Abstract
Studies of secondary sexual ornamentation and its maintenance by sexual selection tend to focus on males; however, females may also possess showy ornaments. For example, female mandrills possess facial coloration that ranges from black to bright pink. We used fortnightly photographs of 52 semi-free-ranging females aged above 3 years over 19 months to evaluate whether colour conveys information concerning female competitive ability, reproductive quality, age or reproductive status. Colour was not related to female rank or quality (body mass index, age at first birth or mean inter-birth interval); however, colour did increase significantly with age and primiparous females were darker than multiparous females. Colour may therefore signal reproductive quality, as younger females are less fertile and produce smaller offspring. Colour was brighter during the follicular phase than during the luteal phase, suggesting that it may signal fertility. Colour also varied across gestation and peaked at four and eight weeks post-parturition, suggesting that it may signal approaching parturition and lactation. Future studies should examine the relationship between colour and the menstrual cycle in more detail, the hormonal basis of female colour, and determine experimentally whether mandrills of both sexes attend to differences in colour between and within females.
Keywords: sexual skin, sexual selection, secondary sexual ornaments, signals
1. Introduction
Studies of secondary sexual ornamentation and their maintenance by sexual selection tend to focus on males (Andersson 1994); however, females may also possess showy ornaments. These have been assumed to represent a by-product, or ‘correlated response’, of selection for ornamentation in males (Lande 1980). However, a review of studies of female ornaments in birds concluded that there is strong evidence that female ornamentation is not severely constrained by selection on males, and that female ornaments are maintained via contest competition and mate choice (Amundsen 2000). This includes cases where females show a less developed version of male ornaments. If both sexes show mate choice, this may give rise to ornaments not only in the less choosy sex (generally males) but also in the choosy sex (generally females; Amundsen 2000).
Many primate species exhibit brightly coloured areas of skin. This is usually limited to red and blue sexual skin on the perineum, genitalia and adjacent areas (Dixson 1983); however, both male and female mandrills (Mandrillus sphinx) show red and blue coloration on the face as well as on the anogenital region. In male mandrills, individual variation in facial red coloration, but not blue, occurs with respect to age, dominance status and levels of circulating testosterone (Wickings & Dixson 1992; Setchell & Dixson 2001a,b, 2002). Female facial coloration is muted by comparison with males, but is highly variable between individuals: ranging from entirely black-faced to a bright pink nasal stripe with blue paranasal ridges. If the coloration serves as a secondary sexual ornament, as in males, then it may serve a signalling function in female contest competition or mate attraction, advertising individual quality in terms of body condition or reproductive quality (Andersson 1994). Such indicator models of sexual selection propose that the expression of secondary sexual traits is correlated with individual condition, and that only high quality individuals can maximally express such traits (Zahavi 1975; Hamilton & Zuk 1982). A non-competing alternative hypothesis is that female colour signals other information to conspecifics, such as reproductive status, including parity, stage of the reproductive cycle and fertile periods during the menstrual cycle.
Mandrills are found only in the dense rainforest of central Africa (Grubb 1973). They are thought to travel in large, semi-disaggregated bands in which males may not have direct knowledge of a female (Abernethy et al. 2002). Indicators may therefore be more important for this species than for other primates, such as macaques or baboons, in which males and females have more stable, longer-term relationships. Forest conditions mean that mandrills are extremely difficult to study and they have, so far, proved impossible to habituate in the wild (Harrison 1988; Abernethy et al. 2002). Most of our knowledge of mandrill reproduction and behaviour therefore comes from a semi-free-ranging colony at the Centre International de Recherches Médicales, Franceville, Gabon (CIRMF).
Studies of the CIRMF colony have shown that mandrill groups are made up of female matrilines (Setchell 1999), while multiple males vary in group association (Wickings & Dixson 1992; Setchell & Dixson 2001a). The alpha male has the most developed secondary sexual characters (Setchell & Dixson 2001a), is responsible for 77–100% of mate-guarding in any one breeding season and sires 33–100% of the offspring born in any one birth season (Setchell et al. 2005a). This reproductive skew results from intense male–male competition, reinforced by female preference for mating with brightly coloured males (Setchell 2005). However, mating is costly for males, in terms of time and energy invested and the risk of aggression from both other males (Setchell & Wickings 2006) and from females (Setchell et al. 2005b), and males also show mate choice, preferentially allocating mating effort to higher quality females (Setchell & Wickings 2006).
