Abstract
The structure of common cuckoo nestling begging calls differs between the two host-races parasitizing reed warblers (reed warbler-cuckoos) and dunnocks (dunnock-cuckoos; longer syllable duration, lower peak and maximum frequency, narrower bandwidth). Cross-fostering experiments demonstrated that this difference is not genetically fixed but develops through experience. When newly hatched reed warbler-cuckoos were transferred to dunnock nests, they developed begging calls more like those of dunnock-cuckoos, whereas controls transferred to the nests of robins or left to be raised by reed warblers developed calls more typical of reed warbler-cuckoos. We tested the effectiveness of these different calls in stimulating host provisioning by placing in host nests a single blackbird or song thrush nestling (of similar size to a young cuckoo, but lacking its exuberant begging calls); when it begged we broadcast, from a small loudspeaker on the nest rim, recordings of either dunnock-cuckoo or reed warbler-cuckoo begging calls. Playback of dunnock-cuckoo begging calls induced higher levels of provisioning by dunnocks, whereas playback of reed warbler-cuckoo begging calls did so for both reed warblers and robins. We suggest that the young cuckoo (which ejects the host's eggs/chicks and so is raised alone) learns by experience which calls best stimulate host provisioning.
Keywords: cuckoo, begging calls, learning, parental care, coevolution
1. Introduction
The common cuckoo, Cuculus canorus, has several female host-races, each specializing on one particular host species and laying a distinctive egg type that usually matches its host's eggs (Chance 1940; Brooke & Davies 1988; Moksnes & Røskaft 1995). Both parentage analysis and studies of genetic differences suggest that these host-races are restricted to female cuckoo lineages, with cross-mating by males maintaining the common cuckoo as one species (Marchetti et al. 1998; Gibbs et al. 2000; Skjelseth et al. 2004).
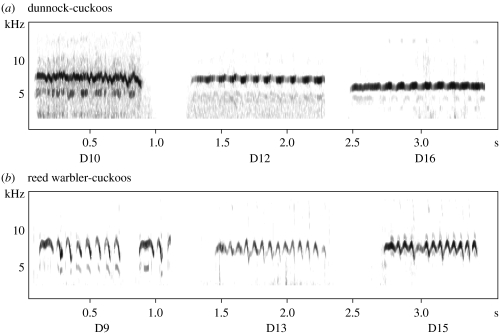
In southern lowland Britain, the two main host-races are those parasitizing reed warblers, Acrocephalus scirpaceus (hereafter referred to as reed warbler-cuckoos) and dunnocks, Prunella modularis (dunnock-cuckoos). These are genetically distinct in mtDNA control region haplotypes (Gibbs et al. 2000). They differ not only in egg colour (Brooke & Davies 1988), but also in the structure of their nestling begging calls. Reed warbler-cuckoo nestlings produce syllables which first rise and then fall in frequency, whereas dunnock-cuckoo nestlings produce syllables of narrower bandwidth, longer duration, and lower peak and maximum frequency (Madden et al. submitted; examples in figure 1). These call differences are evident across all ages, from 6 days old, when the cuckoo nestlings begin to call rapidly during feeds (Kilner & Davies 1999), to 14–17 days old, just before fledging (Madden et al. submitted). Furthermore, they are not confounded by growth, because we found no differences in growth rate between nestling cuckoos of these two host-races (Butchart et al. 2003). Most intriguingly, although in some other cuckoo species the cuckoo nestling mimics the begging calls of its host's young (McLean & Waas 1987; Redondo & Arias de Reyna 1988; Payne & Payne 1998; Langmore et al. 2003), the call differences between reed warbler-cuckoos and dunnock-cuckoos do not reflect mimicry of the different calls of their respective host nestlings or host broods (Madden et al. submitted).
Figure 1.
Typical sonograms of (a) three dunnock-cuckoo nestlings and (b) three reed warbler-cuckoo nestlings, recorded in the field at various ages from 9 to 16 days old.
Here, we explore two questions raised by these host-race differences in begging calls. First, how do they develop? Second, what are their consequences for host provisioning? We imagine two extreme possibilities for development.
Begging call differences are fixed and have a strong genetic basis. Such host-specific nestling adaptations could easily evolve in a brood parasite that specializes on one host species (Payne 2005; Schuetz 2005). However, it is harder to see how these might evolve in a cuckoo species with female host-races targeting a range of host species, each with different traits. Within such a system, a mother could still pass on her egg characteristics to her daughters if genes for egg type were either entirely on the female-specific W sex chromosome (Punnett 1933), or regulated by genes on this chromosome. However, while this mechanism of inheritance could explain the maintenance of host-race differences in egg type, expressed only by females, it could not explain host-race differences in begging calls, expressed by both male (the homogametic sex in birds, ZZ) and female (WZ) offspring. Alternatively, maternal control of offspring behaviour might be possible through expression of a host-race-specific factor in the egg which induces the development of the appropriate begging call. This factor could be genetic (coded for by W chromosome genes) or a non-genetic maternal effect arising from differences in experience (e.g. diet) between females of the different host-races. In birds, for example, females can influence the begging intensity of their offspring by varying the amount of testosterone in their eggs (Schwabl 1993).
