Abstract
Many animal species segregate by sex. Such segregation may be social in nature, or ecological, or both. Grey seals (Halichoerus grypus), like many large mammals, are sexually size dimorphic. In size dimorphic species, allometric differences in morphology, metabolic rate and reproductive costs are likely. Such differences may require the sexes to use different foraging strategies or different habitats. To investigate sexual segregation of habitat in grey seals, we used satellite tracks from 95 (male 46; female 49) adults breeding at Sable Island, Nova Scotia (44 °N, 60 °W) collected from 1995 to 2005. Location estimates were made from satellite fixes using a state-space movement model to estimate true locations and regularize them in time. Location estimates were used to calculate home range kernels of male and female habitat use each month. Month by sex kernel home ranges revealed striking differences and dynamics in habitat use between males and females on spatial scales broader than most terrestrial examples and at temporal and spatial resolutions rarely available for marine species. Differences were most pronounced just before (October–December) and immediately after breeding (February–March). During both periods, males primarily used areas along the continental shelf break, while females mainly used mid-shelf regions. Coupled with previously identified sex-specific seasonal patterns of energy storage, diving and diet, our findings suggest that males and females differ profoundly in their spatial foraging strategies. These differences may serve to maximize fitness by reducing intersexual competition during key foraging periods.
Keywords: sexual segregation, foraging behaviour, kernel analysis, seal, Halichoerus grypus, home range
1. Introduction
In many sexual organisms, differences in morphology and reproductive investment between the sexes are likely to cause divergence in the timing and magnitude of energetic needs for each sex (Perrigo & Bronson 1985; Sibly & Calow 1986; Beck et al. 2003a). Such differences may cause males and females to use different strategies to acquire and defend resources. If differences are sufficiently large, they may be reflected in differing food requirements, foraging strategies and habitat choices for each sex.
Evidence for differential use of habitat is not uncommon, and sexual segregation has been demonstrated in a wide range of species, including birds, cephalopods, fishes and mammals (Clutton-Brock et al. 1982; Smallwood 1988; Voight 1995; Ruckstuhl & Neuhaus 2000, 2002; Bonenfant et al. 2004; Croft et al. 2004, 2006; Catry et al. 2006). Few of these examples, however, demonstrate the temporal and spatial dynamics of segregation. Most of our understanding of such dynamics comes from ungulate research, where Clutton-Brock et al. (1982) suggested that during most of the year male and female red deer (Cervus elaphus) are so separate that they may almost be regarded as separate species. Many species of ungulates similarly segregate into sex-specific social groups when not breeding. A number of hypotheses have been advanced as explanations, including intersexual competition, different time or energy budgets, different predation pressures and different costs of reproduction and rearing young (Ruckstuhl & Neuhaus 2000, 2002; Conradt et al. 2001). In ungulates, however, segregation of sexes occurs by separation into sex-specific social groups, and it is often unclear if social preference itself is the mechanism of segregation, or if underlying ecological differences are the ultimate cause.
Like the well-studied ungulates, grey seals (Halichoerus grypus) are sexually size dimorphic. However, compared to ungulates, they have a more energetically demanding capital breeding strategy (Iverson et al. 1993). During the month-long breeding season, females rely exclusively on blubber stores for their intense 17 day lactation period, and while males do forage some, most of their metabolic needs come from accumulated energy stores (Beck et al. 2003a; Lidgard et al. 2005). In general, grey seals carrying larger energy stores into the breeding season are more likely to sire (males) and wean (females) viable offspring (Mellish et al. 1999; Hall et al. 2001; Lidgard et al. 2001).
Although the general strategy of bulk energy accumulation is the same for both sexes, males and females accumulate and expend energy differently throughout the year (Beck et al. 2003a). These differences include slower post-breeding energy accumulation, deeper dive depths, larger foraging ranges and wider dietary niche breadth in males. Females spend more time diving in smaller foraging ranges, have a narrower niche breadth and faster energy expenditure during the breeding season (Mellish et al. 1999; Beck et al. 2003a–c, 2005; Austin et al. 2004). A larger size and lower basal metabolic rate (BMR) per unit mass may allow males to use lower quality prey than females, allowing them a larger niche breadth. As a consequence of larger size, males must also accumulate greater energy stores in absolute terms than females between breeding seasons. In addition, males may not need to recover as quickly after the breeding season, because, unlike females, they are not supporting early pregnancy (Beck et al. 2003a–c).
These observed differences in behaviour and physiology suggest that the sexes use different overall foraging strategies. If this is the case, we should observe differences in male and female diving, searching and other foraging related behaviours—but not necessarily find them using different habitats. Northwest Atlantic grey seals are conventionally understood to have some difference in habitat use between the sexes, but for the most part the sexes are believed to overlap broadly (Beck 2002; Beck et al. 2003a). However, previous analyses of this population compared ranging only on an annual scale, which, as we will show, obscured strong segregation signals apparent on shorter time-scales.
Given the size dimorphism between the sexes, the demonstrated difference in foraging behaviour, diet and reproductive effort, we tested the hypothesis that males and females use different foraging areas. We further investigated seasonal segregation of foraging areas that appear shared on annual scales. Because many marine mammals and birds express size dimorphism similar to grey seals, the kind of sexual segregation we demonstrate here may be common. If so, species in need of conservation could require different management measures for each sex, while the sexes of abundant species such as grey seals may be having differential ecosystem impacts where top-down ecosystem control is strong.
