Abstract
Young birds and mammals are extremely vulnerable to predators and so should benefit from responding to parental alarm calls warning of danger. However, young often respond differently from adults. This difference may reflect: (i) an imperfect stage in the gradual development of adult behaviour or (ii) an adaptation to different vulnerability. Altricial birds provide an excellent model to test for adaptive changes with age in response to alarm calls, because fledglings are vulnerable to a different range of predators than nestlings. For example, a flying hawk is irrelevant to a nestling in a enclosed nest, but is dangerous to that individual once it has left the nest, so we predict that young develop a response to aerial alarm calls to coincide with fledging. Supporting our prediction, recently fledged white-browed scrubwrens, Sericornis frontalis, fell silent immediately after playback of their parents' aerial alarm call, whereas nestlings continued to calling despite hearing the playback. Young scrubwrens are therefore exquisitely adapted to the changing risks faced during development.
Keywords: alarm calls, vocal communication, predation, fledglings, altricial birds
1. Introduction
In animals with parental care, young may reduce the risk of predation by responding appropriately to parental alarm calls (Davies et al. 2004; Madden et al. 2005a,b). An appropriate response to alarm calls is particularly important to young animals because of their vulnerability to attack and often inability to identify predators (Seyfarth & Cheney 1980; Kullberg & Lind 2002). In addition to thwarting current predators, alarm calls can be important in facilitating learning, so that predators can be recognized in future (Griffin 2004). Playback experiments on mammals and birds show that young can respond appropriately to alarm calls as a result of an innate recognition of conspecific alarms, learning or a combination of the two (Hauser 1988; Miller & Hicinbothom 1991; McCowan et al. 2001; Davies et al. 2004; Griffin 2004; Madden et al. 2005a,b).
Young might be selected to respond to alarm calls differently from adults, either because they are more vulnerable to predators than adults or because they are vulnerable to different predators. However, it has proved difficult to show whether ontogenetic change in response to alarm calls is due to constraint or adaptation. For example, young mammals generally show a gradual convergence on adult responses, over weeks or months, suggesting imperfect responses due to developmental constraints, including time required to learn appropriate responses (Seyfarth & Cheney 1980; Herzog & Hopf 1984; Hauser 1988; Mateo 1996; McCowan et al. 2001). Some changes with age might reflect adaptations to different risks, but the evidence is indirect. For example, juvenile California ground squirrels (Spermophilus beecheyi) usually fled to cover after chatter alarms, given to predators at some distance, whereas adults usually just scanned for danger. The difference might reflect the juveniles' risk of infanticide from other adults, because chatters are also given during agonistic interactions, which can prove lethal to juveniles (Hanson & Coss 2001). Similarly, juvenile bonnet macaques (Macaca radiata) were more likely than adults to flee after playback of both alarm calls and control sounds, which might be adaptive if juveniles are vulnerable to a greater range of threats, but might simply reflect inexperience (Ramakrishnan & Coss 2000).
Altricial birds provide a powerful yet unexploited model to study adaptive changes in alarm call response because they face an abrupt change in life style at fledging that can expose them to new predators, with little opportunity to learn an appropriate response before leaving the nest. For example, young great tits (Parus major) become exposed to sparrowhawks (Accipiter nisus), their major aerial predator, only after they acquire flight and leave their nest (Newton & Marquiss 1982; Gosler 1993). Great and coal tits (Parus ater) then suffer 5–10% mortality in the first 4 days after leaving the nest, with over 80% of deaths probably caused by predation (Naef-Daenzer et al. 2001). Because fledging occurs at a predictable age in altricial birds, one can test for adaptive timing of development of an appropriate response to aerial alarm calls. Despite this explicit prediction, we are aware of no experiments comparing the responses of nestlings and recent fledglings.