Begging calls are plastic and host-race differences develop through experience. In some cuckoo species, the young cuckoo is raised alongside host young, so it could simply copy the begging calls of other young in the brood (Redondo & Arias de Reyna 1988). Common cuckoos have no opportunities for such social learning because they eject the host's eggs (or newly hatched chicks), and so are raised alone in the nest. In any case, the host-race differences we are investigating here do not involve host nestling mimicry. Nevertheless, the young common cuckoo could still learn by first trying out various begging calls and then specializing on those that work best in stimulating provisioning from its particular host species (Payne & Payne 1998). Previous studies have shown that nestlings of other species rapidly learn to modify their begging intensity (postures, duration of begging bouts) in relation to variation in experimental hand-rearing regimes (Kedar et al. 2000; Rodriguez-Girones et al. 2002), but no study has yet tested whether nestlings learn to modify their begging call structure.
Our first aim is to test between these two explanations by cross-fostering reed warbler-cuckoos so that they are raised by dunnocks. According to (i), they should retain their original host-race begging call. According to (ii), they should develop begging calls typical of nestling dunnock-cuckoos. Hypothesis (ii) presupposes that different begging calls will be best for stimulating provisioning by reed warbler and dunnock host parents. This prompts our second topic, namely the function of the difference in calls.
In a reed warbler nest, a cuckoo is provisioned at about the same rate as a whole brood of host young (Brooke & Davies 1989; Grim & Honza 1997). It achieves this by producing unusually rapid begging calls to compensate for its single gape, a deficient visual stimulus for the hosts compared with what they expect from a host brood (Kilner et al. 1999). We previously demonstrated the importance of the vocal component of the cuckoo's begging display by placing a single blackbird, Turdus merula, or song thrush, Turdus philomelos, chick in a reed warbler's nest, to provide these hosts with a nestling the size of a cuckoo but one which lacked the cuckoo's exuberant vocal signals. Reed warblers provisioned a blackbird/thrush chick at a lower rate than a cuckoo chick of the same mass, but could be induced to increase their provisioning to cuckoo-like levels by playback of reed warbler-cuckoo nestling begging calls (Davies et al. 1998). Here, we use this same experimental technique to test whether provisioning by reed warbler hosts is best stimulated by reed warbler-cuckoo begging calls and provisioning by dunnock hosts by dunnock-cuckoo calls. In the discussion, we propose a link between the development and function of host-race differences in cuckoo nestling behaviour.
2. Material and methods
(a) Cross-fostering experiment
We studied reed warbler-cuckoos on Wicken Fen and the surrounding waterways (Cambridgeshire, England) between 2000 and 2005. Only ca 2% of reed warbler nests were parasitized and this sample was then reduced further by predation, so we had a limited availability of cuckoos. Furthermore, we tried to find young originating from different female cuckoos to avoid pseudoreplication. We recognized females by individual differences in their egg markings and distances between host nests. Two lines of evidence validate this method: first, eggs with individually distinctive markings are laid within discrete territories (Chance 1940; Wyllie 1981; Davies & Brooke 1988); second, eggs laid by individually radio-tagged females were all correctly assigned when categorized by appearance (A. Moksnes 2006, personal communication).
This experiment involved 17 cuckoos, all found as eggs in reed warbler nests. Eight were transferred to dunnock (n=5) or robin, Erithacus rubecula (n=3), nests with eggs in the Cambridge University Botanic Garden or hedgerows nearby. One of these cuckoos was a newly laid cuckoo egg, transferred the day after it was laid (and before incubation) to a dunnock nest. The other seven were cuckoo nestlings, transferred within 16 h of hatching (four to dunnock nests, three to robin nests). These eight cross-fostered young came from seven different females. For the female providing two offspring, one was transferred to a dunnock nest and one to a robin nest. The young cuckoos ejected their new fosterer's eggs and were then raised by these new hosts. We chose to cross-foster some cuckoos to robins, which are another regular cuckoo host in southern Britain, because preliminary data (J. R. Madden & N. B. Davies 2006, unpublished) suggest that cuckoos in robin nests have begging calls similar to those in reed warbler nests, so they provided a useful control.
The other nine cuckoos originated from nine other females and they were left in their nest of origin to be raised by reed warblers, their intended host species. It would have been neater to transfer these to other reed warbler nests to control for the transfer procedure itself. However, previous experiments in which we transferred cuckoos between reed warbler nests showed that both host parents and cuckoo chicks adapt rapidly to changes in nest contents, with no significant difference in provisioning rates between cross-fostered cuckoos and those left in their own nests (Davies et al. 1998), so we decided that such strict controls were not necessary. Furthermore, we found no differences in growth between the cuckoos transferred to dunnock/robin nests and those left in reed warbler nests (see §3). The reciprocal cross-fostering of dunnock-cuckoos to reed warbler nests was not possible because dunnock-cuckoos were so hard to find.
We recorded the begging calls of the cuckoo nestlings at 6–9 days old under standard hunger conditions. The cuckoo was temporarily removed from the host nest and replaced with two host chicks from part of a nearby brood to prevent the host parents from deserting during the cuckoo's absence. In the laboratory, the cuckoo was placed in a heated old nest inside a test box, and fed from plastic forceps with Nectarblend rearing mix until it stopped begging. Eighty minutes later, when begging intensity matches that under natural field conditions (Kilner & Davies 1999), the cuckoo was stimulated to beg again, by gentle taps on the side of its bill with forceps. We recorded the begging calls using a Sony ECM-T6 tie-clip microphone placed 10 cm from the nest cup, connected to a Sony WM-D6C tape recorder (2000–2002) or to a Canon MV 500i Digital video and audio camcorder (2003–2005).