2. Material and methods
Grey seals were captured on Sable Island, Nova Scotia, a partially vegetated sand island approximately 300 km east of Halifax, NS (43.55 °N, 60.00 °W), and currently the largest grey seal breeding colony in the world (Bowen et al. 2003). Seals were captured during either January (breeding season), May–June (moult), or September–October from 1995 to 2005. After manual capture with hand-held nets, seals were weighed and anaesthetized with Telazol (Bowen et al. 1999). Once anaesthetized, Argos satellite transmitters (SDR—Wildlife Computers, Redman, WA, USA; ST-18, Telonics, Mesa AZ, USA or SRDL 7000, Sea Mammal Research Unit, St Andrews, UK) were glued to the pelage on the head or upper neck using 5 min epoxy. Some instruments were removed when the animals returned to breed, while others were left on until they failed or were moulted off. Tags were deployed with duty cycles ranging from 1 day on and 2 days off to no duty cycle. In total, we obtained satellite-location tracks from 95 adult grey seals (46 males and 49 females).
(a) State-space location estimates
Different satellite uplink rates, duty cycles, battery life and location error rates caused large differences in both track duration and in the number of locations collected from each animal per unit time. We employed a state-space model to handle erroneous satellite locations and normalize the number of locations per day. State-space models handle erroneous points by estimating the true location using the error structure of the entire set of satellite locations. Using the method described in Jonsen et al. (2005), we used a first difference correlated random walk model with a 480 min time-step (3 pts d−1). The resulting estimated locations include many fewer points on land or in rarely visited deep waters and are evenly spaced in time, so that data-rich tracks do not bias kernel densities (figure 1). Further details of the state-space method employed are given in Jonsen et al. (2003, 2005).
Figure 1.
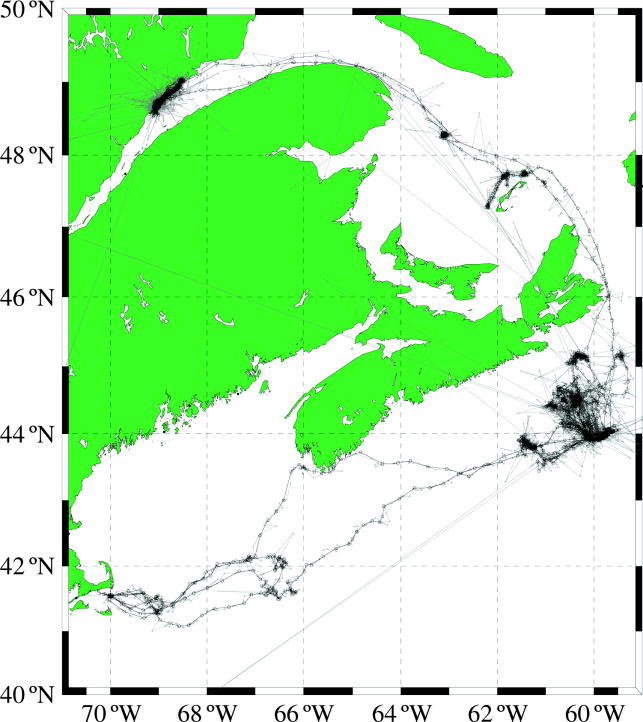
The study area along with two observed and state-space modelled grey seal tracks. Grey lines and points are the observed locations and tracks. Black lines and black circles are state-space estimated locations at 480 min intervals.
(b) Kernel analysis
State-space estimated locations were used to produce kernel home ranges using the adaptive kernel method (Warton 1989). This technique places a normal distribution over each observed location, sums all the distributions together and normalizes them into a single ‘kernel’ distribution. We used a 100 by 100 grid to cover the 1.15×106 km2 area shown in the map panels of figure 2 (115 km2 average cell size). This 10 000-node grid did not account for the curvature of the earth, but since most of the comparable kernel was centred, skewing was minor (the northernmost grid cells were 11% smaller in area than the southernmost grid cells). In order to ensure comparable results between sexes and in randomization tests, we used the same fixed smoothing parameter for all kernel calculations.
Figure 2.