Experimental playbacks to white-browed scrubwrens (Sericornis frontalis) suggest that nestlings respond adaptively by falling silent only to alarm calls indicating danger specifically to nestlings (Platzen & Magrath 2004, 2005). Scrubwrens build domed nests, with a side entrance, usually placed on the ground under leaf litter or low vegetation (Higgins & Peter 2002). The nestlings, and usually the nest, are invisible from above, but accessible to predators on the ground. Consistent with their vulnerability, nestlings responded with silence to playback of parental ‘buzz’ alarm calls, which are mobbing calls that indicate a predator perched or on the ground near the nest, but usually continued calling after playback of ‘trill’ calls, which are aerial alarm calls that are given when raptors or large omnivorous birds are in flight (Platzen & Magrath 2004, 2005; Leavesley & Magrath 2005). Aerial alarms can be given to predators flying even 10–20 m or more away and a predator in flight is unlikely to overhear nestling peep calls, which have a mean of about 31±4 s.d. dB at 50 cm from the nest entrance (n=21 peep calls from seven broods; R. D. Magrath, J. Scarl & E. Parks 2002, unpublished data). In contrast to nestlings, adults become silent and flee for cover after playback of trill alarm calls (Leavesley & Magrath 2005; §2). Playback experiments were carried out when the nestlings were 5, 8 and 11 days old, which covered the bulk of the 15 day nestling period (Magrath et al. 2000). Overall, the nestlings' responses to alarm calls appear adaptively related to their specific risks.
Although the difference in the response of nestling scrubwrens to the two types of alarm call seems related to risk, the key prediction of adaptive development is that young should respond to aerial alarm calls as soon as they become vulnerable to predators in flight; that is, once they have fledged. By contrast, if nestlings fail to respond to aerial alarms merely because of developmental constraints or inadequate time to learn, one would expect a gradual development of the adult response that is not specifically timed to fledging. By analogy with research on mammals, this might take weeks or months (Hauser 1988; Mateo 1996). In this paper, we test the prediction that, in contrast to nestlings, recently fledged scrubwrens should fall silent in response to aerial alarm calls. Testing this prediction raises the challenge of studying fledglings without disturbing either the young or their parents, designing a playback technique that can be used on both nestlings and mobile fledglings and ensuring that both nestlings and fledglings receive alarm call playbacks of equal amplitude despite their different acoustic environments.
2. Material and methods
(a) Study site and species
We studied a colour-marked population of scrubwrens in the Australian National Botanic Gardens, Canberra (Magrath 2001). Scrubwrens are small (ca 13–14 g) passerines in the family Acanthizidae (Schodde & Mason 1999). Nestlings fledge after a mean of 15±1.1 s.d. days and are fed by adults for a further 46±5.7 days (Magrath et al. 2000). In the first week after leaving the nest, fledglings remain together, usually perched in low bushes, with occasional flights from one site of cover to another (Higgins & Peter 2002).
Young scrubwrens have three common call types, one of which first appears around the time of fledging (Higgins & Peter 2002; Maurer et al. 2003). ‘Whines’ are long, broadband begging calls given when parents arrive with food. ‘Peeps’ are short, high frequency (ca 7 kHz), narrow band calls, which are given after feeding and often when parents are absent. Peep rates increase with hunger (Maurer et al. 2003) and a brood of nestlings in the wild can give up to about three peeps per second when the adults are absent (Platzen & Magrath 2004). Fledglings alone also give ‘pipe’ calls that are structurally similar to peeps but louder (about 60 dB at 1 m) and usually given in groups of 2–7 rapidly delivered elements (R. D. Magrath, unpublished data). To the human ear, they can often be heard from 30 m or more.
