesting excess ejaculate (Parker & Simmons 1989; Arnqvist & Nilsson 2000; Arnqvist & Rowe 2005). It is well established that female crickets and bushcrickets will remove and/or eat the spermatophore ampulla before complete ejaculate transfer, in the absence of male hindrance (reviewed in Vahed 1998; see also Laird et al. 2004). There is also evidence that in some bushcricket species, females are able to break down sperm within the spermathecal duct (Viscuso et al. 1996). Females might also have been selected to evolve a larger sperm storage capacity. The capacity of the spermatheca was not measured in the present study, but in scathophagid flies, recent comparative evidence suggests that there has been coevolution between male ejaculate size and the size of the females' sperm storage organs (Minder et al. 2005).
Such female resistance could lead to the further exaggeration of male ejaculate mass and nuptial gift mass, in a coevolutionary arms race between the sexes (Parker & Simmons 1989; Arnqvist & Nilsson 2000; Arnqvist & Rowe 2005). In functioning to deter the female from interfering with insemination, nuptial gifts may be seen as a way in which males overcome female resistance to accepting large ejaculates (reviewed by Vahed 1998; Vahed in press). The positive relationship between relative spermatophylax mass and relative ampulla mass, across species, found in the present study supports this view (see also Wedell 1993; Vahed & Gilbert 1996). Spermatodoses may also function to protect the ejaculate from being destroyed by the female while it is stored in the spermatheca (Vahed 2003). The negative relationship between ejaculate mass and the degree of polyandry found in the present study could suggest that males have the ‘upper hand’ in such a coevolutionary arms race, although this is not necessarily the case. It is not yet clear whether or not the relatively low degree of polyandry of species with large ejaculates in this study is sub-optimal for the female. It could equally be argued that the high levels of polyandry in taxa with small ejaculates, such as Anonconotus (in which males coerce the females to copulate), are above than that which is optimal to the female (Vahed 2002). Further research is required to determine the optimal mating rates for females of different tettigoniid species, and for females of other insect taxa, in order to determine whether or not females really are being manipulated against their reproductive interests (Arnqvist & Nilsson 2000; Arnqvist & Rowe 2005; Arnqvist et al. 2005).
Acknowledgements
I thank Dr N. Wedell for supplying specimens, Dr D. T. Gwynne, Dr K. -G. Heller and three anonymous referees for comments on the manuscript, Prof. G. A. Parker for discussion and Prof. A. Purvis for advice on the comparative analysis. Financial support for the Spanish fieldwork was provided by the European Commission (Human Potential Programme), under BIODIBERIA at the Museo Nacional de Ciencias Naturales. I am grateful to Dr P. Lopez and Dr J. Martin for their support in the field and their hospitality, to Kate Bellis for providing invaluable field assistance in Spain and France and to Fran and Brendan Bellis for field assistance in Devon.
References
- Arnqvist, G. & Andres, J. A. 2006 The effects of experimentally induced polyandry on female reproduction in a monandrous mating system. Ethology (doi:10.1111/j.1439-0310.2006.01211.x)
- Arnqvist G, Nilsson T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. doi:10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Arnqvist G, Nilsson T, Katvala M. Mating rate and fitness in female bean weevils. Behav. Ecol. 2005;16:123–127. doi:10.1093/beheco/arh119 [Google Scholar]
- Baer B, Boomsma J.J. Male reproductive investment and queen mating-frequency in fungus-growing ants. Behav. Ecol. 2004;15:426–432. doi:10.1093/beheco/arh025 [Google Scholar]
- Bateman P.W. Operational sex ratio, female competition and mate choice in the ephippigerine bushcricket Steropleurus stali Bolivar. J. Orthopt. Res. 1997;6:101–104. [Google Scholar]
- Bateman P.W. Changes in phonotactic behaviour of a bushcricket with mating history. J. Insect Behav. 2001;14:333–343. doi:10.1023/A:1011167128430 [Google Scholar]
- Birkhead T.R, Moller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. [Google Scholar]
- Bissoondath C.J, Wiklund C. Protein content of spermatophores in relation to monandry/polyandry in butterflies. Behav. Ecol. Sociobiol. 1995;37:365–371. doi:10.1007/s002650050203 [Google Scholar]