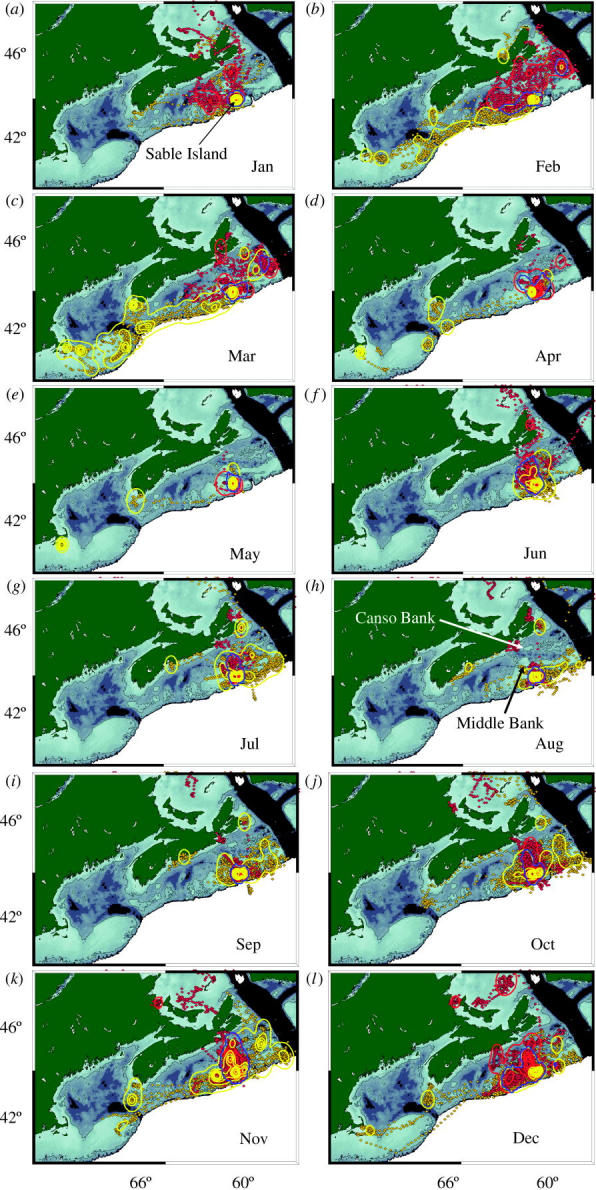
Monthly distributions of adult male and female grey seals. Yellow diamonds represent male and red diamonds female state-space estimated locations. The outer red and yellow contour lines are the 98.5% contour for female and male kernel home ranges, respectively. Blue contour is the overlap of the 98.5% contour of male and female kernels. Density of the estimated locations and the order they are rendered has caused the female (red) locations to obscure some of the male (yellow) locations, but the kernel density contour lines are visible and reflect habitat usage. The 100 m contour line has been added for reference; depths deeper than 500 m (black) are not contoured.
Kernels were computed from points grouped by sex and month. From the 95 tracks, 24 home ranges were produced (12 months×2 sexes). The smallest number of tracks used in a particular month by sex group was 15, the largest was 37 (April and May excluded; table 1). Male and female kernels were plotted for each month, so that differences in habitat usage could be visibly compared. Owing to our choice of gird size and smoothing parameter, our kernels were somewhat peaked, with the 95% contour very near core areas. These parameters worked well in coinciding with natural habitat boundaries, such as bathymetric edges, and resulted in minimal density over land. However, because these settings produced peaked kernels, the 98.5% density contour was a better definition of spatial use boundaries, as these coincide reasonably with natural boundaries, such as coastline and bathymetric edges. Consequently, we chose the overlap of the 98.5% density contour as the operational definition of cohabitated area.
Table 1.
Sample sizes for each month by sex kernel. (N locations indicate the number of state-space estimated locations, not the number of satellite observations. The number of years from which data originated for the construction of each kernel for males (M) and females (F) are the final two columns.)
| month | N male tracks | N female tracks | N male locations | N female locations | no. of years | |
|---|---|---|---|---|---|---|
| M | F | |||||
| Jan | 27 | 27 | 1415 | 1437 | 7 | 6 |
| Feb | 17 | 19 | 1334 | 1599 | 4 | 3 |
| Mar | 17 | 19 | 1241 | 1395 | 4 | 3 |
| Apr | 8 | 8 | 549 | 500 | 2 | 2 |
| May | 4 | 8 | 174 | 106 | 2 | 3 |
| Jun | 15 | 17 | 1164 | 1404 | 4 | 4 |
| Jul | 15 | 17 | 1395 | 1511 | 4 | 4 |
| Aug | 16 | 17 | 1395 | 1554 | 4 | 4 |
| Sep | 27 | 27 | 1892 | 1955 | 7 | 6 |
| Oct | 35 | 38 | 2928 | 3173 | 8 | 8 |
| Nov | 32 | 36 | 2753 | 3227 | 8 | 8 |
| Dec | 27 | 31 | 2320 | 2689 | 7 | 7 |
We employed a randomization analysis to test the null hypothesis that there was no difference in spatial distribution of males and females each month. If the null hypothesis was true, the size of the overlap between male and female kernels should not be significantly different from the size of the overlap if sex were randomly assigned. For each month, the sex of each track was randomly assigned using the same sex ratio as the observations. A kernel density was produced (just as in figure 2) for the two groups of tracks whose sexes had been randomly assigned. We used the area of the overlap region divided by area of the larger home range (operationally defined as 98.5% contour) as the test statistic.
The test was iterated 200 times. Permutations of random sex assignment occurring in earlier iterations were not allowed (i.e. no resampling). A typical randomization test would use several thousand samples, but computing the kernels is computationally intensive and we were able to iterate the test only 200 times. p-value was determined by the proportion of random overlaps that were smaller than the observed overlap, so that if the observed overlap was smaller than all 200 randomly generated overlaps, then p≤0.005. Since we were testing only if the observed overlap was smaller than random overlap, we consider this a one-tailed test.
3. Results
Mean monthly kernels of adult males and females, along with the state-space modelled points, are plotted in figure 2. As expected, during the peak of breeding in January, males are concentrated at Sable Island (figure 2a). Females spend only part of January on Sable, just long enough to give birth and wean their pup. They appear to forage north and west of Sable Island after departing the breeding colony (figure 2a).