Scrubwren adults give their trill aerial alarm call when predatory birds are in flight (Leavesley & Magrath 2005). These are high pitched, narrow-band calls (peak frequency 7–8 kHz; range 6–10 kHz), given at the study site particularly to accipiter hawks (Accipiter spp.), pied currawongs (Strepera graculina) and laughing kookaburras (Dacelo novaeguineae; Leavesley 2003). A greater number of elements in the trill call encodes greater danger and playback of multi-element calls prompts adults to flee for cover and go silent (Leavesley & Magrath 2005). The aerial alarms contrast with broadband buzz mobbing alarms (peak frequency 6–8 kHz; range 3–12 kHz) given to potential predators near the nest, either on the ground or perched nearby, including snakes, large lizards, humans, cats and omnivorous or predatory birds (Higgins & Peter 2002; Maurer et al. 2003; Platzen & Magrath 2004, 2005; unpublished data). The contrasting acoustic features of these calls are typical of avian aerial and mobbing alarm calls (Marler 1955; Bradbury & Vehrencamp 1998).
Fledglings suffer a mortality of 5.1% per day in the week after fledging (n=638 fledglings from 257 broods), with most losses probably caused by avian predators (Leedman & Magrath 2003). Pied currawongs (S. graculina), which are large omnivorous birds, are abundant and a major predator of fledglings at the study site; 21 fledglings have been seen taken by currawongs or their leg bands found in regurgitated pellets (Prawiradilaga 1996; R. D. Magrath unpublished data). Collared sparrowhawks (Accipiter cirrhocephalus) and kookaburras are also likely predators. Nestling broods suffer a mortality of 4.4% per day (n=359 broods hatched), with predation the major cause of failure (Magrath & Yezerinac 1997). Nestlings are taken by pied currawongs, hunting on the ground, mammals and probably snakes and large lizards.
(b) Playback experiments
We broadcast recordings of parental aerial alarm calls to nestlings and then again to fledglings, while simultaneously recording vocalizations of the young. We carried out playbacks midway through the nestling period (mean 7.7 days; range 7–9), and just after the young fledged (mean 2.8 days; range 2–4), from October to December 2003. We chose 7–9 days for nestling playbacks because broods needed to be banded when 9 days old and we wanted to avoid playbacks shortly after handling the brood. Furthermore, we wished to avoid placing a microphone at the nest of old nestlings in case it prompted fledging; young have fledged naturally as young as 12 days old (Magrath et al. 2000). The experiment was based on a matched-pairs design, in which we broadcast alarm calls to nestlings and fledglings from the same 10 social groups. In 8/10 cases, the playbacks were to the same brood. In the remaining two cases, playbacks were to different broods raised by the same group. One brood died before fledging, so we used fledglings from the next brood; in the other, parental alarm calls were not available for nestlings of the first brood, so we used nestlings from the next. Whether the playbacks were carried out to one or two broods of a group had no influence on the results. In addition to these matched data, we performed playbacks solely on fledglings in six groups. These groups were excluded from the main analyses but were used to test whether the fledglings' responses were influenced by prior exposure to playbacks.
The playback alarm call consisted of trill elements recorded from the brood's own parents (figure 1a). Thus every brood received a unique playback, avoiding the problem of pseudoreplication (Kroodsma et al. 2001), and the potential problem of unfamiliar adult calls (Blumstein et al. 2004). We cut two or three high-quality elements from original recordings, and repeated elements at natural intervals to compose six element alarm calls, which are typical of those given by parents to predators flying near the nest (Platzen & Magrath 2005), and would prompt adults to fly immediately to cover (Leavesley & Magrath 2005). Recordings were filtered to remove sound below 2 kHz and broadcast at a natural amplitude of 60 dB, 4 m from the speaker (Leavesley & Magrath 2005). Control playbacks consisted of 16 different recordings of ‘bell’ calls of crimson rosellas, Platycercus elegans (Higgins 1999), broadcast at the same amplitude (figure 1b). Rosellas are abundant parrots that call frequently but do not interact with scrubwrens. We filtered out sounds below 1.5 kHz, which avoids clipping lower-frequency elements, and composed playbacks with a mean of six elements. Bell call elements are longer and more variable than trill call elements, so the mean duration of the control playback was longer than the alarm playback (1255±278 s.d. ms versus 808±75 ms), and the number of elements ranged from 4 to 8. All recordings were made using a Sennheiser ME66 or ME67 directional microphone and Sony TCD-D100 DAT recording at 44.1 kHz. Recordings were downloaded onto a Macintosh computer and edited in Canary 1.2.4 (Charif et al. 1995). Reference recordings of known amplitude, measured with a Bruel and Kjaer 2205 sound level metre re 1 pW m−2, were used to calibrate playbacks and nestling calls for analysis.