Recordings were digitized in mono using WaveLab software, in 16 bits at a sample rate of 44.1 kHz, and sonograms generated using Avisoft-SAS LabPro v. 4.15, using the filter settings in Avisoft (fast Fourier transform=512, frame=50%, window=flattop, overlap=50%). This gave a frequency resolution of 86 Hz and a time resolution of 5.8 ms. A section was taken at least 5 s after the start of a begging bout and 3–10 syllables measured per individual. Call structure was quantified using automatic parameter measurements (APM). We recorded five variables: syllable duration (s), peak, maximum and minimum frequency (kHz) and bandwidth (kHz). Syllables were determined by the APM procedure using automatic element separation with a threshold of −15 dB set relative to the maximum. A mean value for each variable was calculated for an individual and used in the subsequent analyses. Discriminant function analyses were then carried out, using a stepwise procedure, aiming to minimize Wilk's λ, with cuckoo nestlings classified by the host species that raised them. All analyses were performed using SPSS v. 11.
(b) Comparison with naturally raised cuckoos
We compared the begging calls of these experimental and control reed warbler-cuckoos with field recordings made previously from 1997 to 2001 of naturally raised reed warbler-cuckoos (n=14) found in reed warbler nests at various wetland sites in Cambridgeshire and Hertfordshire, and of dunnock-cuckoos (n=9) found at woodland and farmland sites in southern and eastern England. These were recorded with the same equipment as above during host parent feeding visits, and calls were then measured in the same way. Most of these cuckoos were found as nestlings. It is unlikely that any dunnock-cuckoos shared the same maternal origin as samples came from widely spaced nests (12–150 km) over a large geographical area. To minimize the chance that any of the reed warbler-cuckoos shared the same mother, we selected nestlings recorded at sites likely to be separated by at least two laying territories (Davies & Brooke 1988). Most of these reed warbler-cuckoos were recorded 5–7 years before those described in §2a, so, with adult survival of 52% (see in Brooke & Davies 1987), it is unlikely that nestlings in the two samples shared the same mother. To further reduce this possibility, we selected nestlings recorded at sites likely to be separated by at least two laying territories from those described in §2a. The field recordings were of 7–17-day-old nestlings, which included some that were older than our sample of experimental birds (recorded at 6–9 days old). This was necessary to achieve a sufficient sample size. There was no significant difference in mean age between our field samples of the two host-races. The inclusion of some older nestlings in the field samples does not bias the comparison with the experimental cuckoos, because the difference in structure of begging calls of reed warbler-cuckoos and dunnock-cuckoos was evident across all these ages (Madden et al. submitted).
(c) Provisioning experiment
We followed the previously established protocol of Davies et al. (1998). In 2005, we found nests with broods of 3–6-day-old young, of dunnocks and robins (Cambridge University Botanic Garden and nearby hedgerows), and of reed warblers (Wicken Fen and surrounding waterways). On the day of the experiment, a small loudspeaker (2.5 cm diameter) was placed against the outside rim of the nest and connected to a NAPA 128 MB MP3 digital player via a 3 W amplifier. A miniature video camera was also placed about 20 cm above the nest cup and connected to a Canon MV 500i Digital video and audio camcorder. Connecting cables enabled us to sit hidden 15 m from the nest, sufficiently far away to avoid disturbing the parents, and to monitor chick provisioning on the camcorder's video screen.
We allowed an hour for the parents to get used to this equipment. Then their brood was removed from the nest temporarily and kept warm and well fed in a heated old nest for the duration of the experiment. The brood was replaced with a single 3–5-day-old blackbird or song thrush chick (these are similar in size and appearance), taken from part of a brood in a nest nearby. This presented the host parents with a single, large cuckoo-sized chick, but one which lacks the cuckoo's exuberant begging call. Dunnock nests (in bushes) and robin nests (in bushes, crevices or on the ground) provided the blackbird/thrush chicks with a stable nest, like their own, but reed warbler nests are suspended from reed stems and sway in the wind, so we tied the supporting reeds to an anchored bamboo cane to keep the nest steady. The blackbird/thrush chicks settled down readily and were soon begging when the host parents arrived with food. All three host species seemed unconcerned by the sudden change in their nest contents and were usually brooding or feeding the blackbird/thrush chick within a few minutes.
We allowed an hour for both hosts and blackbird/thrush chicks to become accustomed to their new situation (sufficient for provisioning levels to stabilize, see Davies et al. 1998) before we started the playback experiment. We used a paired design in which each nest experienced, in random order, one hour with reed warbler-cuckoo begging call playback and one hour with dunnock-cuckoo playback, with 30 min between these periods during which no playbacks were broadcast. We monitored the nest on the video camera screen; every time the blackbird/thrush opened its gape to beg, we broadcast playback of begging calls through the loudspeaker at a standard, natural volume, and continued the broadcast until the chick closed its gape. Begging mainly occurred when a host parent arrived with food, but we did playbacks whenever the chick begged, even if no parent was at the nest. To start each hour's experimental period, we first waited for a parent to arrive with food and then broadcast the relevant playback while the chick begged and was fed. The hour's recording began as soon as this feed ended, when one parent had experienced the playback stimulus. Visits were recorded on videotape, so we could count the number of feeds during the subsequent hour and, where possible, the type of food delivered. The blackbird/thrush chick was weighed on an electronic balance (to nearest 0.1 g) just before we first placed it in the host nest and then again at the end of the two playback hours so we could relate provisioning frequency to mass gain. We performed just one experiment with each host pair.