February and March show the most striking spatial segregation of habitat of any time of year (figure 2b,c). During this period, males move southwest along the shelf break and forage along the shelf between Sable Island and Cape Cod. Females move north of Sable Island to forage mainly on mid-shelf banks. One male foraged north of Sable during this period, and was responsible for a small amount of overlap, in what was otherwise an area used exclusively by females (figure 2b,c).
Few data are available from April and May (figure 2d,e). During this period of annual moult, tags are shed as pelage is replaced, limiting data and the inferences that can be drawn. The few data available indicate that both sexes used areas near Sable or were hauled out during these months (figure 2d,e).
Summer (June–September; figure 2f–i) was characterized by reduced spatial distribution in both sexes, and areas overlap more broadly than at any other time of year. Following the moult in May, females tended either to travel to feeding areas far from Sable Island (mostly in the Gulf of St Lawrence, northwest of areas depicted in figure 2 panels), or to use Sable Island as a central place from which to make short foraging trips (figure 2f–h). During July–September, females remained close to haulout sites and their home range was so small that it is difficult to pick out under the male ranges in figure 2g–i. During the same period, however, males foraged near the continental shelf edge or on the mid-shelf banks north of Sable typically used by females at other times of the year (i.e. February–March and October–December).
During the three months prior to breeding (October–December), females began to forage intensely on mid-shelf banks near Sable, especially Middle and Canso banks just north of Sable Island (figure 2 h,j,k). By contrast, male foraging activity was more widely distributed across the Scotian Shelf. During October and November, males used essentially the same areas females used during February and March. Males returned to Sable during December to establish dominance before females return. Females generally forage during December, continuing to focus on Middle and Canso banks, but expanding their range to include areas immediately southwest of Sable used by males in November. Most females returned to Sable later than males, near the end of December or in January.
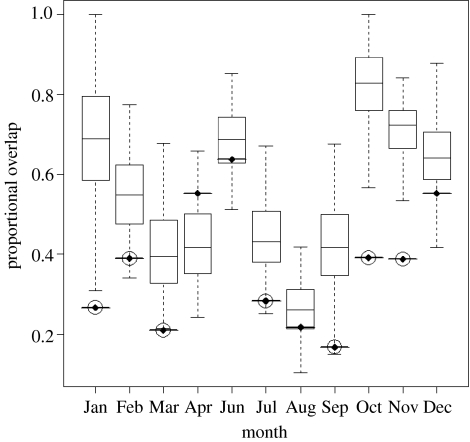
The randomization analysis confirmed the differences that are visually apparent in the kernel density distribution maps of figure 2. In 7 of the 10 months with enough data to test, the degree of habitat segregation between males and females was significantly greater than expected by chance (figure 3). Those differences were most pronounced during the intense periods of foraging immediately before (October–December) and after (February–March) breeding (figure 3).
Figure 3.
Box plots of randomization results for each month (except May due to limited data). Box plots and error bars are normalized overlap for 200 permutations where the sex of each track was randomly assigned. The observed overlap is indicated by the bold bar with diamond centre. Observed overlaps that are significantly different (p≤0.01) from the population of random overlaps are circled.
4. Discussion
In this paper we demonstrate a large scale, seasonally dynamic spatial segregation of male and female grey seal habitat use on the Scotian Shelf in eastern Canada. Differential use of space is likely due to a combination of niche differentiation, differing energetic requirements and intersexual competition. The scale of segregation is much larger than described in almost all of the well-studied terrestrial examples; large enough that males and females may have a differential impact on the ecosystems and foraging areas they inhabit. This has important implications on management and conservation strategies for grey seals and other marine mammals and birds with similar life histories.
(a) Habitat segregation and sexual segregation
The term ‘sexual segregation’ has been used to describe patterns of habitat use and association in a wide range of species (Clutton-Brock et al. 1982; Smallwood 1988; Voight 1995; Ruckstuhl & Neuhaus 2000, 2002; Whitehead 2003; Bonenfant et al. 2004; Croft et al. 2004, 2006). However, it is apparent that these authors are describing a continuum of behaviour with segregation into sex-specific social groups on one end and separation by habitat requirement with no social cohesion on the other. This continuum can be usefully divided into two categories. The first is socially mediated sexual segregation, where one or both sexes segregate into sex-specific social groups. Sex-specific groups may or may not have different dietary requirements or share the same habitat (Clutton-Brock et al. 1982; Ruckstuhl & Neuhaus 2000; Whitehead 2003).
In contrast, some species are solitary foragers—they do not form social groups of any kind, let alone sex-specific social groups. For ecological or physiological reasons, the sexes use differing sets of resources. When these resources are found in different habitats, the sexes separate to acquire them. This is sometimes referred to as ‘sexual habitat segregation.’ This non-social sexual segregation is much more common in birds, while the social variety is more typical of mammals (Ruckstuhl & Neuhaus 2000; Catry et al. 2006).