We examined red facial coloration in female mandrills to evaluate whether red colour conveys information concerning female reproductive quality, age, rank or reproductive status. If red colour acts as a reliable signal of female quality, then we predict that females with an earlier age at first birth (longer reproductive lifespan) and shorter inter-birth intervals (IBIs) should be brighter than females with later ages at first birth and longer IBIs. Similarly, females in better condition should be brighter than females in poor condition, mature females should be brighter than younger females and higher-ranking females should be brighter than lower-ranking females, because in each case the former females are more likely to be fertile and are more able to invest in offspring than the latter (Setchell et al. 2001; Setchell & Wickings 2004). If red colour indicates stages of the reproductive cycle, then we predicted that it should vary across the menstrual cycle, gestation and lactation. If red specifically indicates fertility, then females should be brighter during the follicular phase than during the luteal phase. Finally, we also examined whether female coloration varied with the sex of the offspring in pregnant and lactating females, because female faecal hormones are known to differ with the sex of the offspring during gestation (Altmann et al. 2004).
2. Material and methods
(a) Study population
The CIRMF mandrill colony was established in 1983/1984 when 15 animals (seven males, eight females, originating from the wild) were released into a 6.5 ha naturally rain-forested enclosure (E1). All further additions to the group are due to reproduction of the founder animals although some animals have been removed. A second semi-free-ranging group was established in 1994 (E2, 3.5 ha) by transferring 17 mandrills (including four adult males and six adult females) from the E1. In January 2004 there were 74 animals in E1 and 62 in E2. The animals forage freely in the enclosure and receive daily supplements of monkey chow and seasonal fruits. Water is available ad libitum. The date of birth is recorded for all individuals born into the colony, while the age of founder females was estimated using dental estimates when the animals arrived at CIRMF and their previous history. Photographic images and behavioural data for this study were collected between November 2003 and June 2005. All 52 females aged above 3 years at the beginning of the study contributed data (mean±s.e.m. age 9.7±1.0 yr, range 3.5–26.6 yr).
(b) Reproductive status
Mean gestation in mandrills is 175 days (25 weeks; Setchell et al. 2002), while mean duration of post-partum amenorrhoea following birth of a live infant is 208 days (30 weeks; Setchell & Wickings 2004). Pregnancy was divided into 12 two-week intervals and lactation into 15 two-week intervals for the 30 females that experienced gestation and lactation during the study, with the day of parturition termed day 0. Three pregnant females that aborted were excluded from analyses, as the exact date of the abortion was unknown. Females that displayed sexual swellings were termed cycling females and were subdivided into females in the follicular (sexual swelling increasing in size) and luteal (sexual swelling deflating) phases of the menstrual cycle. Insufficient data were available to examine coloration in females that were pregnant, lactating or cycling. Eighteen females in E2 were implanted with contraceptive implants (melengestrol acetate, provided by Contraception Advisory Group of the American Zoological Association).
(c) Reproductive history and dominance rank
We collated the age and reproductive history (age at first birth, parity and mean IBI following surviving offspring) of the subject females from colony records. It was not possible to investigate the relationship between swelling size and infant survival because mortality rates are very low in this semi-free-ranging population (90% survival to six months; Setchell et al. 2002). Rank relations between females in each enclosure were determined using ad libitum records of avoidance behaviour during daily observation periods. The number of females was similar in the two enclosures, meaning that female ranks were comparable in the two groups.
(d) Female condition
All subjects were captured and anaesthetized for an annual health control during the study (anaesthesia was accomplished using ‘Telinject’ blowpipe to deliver a syringe containing 10 mg kg−1 ‘Imalgene1000’ (Rhone-Mérieux, Lyon, France)). We measured body mass (nearest 100 g) and crown-rump length (using a metal ruler, nearest 0.5 cm). Body condition was calculated as (i) the body mass index (BMI; kg mass per m2 crown-rump length; Cole 1991) and (ii) a standardized residual of a regression of body size (mass) to skeletal size (crown-rump length; Jakob et al. 1996).