The playback cuts were made from the field recordings of reed warbler-cuckoos and dunnock-cuckoos (see above), with no more than two (different) cuts made from the recordings of any one individual. We used a different pair of playback cuts for each experiment within each host species. The use of two different playback cuts from some individuals did not bias our results, because there was no less variation in responses when comparing between those from two cuts of the same individual versus between two cuts from different individuals (p=0.56). Cuckoos raised naturally by dunnocks have a higher begging call rate than those raised by reed warblers (Butchart et al. 2003). To control for rate, we created 12 s playback cuts, each containing six bursts of 15 syllables. This ensured that the rate over a 12 s period was identical for the two playbacks, while preserving the integrity of the bursts of natural begging calls. This calling rate (90 syllables per 12 s) approximates the upper range of natural begging rates for reed warbler-cuckoos and the lower range for dunnock-cuckoos of 4–6 days old (see fig. 4 of Butchart et al. 2003). Playback cuts were looped so that they could be repeated if the begging bout lasted longer than 12 s.
(d) Ethical considerations
Playback and cross-fostering experiments were done under licence from English Nature. Cross-fostered cuckoo nestlings did not differ in growth or mass from those raised normally by reed warblers, and they were all returned to reed warbler nests at 6–9 days old. However, provisioning to the blackbird/song thrush nestlings in both dunnock and robin nests was lower than what they normally experience in their own species' nest, so we reduced sample sizes for these treatments and fed them with minced beef before returning them to their home nests. None of our temporary removals or transfers of blackbird, dunnock, robin or reed warbler nestlings led to desertions, all were returned to their home nests after the experiments, and were readily accepted back by their parents.
3. Results
(a) Cross-fostering experiment: do host-race call differences develop through experience?
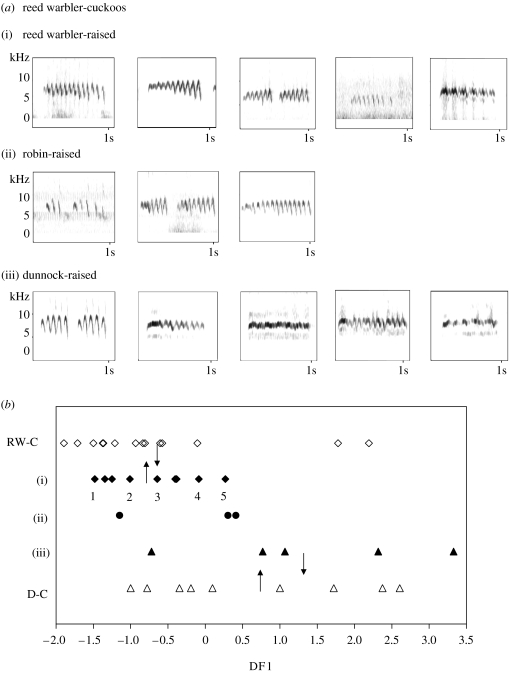
Sonograms showed that four of the five reed warbler-cuckoos cross-fostered to be raised by dunnocks had the ‘narrow frequency band’ call type typical of naturally raised dunnock-cuckoos, whereas those raised by robins or reed warblers retained the ‘frequency rise then fall’ call type more typical of reed warbler-cuckoos (figure 2a). We first compared all five begging call parameters (table 1; ignoring, for the moment, potential problems of multiple comparison and non-independence). Compared with the reed warbler-cuckoos raised by reed warblers, those cross-fostered to dunnocks had calls of significantly longer duration, higher minimum frequency and narrower bandwidth (table 1a). Their calls did not differ in any of the five parameters from those of dunnock-cuckoos naturally raised by dunnocks (table 1b). The calls of the control group of reed warbler-cuckoos raised by reed warblers were similar to those of field recordings of naturally raised reed warbler-cuckoos, but of lower minimum frequency and wider bandwidth (table 1).
Figure 2.
(a) Sonograms of reed warbler-cuckoos from the cross-fostering experiment, comparing those raised by their intended host, reed warblers (n=9: five individuals shown), with those raised by either robins (n=3) or dunnocks (n=5). (b) Discriminant function analysis separating these 17 reed warbler-cuckoos by their raising host species: (i) reed warblers (black diamonds), (ii) robins (black circles) or (iii) dunnocks (black triangles). For each raising host species, the sonograms are arranged in the same order (left to right) as the samples in this graph (the five reed warbler-raised individuals illustrated are numbered). For comparison, we have shown how this discriminant function analysis separates the field recordings of calls of naturally raised reed warbler-cuckoos (RW-C: n=14, open diamonds) and dunnock-cuckoos (D-C: n=9, open triangles). Arrows indicate group centroids.
Table 1.
Begging call measurements of nestling cuckoos in the (a) cross-fostering experiment and of (b) naturally raised cuckoos from two host-races. (In (a), different superscripts within a column indicate significant differences (post hoc least significant difference, p<0.05, after ANOVA comparing the three hosts). In (b), significant differences between the two host-races indicated between the rows (*0.05>p>0.01, two-tailed t-tests) and significant differences from same raising host in (a) indicated next to measurement (*0.05>p>0.01, two-tailed t-tests).)