To date, the only other marine mammals clearly demonstrated to segregate by sex in the non-social fashion are the northern (Mirounga angustirostris) and southern (Mirounga leonina) elephant seals (Slip et al. 1994; LeBoeuf et al. 2000). Given the extreme differences in size and energy requirements between males and females in these species, such differences are not unexpected. Grey seals show relatively modest dimorphism in comparison, with males about 50% larger than females. Although not social foragers, grey seals, elephant seals and many other pinnipeds share a number of ecological traits with the well-studied ungulates. The most important are their polygynous mating systems and associated sexual size dimorphism. Since this is the case, the ecological basis for segregation in both pinnipeds and ungulates appears similar. However, because grey seals are not social foragers, the cause of sexual segregation is not complicated by social factors, and a number of hypotheses posed to explain segregation in ungulates do not need to be considered (Ruckstuhl & Neuhaus 2000), leaving only two realistic possibilities: intersexual competition and niche separation.
Intersexual competition may cause males and females to use different areas, especially when one or both sexes are foraging heavily. Segregation in grey seals is most pronounced in the post-breeding months of February and March, when females forage extensively to regain mass after lactation. However, the basis for competition is unclear, because we cannot observe foraging directly. In ungulates, it has been speculated that females outcompete males because their smaller size and relatively larger teeth in proportion to their body size allow them to graze more effectively and exclude males from the best habitat by scramble competition (Ruckstuhl & Neuhaus 2000). Evidence from research trawl surveys indicates that areas northwest of Sable Island, areas used heavily by females in February–March and October–December, are among the best habitats for seals on the Scotian Shelf. These areas support high densities of sand lance (Frank 1996), the dominant prey of females. However, it is unclear how females would prevent males from making use of the area. Females may simply outcompete males for this small prey species by scramble competition or exploitation.
The dietary flexibility of each sex may be important in understanding how intersexual competition operates in this case. Recent dietary evidence suggests that females are more selective and consume higher-quality prey, and they may be limited to areas of the shelf that contain high-quality prey regardless of the level of intraspecific competition (Beck et al. in preparation). Males, on the other hand, show wider dietary niche breadth. This breadth could be the cause (or maybe the result) of more sensitivity to the presence of conspecifics and a greater probability of abandoning high-quality patches when too many conspecifics are present. Males may be more flexible, because the allometric consequences of their larger size include lower BMR per unit mass and larger gut size, which allow them to more efficiently handle less energy dense prey than females. This hypothesis is further supported by Beck et al. (2003b,c), who observed that females spend more time diving than males, and by Austin et al. (2004), who reported females using smaller home ranges than males. This greater apparent foraging effort in smaller areas may reflect both more selective foraging and a need to remain in high-quality prey patches even when intraspecific competition is keen.
Wider niche breadth in males also fits the ‘forage selection hypothesis’ explanation of sexual segregation and its stronger version niche separation (Ruckstuhl & Neuhaus 2000). In addition to size-related physiological differences between males and females, males, being larger, may be better suited to capture larger (or at least different) prey species. However, in other studies, niche separation between the sexes has only been conclusively demonstrated when mouth parts differ significantly (Stamps 1977; Shine 1991; Herrel et al. 1999; Thom et al. 2004). In grey seals, the allometry of dentition and head morphology has not been studied, but as mouth parts might also be important in male–male competition for mates, any differences between the sexes cannot be unambiguously attributed to niche separation.
Other potential causes of segregation seem unlikely, and the only other mechanism worth mentioning is the ‘predation risk hypothesis’ (Ruckstuhl & Neuhaus 2000). This hypothesis poses that the sexes have different predator tolerances or vulnerabilities, and this differential forces one sex to use poorer but less risky habitat. LeBoeuf et al. (2000) speculated that differential risk of white shark predation was a potential cause of sexual segregation in elephant seals. No studies of predatory shark populations have sufficient spatial or temporal resolution for any areas of the Scotian Shelf frequented by grey seals to test this hypothesis. However, given the physical similarities of the sexes and the similarities of their respective favoured foraging areas, it seems unlikely that males and females experience significantly different predation pressures.
Intersexual competition and niche separation working in concert are the most likely causes of sexual segregation in grey seals, though it is impossible to say which process is more important. They are both ecological consequences of sexual size dimorphism, which is ultimately a product of the mating system and reproductive roles of each sex.
(b) Seasonal dynamics of ranging behaviour
The clear spatial differences in habitat use by males and females are compelling. These differences appear to be related, in part, to the differential timing and magnitude of reproductive expenditure (Beck et al. 2003b). Females exhibit two intense periods of foraging, one in the two months between the breeding season and moult, and another in the three months leading up to the breeding season. In between these two periods (July–September) is a major decrease in foraging effort (Beck et al. 2003b,c). By contrast, although males return to the sea, they exhibit lower levels of foraging behaviour in the several months following the breeding season than females, but also increase apparent foraging effort during the October–November period leading up to the breeding season (Beck et al. 2003b,c).
It is unclear why apparent foraging effort of both sexes decreases during the summer, and why male effort, though low, remains higher than females. Summer may be a period of high prey availability, reducing intraspecific and intersexual competition near Sable, so both sexes need only short foraging trips to meet energy needs. Conversely, lower prey availability during summer might produce a similar effect, with seals preferring to wait until food is more abundant rather than waste energy foraging with minimal success. Foraging effort during summer may also be minimized to avoid exposure to increased numbers of migratory sharks. Large predatory sharks in the North Atlantic are now much less common than historically (Baum et al. 2003; Baum & Myers 2004), but grey seals may have evolved an annual foraging strategy to minimize predation risk (sensu Byers 1997) when sharks were more abundant. If seasonally higher predation risk and/or lower foraging success occur, the observed summer haul out and intense pre-breeding foraging strategy may be optimal. Unfortunately, data are limited to test these hypotheses. As mentioned earlier, shark population data are lacking, and fish community surveys of the area are generally only once a year and unable to detect seasonal dynamics of prey (Frank 2003).