(e) Colour measurements
We attempted to measure female colour every two weeks. We obtained close-up digital images of female faces using a Nikon Coolpix 5700 digital camera with an 8×optical zoom and saved them as ‘fine’ quality JPEGs. All images were taken when females were in either an open grassy area or in an open feeding pen. A total of 1321 photographs were suitable for analysis. Images required calibration, to account for exposure and light drift (Gerald et al. 2001). It was impossible either to get animals in the same frame as a photographic white and black standard, or to place a standard in the same position as the animal and capture a second image immediately following that of the subject. We therefore used only images where colour ranged the full spectrum from white (the white ventral pelage) to black (all animals are black around the eyes), and used the ‘Autolevels’ command in Adobe Photoshop v. 6.0 (Image Mode set to RGB) to define the lightest and darkest pixels in each colour channel as white and black.
Once calibrated, images were analysed using Adobe Photoshop v. 6.0 in randomized order without information concerning reproductive status of the female. The midnasal strip was outlined in a standard fashion using the polygonal lasso tool. The mean luminosity and the red intensity value (the number of pixels at each intensity value) for the highlighted area were then measured using the ‘Image>histogram’ command. Dividing the value for ‘red’ by the luminosity resulted in the closest approximation to the red colour of a photograph. We concentrated on red coloration for two reasons. First, blue coloration is produced by ‘Tyndall scattering’ of light as it is reflected back through the epidermis by an underlying layer of dermal melanin (Dixson 1998) and does not reproduce satisfactorily in photographs. Second, studies of males have shown that red coloration is far more variable than blue and that blue is unrelated to changes in hormonal status (Setchell & Dixson 2001a,b).
(f) Statistical analyses
Where multiple photographs were available for a female at any stage of the reproductive cycle we calculated her mean coloration value for that stage. Data were not available for all females at each defined stage of the female reproductive cycle and no female contributed data at each stage, ruling out the use of repeated measures analyses. To investigate the influence of the female reproductive cycle on colour, we therefore used a generalized linear model (GLM) univariate procedure and included female ID as a random factor, to account for the influence of variation in coloration between individual females. The distribution of females contributing data was essentially random with respect to the time points, meaning that there should be no systematic bias due to the contribution of individual females. We used a paired t-test to compare coloration during the follicular and luteal phases of the menstrual cycle in individual females.
To examine the influence of female variables on female facial colour we calculated a mean residual for each female over all stages of the reproductive cycle. We used a GLM univariate procedure to investigate the effects of the covariates rank and age on these mean residuals (rank and age were not correlated in this sample, r=−0.049, p=0.731, n=52). BMI and the residual of a regression of body size to skeletal size increased significantly with age and we therefore examined the influence of these variables on mean red colour residual separately from age. Both age at first birth (n=34 females) and mean IBI (n=22 females) were co-linear with female rank, and the relationships between these variables and mean residual red coloration were therefore investigated separately, including the influence of age in the model. To evaluate the influence of the sex of an infant on coloration during gestation and lactation, we calculated a mean residual for each female during gestation and lactation and compared residuals for females carrying male and female infants, including maternal age as a covariate.
All statistical analyses were performed using SPSS v. 11.0. A priori predictions (hypothesized positive relationships between red coloration and other variables) were tested using one-tailed significance levels, denoted p1, all other tests were reported with two-tailed significance levels, p2. Means are quoted±standard errors.