| cuckoo host-race | raising host species (n) | syllable duration (s) | begging call measurements (mean±1 s.d.) frequency (kHz) | |||
|---|---|---|---|---|---|---|
| peak | minimum | maximum | bandwidth | |||
| (a) cross-fostering experiment | ||||||
| reed warbler-cuckoo | reed warbler (9) | 0.038±0.012a | 7.0±0.9a | 5.1±1.0a | 8.9±1.0a | 3.9±1.3a |
| robin (3) | 0.050±0.017ab | 7.5±0.2a | 6.3±0.3b | 8.9±0.4a | 2.5±0.7ab | |
| dunnock (5) | 0.080±0.031b | 7.5±0.6a | 6.5±0.5b | 8.6±0.4a | 2.1±0.5b | |
| (b) naturally raised cuckoos | ||||||
| reed warbler-cuckoo | reed warbler (14) | 0.040±0.024 | 7.51±0.4 | 6.2±0.6* | 8.8±0.6 | 2.6±0.9* |
| * | * | * | ||||
| dunnock-cuckoo | dunnock (9) | 0.065±0.027 | 7.1±0.6 | 6.1±1.0 | 8.0±0.6 | 1.9±1.0 |
We used a discriminant function model to try to separate the calls of these reed warbler-cuckoos according to their raising host species. Following a stepwise procedure, only one measure, syllable duration, entered the model and it correctly assigned 76% (76% with cross-validation) of the cuckoos' calls to their raising host species (figure 2b; Wilks λ=0.51, , p=0.009). Those classed as reed warbler raised included all nine cuckoos raised by reed warblers plus one of the five raised by dunnocks. Those classed as dunnock raised included the other four raised by dunnocks (separation by raising host: Fisher's exact test, p=0.005). The calls of the robin-raised cuckoos did not form a distinct category of their own; all three were classified together with those of the reed warbler-raised cuckoos. These call differences were not confounded by differences in development, because we found no differences in mass between cuckoos raised by the three host species (Kruskal–Wallis test; , p=0.71).
How do these call differences compare with those we found previously in cuckoos from the two host-races specializing on reed warblers and dunnocks (Madden et al. submitted)? To make this comparison, we asked how the discriminant function model that separated the cuckoos in the cross-fostering experiment would classify the independent sample of twenty three cuckoos raised naturally by reed warblers or dunnocks (figure 2b). The model classified the calls of 12/14 of the naturally raised reed warbler-cuckoos along with those of the control reed-warbler-raised cuckoos from the experiment. It classified the calls of 5/9 of the naturally raised dunnock-cuckoos alongside those of the experimentally cross-fostered reed warbler-cuckoos raised by dunnocks (Fisher's exact test, p=0.047). We conclude that when reed warbler-cuckoos were raised by dunnocks, their calls became more like those of dunnock-cuckoos.
(b) Playback experiment: do host-race call differences increase host provisioning?
There were marked differences between the three host species in overall provisioning rates to a single blackbird/song thrush chick (table 2; ANOVA, effect of host species, F2,22=24.1, p<0.0001). Dunnocks had the lowest provisioning rate (post hoc LSD tests: compared with robins p=0.032 and with reed warblers p<0.001) and reed warblers had the highest (compared with robins p<0.001). Obviously, provisioning rate could vary with load size per feeding visit. However, all three hosts tended to bring similar-sized loads, usually either single large prey (caterpillars, moths, flies) or billfulls of smaller invertebrates. While mass gain per hour by the blackbird/thrush nestling increased with host-provisioning rate (ANCOVA, F1,20=4.34, p=0.05), there was no interaction with host species (F2,20=1.51, p=0.24). Therefore, provisioning rate was a valid common measure of parental effort across all three hosts and reflected the benefit gained by the nestling blackbird/thrush.
Table 2.
Provisioning rates by dunnocks, robins and reed warblers to a single blackbird or song thrush chick, whose begging was accompanied by playback of either reed warbler-cuckoo begging calls or dunnock-cuckoo begging calls.
| host species (no. of nests) | mean feeding visits per hour (±1 s.e.) | ||
|---|---|---|---|
| reed warbler-cuckoo playback | dunnock-cuckoo playback | Wilcoxon signed-rank test | |
| dunnock (n=7) | 2.1±0.5 | 3.7±0.4 | p=0.041 |
| robin (n=7) | 9.0±0.8 | 7.0±0.8 | p=0.034 |
| reed warbler (n=11) | 17.7±2.2 | 15.1±1.6 | p=0.052 |
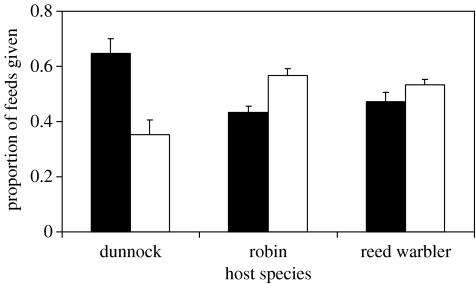
Our most important result was that the effects of the two playbacks differed between host species (table 2; interaction between host and playback type, F2,22=3.55, p=0.046), with dunnocks provisioning at a higher rate when dunnock-cuckoo begging calls were broadcast and both robins and reed warblers provisioning more during playback of reed warbler-cuckoo begging calls. Figure 3 summarizes the proportion of feeds brought during each playback. Responses to the two playbacks differed between hosts (ANOVA on arc-sine transformed data: interaction between host species and playback type, F2,24=11.6, p<0.0001). The responses of robins and reed warblers did not differ (post hoc LSD test, p=0.48) but both differed from that of dunnocks (p<0.001 in both cases). Whereas dunnocks brought a greater proportion of their feeds during the dunnock-cuckoo playback (Wilcoxon signed-rank test, Z=2.04, n=7, p=0.041), both robins and reed warblers did so during the reed warbler-cuckoo playbacks (robins, Z=2.12, n=7, p=0.034; reed warblers, Z=1.68, n=11, p=0.052).
Figure 3.