There may also be physiological limitations and costs to earlier foraging effort. For example, it may be physiologically demanding to carry more blubber than needed during warmer summer months. Thicker blubber layers may cause heat stress when seals are hauled out, and may alter buoyancy that could increase the energetic cost of diving (Beck et al. 2000) or reduce predation avoidance and prey capture ability. Delaying the final accumulation of blubber needed to breed successfully might decrease stress and improve survival.
Apparent foraging effort increases greatly leading up to the breeding season and both sexes exhibit the highest rate of mass gain during autumn and early winter (Beck et al. 2003a). During this time, habitat segregation is pronounced (figures 2 and 3). The most pronounced segregation (February–March) coincides with post-reproductive mass gain by females and continued loss of energy stores in males. Fatty acid data show the greatest differences between male and female diet at the same time (Beck et al. 2005). The immediate post-breeding period is likely critical for the recovery of female body condition required to support viable pregnancy, but it is apparently less important for males.
(c) Limitations and assumptions
Our month-by-sex kernels were constructed with animal tracks that were usually longer than a month, so that a single individual's track typically contributed to multiple months. Such a situation would normally require a repeated-measures analysis. However, repeated-measures tests for this kind of spatial data have not been developed. This should not affect our comparisons between males and females by month, but would tend to underestimate the variance of within-sex temporal patterns. Since De Solla et al. (1999) concluded that removal of autocorrelation from animal tracks does not improve kernel home range estimates, we did not remove autocorrelation from tracks.
Finally, to achieve the relatively large sample sizes used in our study, we combined data over 10 years. This obscures interannual variation in spatial behaviour for both sexes. Such variability could be ecologically important, but considerably larger annual tag deployments would be required to resolve such variation. Even when combining data across years, small sample sizes for some months might not have captured the spatial usage of the whole population. Even if this was the case, the sexual segregation signal is strong enough to be statistically significant during most of the year.
(d) Management and ecosystem implications
Although the Sable Island grey seal population is large, growing, and not in need of conservation, other grey seal populations (e.g. Baltic Sea) are smaller and perhaps less stable. Sex differences in space use may need to be considered in future conservation efforts. Additionally, many other marine mammals, including both pinnipeds and cetaceans, as well as a number of marine birds, are similarly size dimorphic. Since allometric effects are probably driving differences in ecology between male and female grey seals, other size dimorphic marine species might be expected to have the same ecological differences between the sexes. To our knowledge, the only datasets that are large and comprehensive enough to thoroughly test for spatial differences in pinnipeds have been elephant seals and grey seals (LeBoeuf et al. 2000). In both cases, sexual segregation was present.
Evidence of some degree of sexual segregation has been demonstrated in a few other marine species, including giant petrels, albatross and some whales (González-Solís et al. 2000; Hyrenbach et al. 2002; Whitehead 2003; Phillips et al. 2004; Catry et al. 2006; González-Solís & Croxall 2006). In most cases, however, data are not adequate to thoroughly assess spatial differences or annual seasonal dynamic.
Finally, our results have implications for understanding top-down effects of marine mammals on the ecosystems they inhabit. Effects of marine mammal predation on prey populations of commercial importance or conservation concern continue to receive considerable attention (e.g. Mohn & Bowen 1996; Yodzis 2000; Estes et al. 1998; Trzcinski et al. in press). Our results indicate that a better understanding of marine mammal predation as an agent of ecosystem control will often need to consider the seasonally dynamic spatial and temporal distribution of predation by both sexes.
Acknowledgments
We thank Ransom Myers, Ian Jonsen and Joanna Mills-Flemming for developing, providing code and advice with state-space model implementation. We also thank Future of Marine Animal Populations, NSERC, Dalhousie University and DFO Canada for funding. We thank Sara Iverson, Shelly Lang and Patrick O'Laughlin for field assistance, and Mike James and two anonymous reviewers for useful comments on an earlier draft.
References
- Austin D.A, Bowen W.D, McMillan J.I. Intraspecific variation in movement patterns: modeling individual behaviour in a large marine predator. Oikos. 2004;105:15–30. doi:10.1111/j.0030-1299.1999.12730.x [Google Scholar]
- Baum J.K, Myers R.A. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 2004;7:135–145. doi:10.1111/j.1461-0248.2003.00564.x [Google Scholar]
- Baum J.K, Myers R.A, Kehler D.G, Worm B, Harley S.J, Doherty P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. doi:10.1126/science.1079777 [DOI] [PubMed] [Google Scholar]
- Beck, C. A. 2002 Sex differences in the foraging ecology of a size dimorphic marine carnivore. Ph.D. thesis, Dalhousie University, Halifax, Nova Scotia.