- Boldyrev B.T. Contributions a` l'e´tude de la structure des spermatophores et des particularite´s de la copulation chez locustidae et Gryllodea. Horae Soc. Entomol. Rossicae. 1915;41:1–245. [Google Scholar]
- Bretman A, Tregenza T. Measuring polyandry in wild populations: a case study using promiscuous crickets. Mol. Ecol. 2005;14:2169–2179. doi: 10.1111/j.1365-294X.2005.02556.x. doi:10.1111/j.1365-294X.2005.02556.x [DOI] [PubMed] [Google Scholar]
- Byrne P.G, Roberts J.D, Simmons L.W. Sperm competition selects for increased tetes mass in Australian frogs. J. Evol. Biol. 2002;15:347–355. doi:10.1046/j.1420-9101.2002.00409.x [Google Scholar]
- Cholodkovsky N. Uber die spermatodosen der Locustiden. Zool. Anz. 1913;41:615–619. [Google Scholar]
- Colonello N.A, Hartfelder K. She's my girl—male accessory gland products and their function in the reproductive biology of social bees. Apidologie. 2005;36:231–244. doi:10.1051/apido:2005012 [Google Scholar]
- Cook P.A, Wedell N. Non-fertile sperm delay female remating. Nature. 1999;397:486. doi:10.1038/17257 [Google Scholar]
- Cordero C. Ejaculate substances that affect female insect reproductive physiology and behavior: honest or arbitrary traits? J. Theor. Biol. 1995;174:453–461. doi:10.1006/jtbi.1995.0111 [Google Scholar]
- Diekötter T, Csencsics D, Rothenbűhler C, Billeter R, Edwards P.J. Movement and dispersal patterns in the bush cricket Pholidoptera griseoaptera: the role of developmental stage and sex. Ecol. Entomol. 2005;30:419–427. doi:10.1111/j.0307-6946.2005.00714.x [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Gage M.J.G. Associations between body size, mating pattern, testes size and sperm lengths across butterflies. Proc. R. Soc. B. 1994;258:247–254. [Google Scholar]
- Garcia-Gonzalez F, Simmons L.W. The evolution of polyandry: intrinsic sire effects contribute to embryo viability. J. Evol. Biol. 2005;18:1097–1103. doi: 10.1111/j.1420-9101.2005.00889.x. doi:10.1111/j.1420-9101.2005.00889.x [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. doi:10.1146/annurev.ento.48.091801.112657 [DOI] [PubMed] [Google Scholar]
- Gorochov A.V. The classification and phylogeny of grasshoppers (Orthoptera, Tettigonioidea) In: Pomerenko A, editor. The Cretaceous biocoenotic crisis and the evolution of insects. Hayka; Moscow, Russia: 1988. pp. 145–190. [Google Scholar]
- Gwynne D.T. Sexual selection and sexual differences in Mormon crickets (Orthoptera: Tettigoniidae, Anabrus simplex) Evolution. 1984;38:1011–1022. doi: 10.1111/j.1558-5646.1984.tb00371.x. doi:10.2307/2408435 [DOI] [PubMed] [Google Scholar]
- Gwynne D.T. Courtship feeding in katydids: investment in offspring or in obtaining fertilisations? Am. Nat. 1986;128:342–352. doi:10.1086/284566 [Google Scholar]
- Gwynne D.T. Food quality controls sexual selection in Mormon crickets by altering male mating investment. Ecology. 1993;74:1406–1413. doi:10.2307/1940070 [Google Scholar]
- Gwynne D.T. Cornell University Press; Ithaca, NY: 2001. Katydids and bush-crickets: reproductive behaviour and evolution of the Tettigoniidae. [Google Scholar]
- Harris W.E, Moore P.J. Sperm competition and male ejaculate investment: effects of social environment during development. J. Evol. Biol. 2005;18:474–480. doi: 10.1111/j.1420-9101.2004.00816.x. doi:10.1111/j.1420-9101.2004.00816.x [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Heller K.G, Helversen D. von. Operational sex ratio and individual mating frequencies in two bushcricket species (Orthoptera, Tettigonioidea, Poecilimon) Ethology. 1991;89:211–228. [Google Scholar]
- Heller K.G, Reinhold K. Mating effort function of the spermatophore in the bushcricket Poecilimon veluchianus (Orthoptera: Phaneropteridae): support from a comparison of the mating behaviour of two subspecies. Biol. J. Linn. Soc. 1994;53:153–163. doi:10.1006/bijl.1994.1065 [Google Scholar]
- Heller K.G, Willemse F, Sevgili H. Poecilimon mytilenensis Werner, a polytypic phaneropterid bushcricket from the Aegean Island of Lesbos (Orthoptera, Tettigonioidea), differing in male mating structures. J. Orthopt. Res. 2004;13:221–230. [Google Scholar]
- Hockham L.R, Graves J.A, Ritchie M.G. Sperm competition and the level of polyandry in a bushcricket with large nuptial gifts. Behav. Ecol. Sociobiol. 2004;57:149–154. doi:10.1007/s00265-004-0838-x [Google Scholar]
- Hosken D.J, Stockley P. Benefits of polyandry: a life history perspective. Evol. Biol. 2003;33:173–194. [Google Scholar]
- Hosken D.J, Ward P.I. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 2001;4:10–13. doi:10.1046/j.1461-0248.2001.00198.x [Google Scholar]