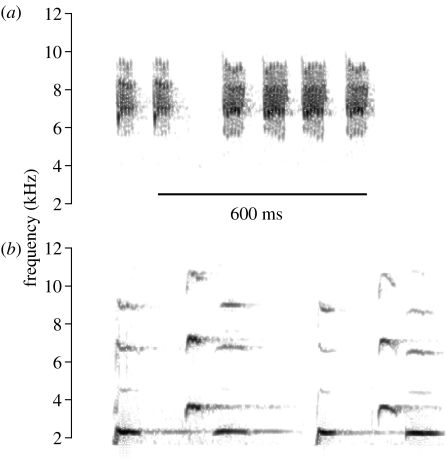
Figure 1.
Spectrograms of playback sounds used for one brood, showing (a) a six element scrubwren aerial alarm call and (b) a six element rosella ‘bell’ call. Spectrograms were created using a filter bandwidth of 684 Hz, frame length 5.81 ms and grid resolution of 0.36 ms and 86.1 Hz with 94% overlap. Each brood received a unique example of each type of call (§2).
Playbacks were conducted using a Sony CD Walkman D-EJ751 connected via an amplifier and a 15 m cable to a Response Dome Tweeter speaker (1.5–20 kHz) mounted on a tripod facing the young. The speaker was 7.0±1.4 m (mean±s.d.) from the fledglings and 1.3±0.3 m above the ground. The speaker was deliberately placed slightly closer to the nestlings, at a distance of 5.8±1.1 m (below), at the same height. The variability in speaker placement at each age was due to constraints of topography and vegetation. Playback of the alarm call and control was carried out from the same location, or in the three cases when fledglings moved, from a similar distance. Once the equipment was in place, we waited until the young had been fed at least once before conducting any playback. Playbacks were carried out when the young were giving peep calls, and the parents were well clear (at least 10 m) and not vocalizing. The second playback was broadcast after at least one parental feeding visit or 10 min had elapsed. The order of playbacks was alternated between broods.
We recorded young during playbacks with a Sony TCD-D100 DAT, using an Audio-technica ATM15a condenser microphone placed 16 cm from the nest entrance for nestlings and a Sennheiser ME66 directional microphone placed on a tripod 3.7±2.2 m from fledglings, which was as close as we could get without disturbing fledglings or adults.
We compared the brood's vocalizations in a 10 s period before and after playback of the alarm call compared to the control. We measured the (i) total number, (ii) mean duration and (iii) mean amplitude of calls. Young gave only peep calls because the adults were absent during playbacks and piping calls were relatively infrequent. We also measured the number and duration of calls given by nestlings during the alarm call playback itself and in the period immediately before the playback corresponding the duration of the playback. Periods ranged from 704 to 908 ms. We did this to assess whether the nestlings ceased calling during the playback itself, indicating they had heard the playback.
A potential problem with the playback experiment is that nestlings and fledglings live in different acoustic environments, so we carried out a second experiment to test whether nestlings and fledglings experienced the same sound levels. We anticipated some additional attenuation of calls to nestlings, which are lower and in enclosed nests and so placed the speaker about 20% closer than to fledglings. During the second set of playbacks, we put the speaker in the same position it had been during the playback to young, and placed a lapel microphone in the nest, or ‘perched’ on a branch where the fledglings had been. We then carried out the playbacks as before and measured amplitude using calibrated files in Canary.