3. Results
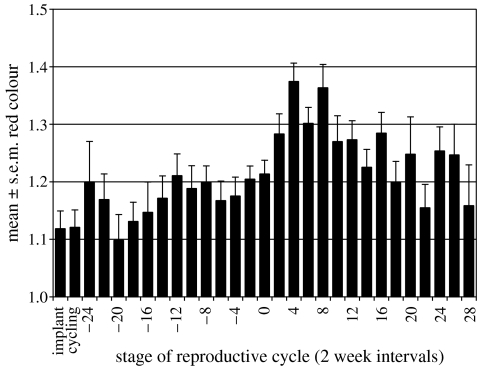
Red coloration score ranged from 0.75 (entirely black faced) to 1.88 (bright pink nasal strip; see electronic supplementary material). The mean for individual females across the study period ranged from 0.96 to 1.50 (mean 1.16, n=52). Female ID significantly influenced colour (F53,406=10.206, p2<0.001), implying that within-female variation was smaller than between-female variation in colour. Colour was significantly brighter during the follicular phase (1.19±0.02) than during the luteal phase (1.11±0.03, t6=2.030, p1=0.045). Stage of the reproductive cycle significantly influenced colour (F28,378=7.229, p2<0.001, figure 1). Implanted females were the same colour as cycling females. Colour increased in comparison to cycling females at the very beginning of gestation, fell again to a low at 20 weeks before parturition, then increased to previous levels by 12 weeks before parturition and remained stable. Colour increased to a peak at four and eight weeks post-parturition, when females were noticeably brighter than previously. This effect decreased by the end of lactation.
Figure 1.
Patterns of red coloration across the female reproductive cycle. 0=parturition.
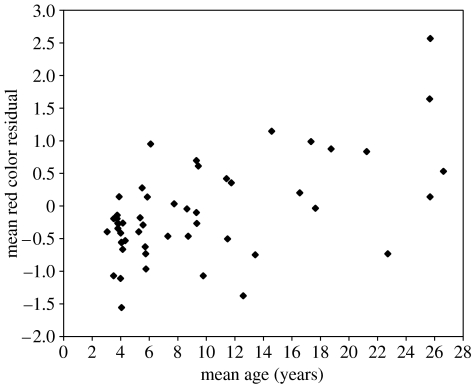
The mean colour residual for an individual female increased significantly with age (F1,48=23.928, p1<0.001, figure 2), and females that had not previously given birth (mean residual −0.59±0.12, n=11) were darker faced than those that were multiparous (0.14±0.14, n=25, t34=3.293, p1=0.002, excluding females with implants). Female colour was not related to rank (F1,49=0.108, p1=0.372), BMI (F1,50=1.133, p1=0.146), the residual of a regression of body size to skeletal size (F1,50=0.163, p1=0.344), age at first birth (F1,32=0.002, p1=0.483) or mean IBI (F1,19=0.662, p=0.213). Finally, pregnant mothers carrying male infants were somewhat brighter than mothers carrying female infants, but this difference was non-significant (F1,24=12.896, p=0.102). There was no difference in maternal colour during lactation between females suckling males and females (F1,26=1.265, p=0.315).
Figure 2.
Female mean colour residual versus mean age during the study.
4. Discussion
Facial colour does not appear to act as a ‘badge of status’, signalling competitive ability in terms of rank, in female mandrills. This is in contrast to males, where facial colour is clearly linked to dominance rank and signals fighting ability (Setchell & Dixson 2001a,b; Setchell & Wickings 2005). This difference between the sexes is not surprising because reproductive priorities in male and female primates are very different, and we can expect their ornaments to signal different information. Furthermore, whereas male rank is determined via contest competition, female rank is inherited matrilinearly in mandrills and is not related to body size (Setchell 1999). Female colour was also unrelated to female quality, in terms of either reproductive history or body condition, although caution should be applied when interpreting correlational relationships between body condition and sexually selected traits in the absence of experimental manipulation (Cotton et al. 2004).
Young females, and those that had not yet produced an offspring, were significantly darker than older and parous females. These relationships are due mostly to females brightening for the first few years following the birth of their first offspring. Females also continue to grow in mass and body length until the age of 7 years, and increase slowly in mass after this age (Setchell et al. 2001). Although the oldest females appeared to be brighter than younger full-grown females, this is likely to be an artefact of the small sample size of elderly females, as full-grown females do not change colour from year to year (J. M. Setchell 2006, unpublished data). Female colour may signal reproductive quality in this context, in that younger and nulliparous females are less fertile (Setchell & Wickings 2004) and produce smaller offspring (Setchell et al. 2001). However, older females can also be dark-faced, and many other cues exist that can inform males about the age and parity of females, including body size (Setchell et al. 2001) and the size of their sexual swelling (Setchell & Wickings 2003).