Proportion of feeds brought by dunnocks, robins and reed warblers to a single blackbird or song thrush nestling placed in their nests, during periods when this nestling's begging was accompanied by playback of either reed warbler-cuckoo begging calls (white columns) or dunnock-cuckoo begging calls (black columns). Bars indicate means (+1 s.e.) for 7 dunnock, 7 robin and 11 reed warbler nests.
We considered two factors which could confound the interpretation of these results. First, there was no evidence that playback type influenced load size. We could examine this only for reed warblers, where loads were most visible on the video recordings. There was no difference between playback type in either the percentage of visits involving billfulls of small prey (Wilcoxon signed-rank test, Z=0.91, n=10, p=0.36), or the percentage of visits with a single large prey item (Z=0.20, n=10, p=0.84). Second, there was no evidence that the playback influenced the behaviour of the nestling blackbird/thrush. We timed the duration of the begging bouts under each playback type. Although there were differences between host species (general linear model host, F2,12=4.69, p=0.03), there was no effect of playback type (F1,7=1.34, p=0.28), nor any interaction between host species and playback type (F2,12=0.35, p=0.71). Furthermore, when we tested the responses of each nestling blackbird/thrush to a 6 s playback during the host parents' absence, none of them begged. We conclude that the playbacks stimulated the host parents directly rather than indirectly via a change in nestling begging behaviour.
4. Discussion
(a) Learning to beg profitably
Our results suggest two main conclusions. First, the host-race differences in nestling cuckoo begging calls are not fixed, either genetically or by a maternal effect, but may develop through differences in nestling experience. When reed warbler-cuckoos were cross-fostered to dunnock nests, four of the five developed begging calls typical of dunnock-cuckoos raised naturally by dunnocks. Second, the differences in begging calls increase host provisioning, with dunnock-cuckoo begging calls being more stimulating for dunnock hosts and reed warbler-cuckoo begging calls for reed warbler hosts.
The second conclusion immediately suggests a mechanism for how the different calls might develop: the young cuckoo could first try out various begging calls and then learn by experience which calls work best in stimulating host provisioning (Payne & Payne 1998). There is evidence for this kind of vocal learning in another brood parasite, the brown-headed cowbird, Molothrus ater, where naive yearling males learnt to repeat the song types to which females responded most strongly through a visual display (West & King 1988). The cuckoo's problem is analogous to that of a naive forager faced with a choice of feeding options which differ in profitability. For example, when foraging great tits, Parus major, were faced with two feeding places the sampling period needed to decide which was best increased as the patches became more similar, but the birds could readily choose the best even when it was only 1.5 times better than the other patch (Krebs et al. 1978). This difference is similar in magnitude to that between the profitabilities of the two types of cuckoo begging calls, as revealed by the playback experiment (more effective call increasing provisioning by 1.2–1.8 times that in response to the less effective call). It would be interesting to record the calls of nestling cuckoos throughout their development to see whether they first sample call effectiveness and, if so, the range of calls they try before choosing.
There is, perhaps, a simpler potential mechanism for how cuckoo begging calls might vary. Although dunnock-cuckoo and reed warbler-cuckoo nestlings grow at the same rate, dunnock-cuckoo nestlings produce more rapid begging calls (Butchart et al. 2003). The likely explanation is that dunnock chicks themselves call more rapidly than the young of other host species, so to tune into this host's communication system the cuckoo, too, has to call more rapidly (Butchart et al. 2003). Dunnock broods often have mixed paternity, so dunnock chicks often compete with half sibs rather than full sibs (Davies 1992). This leads not only to more selfish begging by the young (Briskie et al. 1994), but also to the parents, in turn, demanding more intensive begging for a given amount of provisioning (Godfray 1991). An increase in the nestling cuckoo's calling rate might inevitably lead to changes in call structure for the following reason. Studies of bird song have shown that as syllable repetition rate increases, frequency bandwidth decreases. This is because syllables with a wider frequency bandwidth require higher magnitude movements of the vocal tract (which modifies resonance). To produce syllables at a faster rate requires more rapid vocal tract oscillations, which are therefore constrained to be of lower magnitude, hence reducing the frequency bandwidth (Nowicki & Searcy 2005). If similar constraints apply to nestling begging calls, the narrower bandwidth of the dunnock-cuckoo's begging call (and perhaps other differences too) might be an inevitable consequence of more rapid calling.
In our playback experiments we standardized call rate, so our results show that dunnocks and reed warblers are best stimulated by cuckoo begging calls of different structure. Nevertheless, further experiments are needed to test how call rate and call structure interact to influence host provisioning. We are also still left with the puzzle of why the cuckoo calls differ from those of the host young (Madden et al. submitted). It would be illuminating to compare the stimulating effect of the begging calls of the host species' own young with those of its cuckoo host-race. A cuckoo might be able to produce an even more effective begging call than a host nestling because it is larger and does not have to compete for food in the nest, both of which may influence begging signals (Morton 1975; Leonard & Horn 2001). Perhaps different host species are responsive to different call features and their respective cuckoo host-races exaggerate these rather than mimicking the overall call structure of their host young.
We found marked differences between host species in overall provisioning rate in our playback experiment (table 2). The lower provisioning rate by dunnocks might, in part, result from their variable mating system. First, polygynous females are often left to feed a brood alone and polygynandrous females get only the part-time help of a male (Davies & Hatchwell 1992). By contrast, both robins and reed warblers usually breed in monogamous pairs where male and female provision a brood together. Second, our standardized playback rate was at the lower limit of the natural range for dunnock-cuckoos and at the upper limit for reed warbler-cuckoos, which may have contributed to a relatively lower overall rate of provisioning by dunnocks. Finally, it is possible that dunnocks (and perhaps robins too) were simply more reluctant to feed a blackbird/thrush nestling than were reed warblers. Although the blackbird/thrush plus playback provides a useful experimental model system, it does not mimic the stimulus of a cuckoo exactly; for example, the blackbird/thrush has a different gape colour and begging posture, both of which could interact with begging calls to influence host provisioning (Kilner 2002; Alvarez 2004).