Scrubwrens naturally give aerial alarm calls about 4.8 times per hour (Leavesley & Magrath 2005), so it is unlikely that playback of two additional alarms would cause detectable stress. We protected all nests with a ‘cage’ of 50 mm green wire mesh, which allowed access by parents but excluded large predators. Cages were installed during the incubation period when the female was off the nest and appeared to cause no lasting disturbance. They had the ethical benefit of thwarting predators that might have been alerted to the nest during experiments, and the practical benefit of ensuring that most broods fledged. After placing the speaker and microphone we remained about 15 m clear of young, which appeared to cause no disturbance to adults or young.
(c) Statistical analyses
The analyses were based on a matched-pairs design. We used Wilcoxon Matched Pairs Sign Ranks tests to compare calls given before and after playbacks and used the difference after-before to test for a response to alarm calls compared with controls. In many cases broods fell silent after playback and so we used a McNemar test to analyse the dichotomous outcome of whether broods continued to call or not. We used repeated measures ANOVA for the second experiment on sound attenuation, as the residuals were normally distributed. We used 2-tailed tests except when testing the directional hypothesis that fledglings would reduce calling to alarm calls more than nestlings. Statistical procedures were carried out in SPSS 10.0 (Norusis 2000), in consultation with Siegel & Castellan (1988).
3. Results
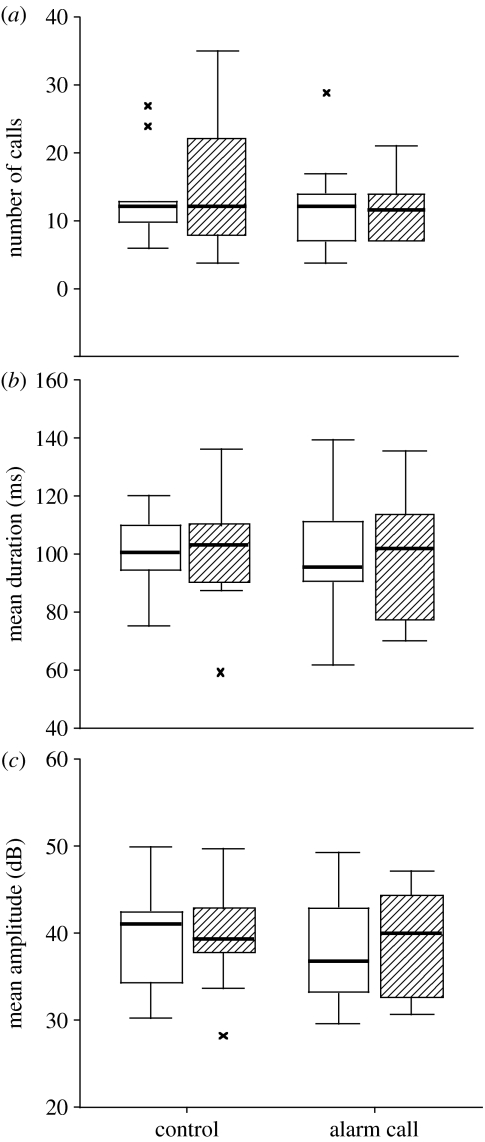
The results supported our prediction that recent fledglings would fall silent after playback of aerial alarm calls. All but one of 10 broods of fledglings fell completely silent after playback of the aerial alarm call, but none did so after playback of the control (figure 2; McNemar exact 1-tailed p=0.002). By contrast, nestlings continued to give peep calls in all 10 broods after playback of both aerial alarm calls and controls (figure 2). This response by fledglings differed significantly from the lack of response by nestlings (McNemar exact 1-tailed p=0.002). Furthermore, there was no effect of the alarm call on the number of nestling calls, their duration or amplitude (figure 3; Wilcoxon Signed Ranks Tests: number, Z=0.66, p=0.5; mean duration, Z=0.26, p=0.8; mean amplitude, Z=0.56, p=0.6).
Figure 2.