Colour was brighter during the follicular phase than during the luteal phase of the menstrual cycle, suggesting that colour may advertise fertility. This is in contrast to the bright pink skin on the neck and chest of female Theropithecus gelada and facial red colour in Macaca mulatta, which are poor predictors of the menstrual cycle (Baulu 1976; Dixson 1983). Colour in mandrills may act as a graded signal of fertility, representing the probability of ovulation, as proposed for sexual swellings (Nunn 1999). Under this hypothesis changing female ornaments allow females to manipulate male behaviour by altering the costs and benefits of mating effort, biasing paternity towards preferred males, but also confusing paternity certainty by mating with multiple males. Testing this hypothesis would require detailed hormonal data paired with colour measurements across the menstrual cycle. However, female mandrills also possess sexual swellings, and swelling size is likely to be a more obvious clue to males as to stage of the reproductive cycle and fertility than relatively small within-female changes in facial colour.
Red colour also signalled approaching parturition and peaked when females were nursing a dependent infant. Similar colour increases during pregnancy have been reported for the perineal sexual skin in other primates (Scruton & Herbert 1970; Fooden 1971; Rowell 1972). In Macaca mulatta these colour changes co-occur with increases in oestrogen across gestation (Bielert et al. 1976). However, patterns of red coloration in female mandrills are not simply linked to levels of circulating oestrogens. While red colour increases during gestation, in line with increases in oestrogen, colour peaked post-parturition when circulating levels of oestrogen rapidly decrease. Possible adaptive explanations for this peak in coloration include signalling the presence of a ventral infant to attract paternal care from candidate sires who will protect infants vulnerable to infanticide (van Schaik 2000; Buchan et al. 2003), which is known to occur in the CIRMF colony where sires are not present (Setchell et al. submitted).
The possible relationship between offspring sex and female colour is interesting. Trivers & Willard (1973) have suggested that parents should favour the production of sons when conditions are favourable and when variance in male reproductive success is higher than that of females, and honestly signalling the sex of offspring may be adaptive in this context. The polygymous mating system and extreme sexual dimorphism of mandrills predict that this hypothesis should apply to this species. However, the predictions of the Trivers–Willard hypothesis are not supported in mandrills, where neither birth sex ratio nor maternal investment vary with offspring sex (Setchell et al. 2001, 2002).
Our results suggest that female colour may signal information concerning female age, parity and reproductive status to conspecifics. It would now be of interest to examine whether conspecifics attend to differences in female colour. Experiments using attention as a means of measuring preferences show that females of other primate species prefer more brightly coloured males (Cooper & Hosey 2003; Waitt et al. 2003). Interestingly, male rhesus macaques (Macaca mulatta) appear to prefer females with redder hind-quarters, but show no such preference for redder-faced females, suggesting that the signal function of sexual skin may vary across anatomical regions (Waitt et al. in press).
Finally, within-female differences in colour were considerably smaller than between-female differences. The possibility remains that female red colour represents a genetically correlated response to selection on males (Lande 1980) and that there are no adaptive explanations for within-female changes in colour. As most of the genome is shared by males and females, females as well as males inherit the genetic basis for ornamentation. Unless consistent and strong selection acts to inhibit the expression of coloration in females, vestigial versions of male traits can be expected to occur in females that will reflect hormonal changes associated with reproduction. This may explain why female coloration is more muted than in adult males, although ranges overlap considerably. Future studies should therefore examine similarities and differences between hormone profiles and facial colour in males and females.
Acknowledgments
We thank CIRMF for making this study possible. We are grateful to O. Bourry, P. Bamba, P. Engandja, C. Mbou and other staff of the Primate Centre for their help during the study. CIRMF is financed by the Gabonese government, Total Gabon and the Ministère Français des Affaires Etrangères. Observations of the mandrill colony are financed by Leverhulme Trust project grant award no. F/01576/B. We thank two anonymous reviewers for their constructive comments on a previous version of the manuscript.