- Beck C.A, Bowen W.D, Iverson S.J. Seasonal changes in buoyancy and diving behaviour of grey seals. J. Exp. Biol. 2000;203:2323–2330. doi: 10.1242/jeb.203.15.2323. [DOI] [PubMed] [Google Scholar]
- Beck C.A, Bowen W.D, Iverson S.J. Sex differences in the seasonal patterns of energy storage and expenditure in a phocid seal. J. Anim. Ecol. 2003a;72:280–291. doi:10.1046/j.1365-2656.2003.00704.x [Google Scholar]
- Beck C.A, Bowen W.D, McMillan J.I, Iverson S.J. Sex differences in the diving behaviour of a size dimorphic capital breeder: the grey seal. Anim. Behav. 2003b;66:777–789. doi:10.1006/anbe.2003.2284 [Google Scholar]
- Beck C.A, Bowen W.D, McMillan J.I, Iverson S.J. Sex differences in diving at multiple temporal scales in a size-dimorphic capital breeder. J. Anim. Ecol. 2003c;72:979–993. doi:10.1046/j.1365-2656.2003.00761.x [Google Scholar]
- Beck C.A, Iverson S.J, Bowen W.D. Blubber fatty acids of grey seals reveal sex differences in diet of a size-dimorphic marine carnivore. Can. J. Zool. 2005;83:377–388. doi:10.1139/z05-021 [Google Scholar]
- Beck, C. A., Iverson, S. J., Bowen, W. D. & Blanchard, W. In preparation. Sex differences in diet reflect seasonal variation in foraging behaviour and energy balance: evidence from quantitative fatty acid signature analysis. [DOI] [PubMed]
- Bonenfant C, Loe L.E, Mysterud A, Langvatn R, Stenseth N.C, Gaillard J.-M, Klein F. Multiple causes of sexual segregation in European red deer: enlightenments from varying breeding phenology at high and low latitude. Proc. R. Soc. B. 2004;271:883–892. doi: 10.1098/rspb.2003.2661. doi:10.1098/rspb.2003.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W.D, Beck C.A, Iverson S.J. Bioelectrical impedance analysis as a means of estimating total body water in grey seals. Can. J. Zool. 1999;77:418–422. doi:10.1139/cjz-77-3-418 [Google Scholar]
- Bowen W.D, McMillan J, Mohn R. Sustained exponential population growth of grey seals at Sable Island, Nova Scotia. ICES J. Mar. Sci. 2003;60:1265–1274. doi:10.1016/S1054-3139(03)00147-4 [Google Scholar]
- Byers J.A. University of Chicago Press; Chicago, IL: 1997. American pronghorn. Social adaptations and the ghosts of predators past. [Google Scholar]
- Catry P, Phillips R.A, Croxall J.P. Sexual segregation in birds: patterns, processes and implications for conservation. In: Ruckstuhl K.E, Neuhaus P, editors. Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press; Cambridge, UK: 2006. pp. 351–378. [Google Scholar]
- Clutton-Brock T.H, Guinness F.E, Albon S.D. University of Chicago Press; Chicago, IL: 1982. Red deer, behavior and ecology of two sexes. [Google Scholar]
- Conradt L, Gordon I.J, Clutton-Brock T.H, Thompson D, Guinness F.E. Could the indirect competition hypothesis explain inter-sexual site segregation in red deer (Cervus elaphus L.)? J. Zool. (Lond.) 2001;254:185–193. [Google Scholar]
- Croft D.P, Botham M.S, Krause J. Is sexual segregation in the guppy, Poecilia reticulata, consistent with the predation risk hypothesis? Environ. Biol. Fishes. 2004;71:127–133. [Google Scholar]
- Croft D.P, James R, Krause J. Predator avoidance as a factor for size assortative shoaling and its implications for sex segregation in fish. In: Ruckstuhl K.E, Neuhaus P, editors. Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press; Cambridge, UK: 2006. pp. 115–126. [Google Scholar]
- De Solla S.R, Bonduriansky R, Brooks R.J. Eliminating autocorrelation reduces biological relevance of home range estimates. J. Anim. Ecol. 1999;68:221–234. doi:10.1046/j.1365-2656.1999.00279.x [Google Scholar]
- Estes J.A, Tinker M.T, Williams T.M, Doak D.F. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. doi:10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- Frank, K. 1996 DFO Atlantic fisheries stock status report 96/77E. Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada.
- Frank, K. 2003 State of the Eastern Scotian Shelf ecosystem. DFO Can. Sci. Advis. Sec. Ecosystem Status Report 2003/2004. Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada.