- Ivy T.M, Sakaluk S.K. Polyandry promotes enhanced offspring survival in decorated crickets. Evolution. 2005;59:152–159. [PubMed] [Google Scholar]
- Karlsson B. Resource allocation and mating strategies in butterflies. Evolution. 1995;49:955–961. doi: 10.1111/j.1558-5646.1995.tb02330.x. doi:10.2307/2410417 [DOI] [PubMed] [Google Scholar]
- Kindvall O, Vessby K, Berggren A. Individual mobility prevents an Allee effect in sparse populations of the bush cricket Metrioptera roeseli: an experimental study. Oikos. 1998;81:449–457. [Google Scholar]
- Kondoh M. Co-evolution of nuptial gift and female multiple mating resulting in diverse breeding systems. Evol. Ecol. Res. 2001;3:75–89. [Google Scholar]
- Laird G, Gwynne D.T, Andrade M.C.B. Extreme repeated mating as a counter-adaptation to sexual conflict? Proc. R. Soc. B. 2004;271(Suppl. 6):S402–S404. doi: 10.1098/rsbl.2004.0198. doi:10.1098/rsbl.2004.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann G.U.C, Lehmann A.W. Female bushcrickets mated with parasitized males show rapid re-mating and reduced fecundity (Orthoptera: Phaneropteridae: Poecilimon mariannae) Naturwissenschaften. 2000;87:404–407. doi: 10.1007/s001140050750. doi:10.1007/s001140050750 [DOI] [PubMed] [Google Scholar]
- Mikheyev A.S. Male accessory gland size and the evolutionary transition from single to multiple mating in the fungus-gardening ants. J. Insect Sci. 2004;4:37. doi: 10.1093/jis/4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minder A.M, Hosken D.J, Ward P.I. Co-evolution of male and female reproductive characters across the Scatophagidae (Diptera) J. Evol. Biol. 2005;18:60–69. doi: 10.1111/j.1420-9101.2004.00799.x. doi:10.1111/j.1420-9101.2004.00799.x [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Moller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 3–54. [Google Scholar]
- Parker G.A. Sexual conflict over mating and fertilisation: an overview. Phil. Trans. R. Soc. B. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. doi:10.1098/rstb.2005.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Ball M.A. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol. Lett. 2005;1:235–238. doi: 10.1098/rsbl.2004.0273. doi:10.1098/rsbl.2004.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Simmons L.W. Nuptial feeding in insects: theoretical models of male and female interests. Ethology. 1989;82:3–26. [Google Scholar]
- Pitcher T.E, Dunn P.O, Whittingham L.A. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005;18:557–567. doi: 10.1111/j.1420-9101.2004.00874.x. doi:10.1111/j.1420-9101.2004.00874.x [DOI] [PubMed] [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. CABIOS. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Reinhold K, Helversen D. von. Sperm number, spermatophore weight and remating in the bushcricket Poecilimon veluchianus. Ethology. 1997;103:12–18. [Google Scholar]
- Rentz D.C.F, Coless D.H. A classification of the shield-back katydids (Tettigoniinae) of the world. In: Bailey W.J, Rentz D.C.F, editors. The Tettigoniidae: biology, systematics and evolution. Crawford House Press; Bathurst, Australia: 1990. pp. 352–377. [Google Scholar]
- Ridley M. Mating frequency and fecundity in insects. Biol. Rev. 1988;63:509–549. [Google Scholar]
- Sakaluk S.K, Avery R.L, Weddle C.B. Cryptic sexual conflict in gift-giving insects: chasing the chase-away. Am. Nat. 2006;167:94–104. doi: 10.1086/498279. doi:10.1086/498279 [DOI] [PubMed] [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;36:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Simmons L.W, Achmann R. Microsatellite analysis of sperm-use patterns in the bushcricket Requena verticalis. Evolution. 2000;54:942–952. doi: 10.1111/j.0014-3820.2000.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Simmons L.W, Gwynne D.T. The refractory period of female katydids (Orthoptera: Tettigoniidae): sexual conflict over the re-mating interval? Behav. Ecol. 1991;2:276–282. [Google Scholar]
- Simmons L.W, Siva-Jothy M.T. Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead T.R, Moller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 341–432. [Google Scholar]
- Svärd L, Wiklund C. Mass and production rate of ejaculates in relation to monandry–polyandry in butterflies. Behav. Ecol. Sociobiol. 1989;24:395–402. doi:10.1007/BF00293267 [Google Scholar]
- Tregenza T, Wedell N. Polyandrous females avoid costs of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. doi:10.1038/415071a [DOI] [PubMed] [Google Scholar]
- Vahed, K. 1994 The evolution and function of the spermatophylax in bushcrickets (Orthoptera: Tettigoniidae). Ph.D. thesis, University of Nottingham, UK.