Supplementary Material
Females 16 (left, aged 23 years) and 6 (right, aged 26 years)
References
- Abernethy K.A, White L.J.T, Wickings E.J. Hordes of mandrills (Mandrillus sphinx): extreme group size and seasonal male presence. J. Zool. 2002;258:131–137. doi:10.1017/S0952836902001267 [Google Scholar]
- Altmann J, Lynch J.W, Nguyen N, Alberts S.C, Gesquiere L.R. Life-history correlates of steroid concentrations in wild peripartum baboons. Am. J. Primatol. 2004;64:95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- Amundsen T. Why are female birds ornamented? Trends Ecol. Evol. 2000;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. [DOI] [PubMed] [Google Scholar]
- Andersson M.Sexual Selection1994Princeton University Press; Princeton, NJ [Google Scholar]
- Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm. Behav. 1976;7:495–507. doi: 10.1016/0018-506x(76)90019-2. doi:10.1016/0018-506X(76)90019-2 [DOI] [PubMed] [Google Scholar]
- Bielert C, Czaja J.A, Eisele S, Scheffler G, Robinson J.A, Goy R.W. Mating in the rhesus monkey (Macaca mulatta) after conception and its relationship to oestradiol and progesterone levels throughout pregnancy. J. Reprod. Fertil. 1976;46:179–182. doi: 10.1530/jrf.0.0460179. [DOI] [PubMed] [Google Scholar]
- Buchan J.C, Alberts S.C, Silk J.C, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179. doi: 10.1038/nature01866. doi:10.1038/nature01866 [DOI] [PubMed] [Google Scholar]
- Cole T.J. Weight-stature indices to measure underweight, overweight and obesity. In: Himes J.H, editor. Anthropometric assessment of nutritional status. Wiley Liss; New York, NY: 1991. pp. 83–111. [Google Scholar]
- Cooper V.J, Hosey G.R. Sexual dichromatism and female preference in Eulemur fulvus subspecies. Int. J. Primatol. 2003;24:1177–1188. doi:10.1023/B:IJOP.0000005986.21477.ad [Google Scholar]
- Cotton S, Fowler K, Pomiankowski A. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. B. 2004;271:771–783. doi: 10.1098/rspb.2004.2688. doi:10.1098/rspb.2004.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson A.F. Observations on the evolution and behavioural significance of ‘sexual skin’ in female primates. Adv. Study Behav. 1983;13:63–106. [Google Scholar]
- Dixson A.F.Primate sexuality: comparative studies of the prosimians, monkeys, apes and human beings1998Oxford University Press; Oxford, UK [Google Scholar]
- Fooden J. Female genitalia and taxonomic relationships of Macaca assamensis. Primates. 1971;12:63–73. doi:10.1007/BF01730382 [Google Scholar]
- Gerald M.S, Bernstein J, Hinkson R, Fosbury R.A.E. Formal method for objective assessment of primate color. Am J. Primatol. 2001;53:79–85. doi: 10.1002/1098-2345(200102)53:2<79::AID-AJP3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Grubb P. Distribution, divergence and speciation of the drill and mandrill. Folia Primatol. 1973;20:161–177. doi: 10.1159/000155574. [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Harrison M.J.S. The mandrills in Gabon's rain forest: ecology, distribution and status. Oryx. 1988;22:218–228. [Google Scholar]
- Jakob E.M, Marshall S.D, Uetz G.W. Estimating fitness: a comparison of body condition indices. Oikos. 1996;77:61–67. [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Nunn C.L. The evolution of exaggerated sexual swellings in primates and the graded signal hypothesis. Anim. Behav. 1999;58:299–246. doi: 10.1006/anbe.1999.1159. doi:10.1006/anbe.1999.1159 [DOI] [PubMed] [Google Scholar]
- Rowell T.E. Female reproductive cycles and social behavior in primates. Adv. Study Behav. 1972;4:69–105. [Google Scholar]
- Scruton D.M, Herbert J. The menstrual cycle and its effect upon behaviour in the talapoin monkey (Miopithecus talapoin) J. Zool. 1970;162:419–436. doi: 10.1111/j.1469-7998.1975.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Setchell, J. M. 1999 Socio-sexual development in the male mandrill (Mandrillus sphinx) Ph.D. thesis, University of Cambridge, Cambridge, UK.