(b) Flexible begging calls but fixed alarm tuning
Has the ability to develop different begging calls in different host nests evolved as a special adaptation to enable the common cuckoo to exploit different host species, within the restrictions imposed by female host-races within one cuckoo species? If so, then cuckoo species that specialize on one host species should lack this flexibility in development. Further studies are needed before we can make this comparison. However, there are reports that some other cuckoo species likely to have host-races also exhibit different cuckoo nestling begging calls in the nests of different host species (Reed 1968; Payne & Payne 1998). By contrast, the shining bronze-cuckoo, Chrysococcyx lucidus, specializes mainly on thornbills, Acanthiza spp., and the begging calls of the nestling cuckoo resemble those of thornbill young (Payne & Payne 1998). When shining bronze-cuckoos were transferred, as eggs, to the nests of superb fairy wrens, Malurus cyaneus, they retained thornbill-like begging calls and were rejected by the fairy wren hosts. The fairy wrens are normally parasitized by another cuckoo species with begging calls that mimic those of fairy wren young (Langmore et al. 2003). This suggests that some specialist cuckoos lack the flexibility in begging call development necessary to exploit a range of host species.
The developmental flexibility we have described here for reed warbler-cuckoo nestling begging calls contrasts with their predisposition to cease begging calls only in response to reed warbler parental alarm calls, given when a predator is near the nest. Reed warbler-cuckoos cross-fostered to dunnock or robin nests did not tune into the different alarms of these new foster parents; instead, they retained a specific response to reed warbler alarms (Davies et al. 2006). Why is there more developmental flexibility in a begging call than in an alarm response? We suggest that learning is less costly for begging calls. During the sampling period, the nestling suffers a reduction in food intake but frequent provisioning visits are likely to lead to rapid learning by reinforcement. With alarm calls, however, there is both less opportunity for learning (predator visits are less frequent) and the costs are greater—a cuckoo may not live to learn from its mistake if its begging calls attract a predator. Although reed warbler-cuckoo nestlings fine-tune their alarm response by exposure to reed warbler alarms, neural pre-tuning seems essential to enable them to pick out a life-saving signal against a background of irrelevant sounds. Host nestlings, too, had an innate predisposition to respond to their own species alarms and did not tune into another species alarms when cross-fostered (Davies et al. 2004). If common cuckoo host-races are indeed restricted to female lineages, then alarm tuning must be under maternal control. Alternatively, reed warbler-cuckoos might have evolved into a cryptic species which has retained the ability to develop various begging calls through learning. Future studies of nestling adaptations are likely to throw new light on specialization and speciation in brood parasites.
Acknowledgments
We thank: the Natural Environment Research Council for funding; the Director, John Parker, for permission to work in the Cambridge University Botanic Garden; the National Trust for permission to work on Wicken Fen and Chris Thorne and the Wicken Fen Ringing Group for facilities there; and the following for help with finding nests, with experiments or with the manuscript: Stephen Browne, Stuart Butchart, Jan and Alice Davies, Derek Gruar, Alan Harris, Camilla Hinde, Ann Jeffrey, Rebecca Kilner, Oliver Krüger, Helen Markland, David McKee, Vee Mead, Andy Radford, Philip Radford, Stephen Rumsey, Trevor Squire, Chris Stoate, Justin Welbergen and Helen Withers.
References
- Alvarez F. The conspicuous gape of the nestling common cuckoo Cuculus canorus as a supernormal stimulus for rufus bush chat Cercotrichas galactotes hosts. Ardea. 2004;92:63–68. [Google Scholar]
- Briskie J.V, Naugler C.T, Leech S.M. Begging intensity of nestling birds varies with sibling relatedness. Proc. R. Soc. B. 1994;258:73–78. [Google Scholar]
- Brooke M.de L, Davies N.B. Recent changes in host usage by cuckoos Cuculus canorus in Britain. J. Anim. Ecol. 1987;56:873–883. [Google Scholar]
- Brooke M.de L, Davies N.B. Egg mimicry by cuckoos, Cuculus canorus, in relation to discrimination by hosts. Nature. 1988;335:630–632. doi:10.1038/335630a0 [Google Scholar]
- Brooke M.de L, Davies N.B. Provisioning of nestling cuckoos Cuculus canorus by reed warbler Acrocephalus scirpaceus hosts. Ibis. 1989;131:250–256. [Google Scholar]
- Butchart S.H.M, Kilner R.M, Fuisz T, Davies N.B. Differences in the nestling begging calls of hosts and host-races of the common cuckoo, Cuculus canorus. Anim. Behav. 2003;65:345–354. doi:10.1006/anbe.2003.2066 [Google Scholar]
- Chance E.P. Country Life; London: 1940. The truth about the cuckoo. [Google Scholar]
- Davies N.B. Oxford University Press; Oxford, UK: 1992. Dunnock behaviour and social evolution. [Google Scholar]
- Davies N.B, Brooke M.de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;36:262–284. [Google Scholar]