- Vahed K. The function of nuptial feeding in insects: a review of empirical studies. Biol. Rev. 1998;73:43–78. doi:10.1017/S0006323197005112 [Google Scholar]
- Vahed K. Coercive copulation in the alpine bushcricket Anonconotus alpinus Yersin (Tettigoniidae: Tettigoniinae: Platycleidini) Ethology. 2002;108:1065–1075. doi:10.1046/j.1439-0310.2002.00838.x [Google Scholar]
- Vahed K. Structure of spermatodoses in shield-back bushcrickets (Tettigoniidae, Tettigoniinae) J. Morphol. 2003;257:45–52. doi: 10.1002/jmor.10110. doi:10.1002/jmor.10110 [DOI] [PubMed] [Google Scholar]
- Vahed, K. In press. All that glistens is not gold: sensory bias, sexual conflict and nuptial feeding in insects and spiders. Ethology
- Vahed K, Gilbert F.S. Differences across taxa in nuptial gift size correlate with differences in sperm number and ejaculate volume in bushcrickets (Orthoptera: Tettigoniidae) Proc. R. Soc. B. 1996;263:1257–1265. [Google Scholar]
- Viscuso R, Barone N, Sottile L, Narcisi L. Spermiolytic activity of the epithelium of the spermathecal duct of Rhacocleis annulata Fieber (Orthoptera: Tettigoniidae) Int. J. Insect Morphol. Embryol. 1996;25:135–144. doi:10.1016/0020-7322(95)00025-9 [Google Scholar]
- Viscuso R, Brundo M.V, Sottile L. Mode of transfer of spermatozoa in Orthoptera Tettigoniidae. Tissue Cell. 2002;34:337–348. doi: 10.1016/s0040816602000344. doi:10.1016/S0040816602000344 [DOI] [PubMed] [Google Scholar]
- Voigt C.C, Michener R, Kunz T.H. The energetics of trading nuptial gifts for copulations in katydids. Physiol. Biochem. Zool. 2005;78:417–423. doi: 10.1086/430224. doi:10.1086/430224 [DOI] [PubMed] [Google Scholar]
- Wedell N. Spermatophore size in bushcrickets: comparative evidence for nuptial gifts as sperm protection devices. Evolution. 1993;47:1203–1212. doi: 10.1111/j.1558-5646.1993.tb02147.x. doi:10.2307/2409986 [DOI] [PubMed] [Google Scholar]
- Wedell N, Arak A. The wartbiter spermatophore and its effect on female reproductive output (Orthoptera: Tettigoniidae, Decticus verrucivorus) Behav. Ecol. Sociobiol. 1989;24:117–125. doi:10.1007/BF00299643 [Google Scholar]
- Wedell N, Ritchie M. Male age, mating status and nuptial gift quality in a bushcricket. Anim. Behav. 2004;67:1059–1065. doi:10.1016/j.anbehav.2003.10.007 [Google Scholar]
- Wedell N, Gage J.G, Parker G. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. doi:10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Wiklund C, Fosberg J. Sexual size dimorphism in relation to female polygamy and protandry in butterflies: a comparative study of Swedish Pieridae and Satyridae. Oikos. 1991;60:373–381. [Google Scholar]
- Zeh J.A, Zeh D.W. Toward a new sexual selection paradigm: polyandry, conflict and incompatibility. Ethology. 2003;109:929–950. doi:10.1046/j.1439-0310.2003.00945.x [Google Scholar]