- Setchell J.M. Do female mandrills (Mandrillus sphinx) prefer brightly coloured males? Int. J. Primatol. 2005;26:713–732. doi:10.1007/s10764-005-5305-7 [Google Scholar]
- Setchell J.M, Dixson A.F. Arrested development of secondary sexual adornments in subordinate adult male mandrills (Mandrillus sphinx) Am. J. Phys. Anthropol. 2001a;115:245–252. doi: 10.1002/ajpa.1079. doi:10.1002/ajpa.1079 [DOI] [PubMed] [Google Scholar]
- Setchell J.M, Dixson A.F. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm. Behav. 2001b;39:177–184. doi: 10.1006/hbeh.2000.1628. doi:10.1006/hbeh.2000.1628 [DOI] [PubMed] [Google Scholar]
- Setchell J.M, Dixson A.F. Developmental variables and dominance rank in male mandrills (Mandrillus sphinx) Am J. Primatol. 2002;56:9–25. doi: 10.1002/ajp.1060. [DOI] [PubMed] [Google Scholar]
- Setchell J.M, Wickings E.J. Sexual swellings in mandrills (Mandrillus sphinx): a test of the reliable indicator hypothesis. Behav. Ecol. 2003;15:438–445. doi:10.1093/beheco/arh027 [Google Scholar]
- Setchell J.M, Wickings E.J. Social and seasonal influences on the reproductive cycle in female mandrills (Mandrillus sphinx) Am. J. Phys. Anthropol. 2004;125:73–84. doi: 10.1002/ajpa.10375. doi:10.1002/ajpa.10375 [DOI] [PubMed] [Google Scholar]
- Setchell J.M, Wickings E.J. Dominance, status signals and coloration in mandrills (Mandrillus sphinx) Ethology. 2005;111:25–50. doi:10.1111/j.1439-0310.2004.01054.x [Google Scholar]
- Setchell J.M, Wickings E.J. Mate choice in male mandrills. Ethology. 2006;112:91–99. doi:10.1111/j.1439-0310.2006.01128.x [Google Scholar]
- Setchell J.M, Lee P.C, Wickings E.J, Dixson A.F. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx) Am. J. Phys. Anthropol. 2001;115:349–360. doi: 10.1002/ajpa.1091. doi:10.1002/ajpa.1091 [DOI] [PubMed] [Google Scholar]
- Setchell J.M, Lee P.C, Wickings E.J, Dixson A.F. Reproductive parameters and maternal investment in mandrills (Mandrillus sphinx) Int. J. Primatol. 2002;23:51–68. doi:10.1023/A:1013245707228 [Google Scholar]
- Setchell J.M, Charpentier M, Wickings E.J. Mate-guarding and paternity in mandrills (Mandrillus sphinx): factors influencing monopolisation of females by the alpha male. Anim. Behav. 2005a;70:1105–1120. doi:10.1016/j.anbehav.2005.02.021 [Google Scholar]
- Setchell J.M, Knapp L.A, Wickings E.J. Violent coalitionary attack by female mandrills against an injured alpha male. Am. J. Primatol. 2005b;68:411–418. doi: 10.1002/ajp.20234. doi:10.1002/ajp.20234 [DOI] [PubMed] [Google Scholar]
- Setchell, J. M., Wickings, E. J. & Knapp, L. A. Submitted. Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank and group association. [DOI] [PubMed]
- Trivers R.L, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P. Infanticide by male primates: the sexual selection hypothesis revisited. In: van Schaik C.P, Janson C.H, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge, UK: 2000. pp. 27–60. [Google Scholar]
- Waitt C, Little A.C, Wolfensohn S, Honess P, Brown A.P, Buchanan-Smith H.M, Perret D.I. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc. R. Soc. B. 2003;270(Suppl. 2):S144–S146. doi: 10.1098/rsbl.2003.0065. doi:10.1098/rsbl.2003.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt, C., Gerald, M. S., Little, A. C. & Kraiselburd, E. In press. Selective attention toward female secondary sexual color in male rhesus macaques. Am. J. Primatol [DOI] [PubMed]
- Wickings E.J, Dixson A.F. Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx) Physiol. Behav. 1992;52:909–916. doi: 10.1016/0031-9384(92)90370-h. doi:10.1016/0031-9384(92)90370-H [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection—a selection for handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Females 16 (left, aged 23 years) and 6 (right, aged 26 years)