- Davies N.B, Hatchwell B.J. The value of male parental care and its influence on reproductive allocation by male and female dunnocks Prunella modularis. J. Anim. Ecol. 1992;61:259–272. [Google Scholar]
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos Cuculus canorus exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. B. 2004;271:2297–2304. doi: 10.1098/rspb.2004.2835. doi:10.1098/rspb.2004.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M, Rutila J. A host-race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. Proc. R. Soc. B. 2006;273:693–699. doi: 10.1098/rspb.2005.3324. doi:10.1098/rspb.2005.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs H.L, Sorenson M.D, Marchetti K, Brooke M.de L, Davies N.B, Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. doi:10.1038/35025058 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. doi:10.1038/352328a0 [Google Scholar]
- Grim T, Honza M. Differences in parental care of reed warbler Acrocephalus scirpaceus to its own nestlings and parasitic cuckoo Cuculus canorus chicks. Folia Zoolog. 1997;46:135–142. [Google Scholar]
- Kedar H, Rodriguez-Girones M, Yedvab S, Winkler D.W, Lotem A. Experimental evidence for offspring learning in parent–offspring communication. Proc. R. Soc. B. 2000;267:1723–1727. doi: 10.1098/rspb.2000.1201. doi:10.1098/rspb.2000.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R.M. The evolution of complex begging displays. In: Wright J, Leonard M.L, editors. The evolution of begging: competition, cooperation and communication. Kluwer; Dordrecht: 2002. pp. 87–106. [Google Scholar]
- Kilner R.M, Davies N.B. How selfish is a cuckoo chick? Anim. Behav. 1999;58:797–808. doi: 10.1006/anbe.1999.1197. doi:10.1006/anbe.1999.1197 [DOI] [PubMed] [Google Scholar]
- Kilner R.M, Noble D.G, Davies N.B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature. 1999;397:667–672. doi:10.1038/17746 [Google Scholar]
- Krebs J.R, Kacelnik A, Taylor P. Test of optimal sampling by foraging great tits. Nature. 1978;275:27–31. doi:10.1038/275027a0 [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Leonard M.L, Horn A.G. Dynamics of calling by tree swallow (Tachycineta bicolor) nestmates. Behav. Ecol. Sociobiol. 2001;50:430–435. doi:10.1007/s002650100380 [Google Scholar]
- Madden, J. R., Butchart, S. H. M., Kilner, R. M. & Davies, N. B. Submitted. Nestling begging calls of the cuckoo Cuculus canorus: host-race differences but no host nestling mimicry.
- Marchetti K, Nakamura H, Gibbs H.L. Host race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. doi:10.1126/science.282.5388.471 [DOI] [PubMed] [Google Scholar]
- McLean I.G, Waas J.R. Do cuckoo chicks mimic the begging calls of their hosts? Anim. Behav. 1987;35:1896–1898. doi:10.1016/S0003-3472(87)80083-0 [Google Scholar]
- Moksnes A, Røskaft E. Egg-morphs and host preference in the common cuckoo Cuculus canorus: an analysis of cuckoo and host eggs from European museum collections. J. Zool. Lond. 1995;236:625–648. [Google Scholar]
- Morton E.S. Ecological sources of selection on avian sounds. Am. Nat. 1975;109:17–34. doi:10.1086/282971 [Google Scholar]
- Nowicki S, Searcy W.A. Song and mate choice in birds: how the development of behaviour helps us understand function. Auk. 2005;122:1–14. [Google Scholar]
- Payne R.B. Nestling mouth markings and colors of old world finches Estrildidae: mimicry and coevolution of nesting finches and their Vidua brood parasites. Misc. Publ. Mus. Zool., Univ. Mich. 2005;194:1–45. [Google Scholar]
- Payne R.B, Payne L.L. Nestling eviction and vocal begging behaviours in the Australian glossy cuckoos Chrysococcyx basalis and C. lucidus. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts: studies in coevolution. Oxford University Press; Oxford, UK: 1998. pp. 152–169. [Google Scholar]
- Punnett R.C. Inheritance of egg-colour in the parasitic cuckoos. Nature. 1933;132:892. [Google Scholar]
- Redondo T, Arias de Reyna L. Vocal mimicry of hosts by great spotted cuckoo, Clamator glandarius: further evidence. Ibis. 1988;130:540–544. [Google Scholar]
- Reed R.A. Studies of the diederik cuckoo Chrysococcyx caprius in the Transvaal. Ibis. 1968;110:321–331. [Google Scholar]
- Rodriguez-Girones M.A, Zuniga J.M, Redondo T. Feeding experience and relative size modify the begging strategies of nestlings. Behav. Ecol. 2002;13:782–785. doi:10.1093/beheco/13.6.782 [Google Scholar]
- Schuetz J.G. Reduced growth but not survival of chicks with altered gape patterns: implications for the evolution of nestling similarity in a parasitic finch. Anim. Behav. 2005;70:839–848. doi:10.1016/j.anbehav.2005.01.007 [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelseth S, Moksnes A, Røskaft E, Gibbs H.L, Taborsky M, Taborsky B, Honza M, Kleven O. Parentage and host preference in the common cuckoo Cuculus canorus. J. Avian Biol. 2004;35:21–24. doi:10.1111/j.0908-8857.2004.03219.x [Google Scholar]
- West M.J, King A.P. Female visual displays affect the development of male song in the cowbird. Nature. 1988;334:244–246. doi: 10.1038/334244a0. doi:10.1038/334244a0 [DOI] [PubMed] [Google Scholar]
- Wyllie I. Batsford; London: 1981. The cuckoo. [Google Scholar]