- González-Solís J, Croxall J.P. Differences in foraging behaviour and feeding ecology in giant petrels. In: Ruckstuhl K.E, Neuhaus P, editors. Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press; Cambridge, UK: 2006. pp. 92–111. [Google Scholar]
- González-Solís J, Croxall J.P, Wood A.G. Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos. 2000;90:390–398. doi:10.1034/j.1600-0706.2000.900220.x [Google Scholar]
- Hall A.J, McConnell B.J, Barker R.J. Factors affecting first-year survival in grey seals and their implications for life history strategy. J. Anim. Ecol. 2001;70:138–149. doi:10.1046/j.1365-2656.2001.00468.x [Google Scholar]
- Herrel A, Spithoven L, Van Damme R, De Vree F. Sexual dimorphism of head size in Gallotia galloti: testing the niche divergence hypothesis by functional analyses. Funct. Ecol. 1999;13:289–297. doi:10.1046/j.1365-2435.1999.00305.x [Google Scholar]
- Hyrenbach K.D, Fernández P, Anderson D.J. Oceanographic habitats of two sympatric North Pacific albatrosses during the breeding season. Mar. Ecol. Prog. Ser. 2002;233:283–301. [Google Scholar]
- Iverson S.J, Bowen W.D, Boness D.J, Oftedal O.T. The effect of maternal size and milk energy output on pup growth in grey seals (Halichoerus grypus) Phys. Zool. 1993;66:61–88. [Google Scholar]
- Jonsen I.D, Myers R.A, Mills-Flemming J. Meta-analysis of animal movement using state-space models. Ecology. 2003;84:3055–3063. [Google Scholar]
- Jonsen I.D, Mills-Flemming J, Myers R.A. Robust state-space modeling of animal movement data. Ecology. 2005;86:2874–2880. [Google Scholar]
- LeBoeuf B.J, Crocker D.E, Costa D.P, Blackwell S.B, Webb P.M, Houser D.S. Foraging ecology of northern elephant seals. Ecol. Monogr. 2000;70:353–382. doi:10.2307/2657207 [Google Scholar]
- Lidgard D.C, Boness D.J, Bowen W.D. A novel mobile approach to investigating mating tactics in male grey seals (Halichoerus grypus) J. Zool. (Lond.) 2001;255:313–320. [Google Scholar]
- Lidgard D.C, Boness D.J, Bowen W.D, McMillan J.I. State-dependent male mating tactics in the grey seal: the importance of body size. Behav. Ecol. 2005;16:541–549. doi:10.1093/beheco/ari023 [Google Scholar]
- Mellish J.E, Iverson S.J, Bowen W.D. Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol. Biochem. Zool. 1999;72:677–690. doi: 10.1086/316708. doi:10.1086/316708 [DOI] [PubMed] [Google Scholar]
- Mohn B, Bowen W.D. Grey seal predation on the Eastern Scotian Shelf: modelling the impact on Atlantic cod. Can. J. Fish. Aquat. Sci. 1996;53:2722–2738. doi:10.1139/cjfas-53-12-2722 [Google Scholar]
- Perrigo G, Bronson F.H. Sex differences in the energy allocation strategies of house mice. Behav. Ecol. Sociobiol. 1985;17:297–302. doi:10.1007/BF00300150 [Google Scholar]
- Phillips R.A, Silk J, Phalan R.D.B, Catry P, Croxall J.P. Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc. R. Soc. B. 2004;271:1283–1291. doi: 10.1098/rspb.2004.2718. doi:10.1098/rspb.2004.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckstuhl K.E, Neuhaus P. Causes of sexual segregation in ungulates: a new approach. Behaviour. 2000;137:361–377. doi:10.1163/156853900502123 [Google Scholar]
- Ruckstuhl K.E, Neuhaus P. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. Camb. Phil. Soc. 2002;77:77–96. doi: 10.1017/s1464793101005814. [DOI] [PubMed] [Google Scholar]
- Shine R. Intersexual dietary divergence and the evolution of sexual dimorphism in snakes. Am. Nat. 1991;138:103–122. doi:10.1086/285207 [Google Scholar]
- Sibly R.M, Calow P. Blackwell Science; Oxford, UK: 1986. Physiological ecology of animals. [Google Scholar]
- Slip D.J, Hindell M.A, Burton H.R. Diving behaviour of southern elephant seals from Macquarie Island: an overview. In: Le Boeuf B, Laws R.M, editors. Elephant seals: population ecology, behaviour, and physiology. University of California Press; Berkeley, CA: 1994. pp. 253–270. [Google Scholar]
- Smallwood J.A. A mechanism of sexual segregation by habitat in American Kestrels (Falco sparverius) wintering in south-central Florida. Auk. 1988;105:36–46. [Google Scholar]
- Stamps J.A. Social behavior and spacing patterns in lizards. In: Gans C, Tinkle D.W, editors. Biology of the Reptilia. vol. 7. Academic Press; London, UK: 1977. pp. 265–334. [Google Scholar]
- Thom M.D, Harrington L.A, Macdonald D.W. Why are American mink sexually dimorphic? A role for niche separation. Oikos. 2004;105:525–535. doi:10.1111/j.0030-1299.2004.12830.x [Google Scholar]
- Trzcinski, M. K., Mohn, R. & Bowen, W. D. In press. Continued decline of the threatened Eastern Scotian Shelf Atlantic cod population: how important is grey seal predation. J. Appl. Ecol [DOI] [PubMed]
- Voight J.R. Sexual dimorphism and prey partitioning in mid-water octopus (Cephalopoda: Bolitaenidae) Biol. Bull. 1995;189:113–119. doi: 10.2307/1542461. [DOI] [PubMed] [Google Scholar]
- Warton B.J. Kernel methods for estimating the utilization distribution in home range studies. Ecology. 1989;70:164–168. doi:10.2307/1938423 [Google Scholar]
- Whitehead H. University of Chicago Press; Chicago, IL: 2003. Sperm whale societies; social evolution in the ocean. [Google Scholar]
- Yodzis P. Diffuse effects in food webs. Ecology. 2000;81:261–266. doi:10.2307/177149 [Google Scholar]