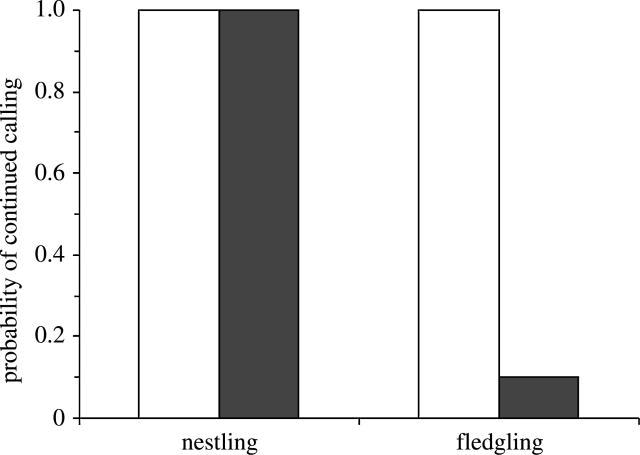
The probability of continued calling by nestling and fledgling scrubwrens after playback of control sounds (open bars) or parental aerial alarm calls (black bars); n=10 groups.
Figure 3.
The response of nestlings to playback: (a) the number, (b) mean duration and (c) mean amplitude of ‘peep’ calls given by nestlings in a 10 s period before (open bars) and after (shaded bars) playback of control sounds or aerial alarm calls. The figure shows box plots in which the heavy line is the median, the boxes show the inter-quartile range and crosses are more extreme values; n=10 groups.
The different response of fledglings and nestlings to alarm calls was not a consequence of fledglings having had prior exposure to the alarm call playback. Overall, eight broods of fledglings had heard the alarm call playback as nestlings and eight had not. The latter group includes the six groups whose nestlings never received playback and the two groups in which playback to nestlings and fledglings involved different broods. In each case 7/8 broods of fledglings fell silent after playback of alarm calls, showing that prior exposure had no effect.
The greater response by fledglings was not due to reduced attenuation of playbacks compared to nestlings. There was no difference in the amplitude of playbacks re-recorded in the nest compared to where the fledglings had been (means±s.e. dB; nest: control 51.1±1.12, alarm 51.4±1.45; fledgling site: control 50.0±1.35, alarm 50.7±0.78; repeated measures ANOVA: playback type, F1,18=0.36, p=0.6; brood age, F1,18=0.34, p=0.6; interaction, F1,18=0.05, p=0.8). Furthermore, although nestlings continued calling at the same rate in the 10 s after alarm call playbacks compared to the 10 s before, they usually went silent during the alarm call itself. We tested for a pause in vocalization by comparing calling in the short period before and during the playback corresponding the duration of the playback itself (§2). Overall, seven broods called before but not during playback, none did the opposite, two called both before and during and one called in neither period (McNemar, 2-tailed p=0.02). Nestlings clearly heard the alarm playback but resumed calling.
4. Discussion
Our study supports the idea of adaptive ontogenetic change in response to alarm calls. In contrast to nestlings, recently fledged scrubwrens responded with silence to aerial alarm calls, coinciding with becoming vulnerable to aerial attack. The results therefore complement previous work on the response by scrubwren nestlings to different alarm calls (§1). The differential response by nestlings to alarm calls signalling different risk and the change in response to aerial alarm calls with changing exposure, both support the hypothesis of adaptive response to parental alarm calls. Together the results suggest the response to alarm calls by young is exquisitely adapted to current need rather than resulting merely from imperfect stages of development, and adds to other studies showing adaptive changes around fledging in communication in altricial birds (Beecher et al. 1981a; Loesche et al. 1991; Insley et al. 2003).
Suppression of calling by fledglings probably reduces the risk of being overheard by predators, as falling silent is a common response to alarm calls for both adult and nestling birds (Klump & Curio 1983; Davies et al. 2004; Madden et al. 2005a,b), and playback of nestling begging calls from artificial nests can attract predators (Haskell 1994; Leech & Leonard 1997). Eavesdropping by predators might be an even greater problem after fledging, when young can develop loud calls to enable location and recognition by parents (Beecher et al. 1981a,b; §2). Eavesdropping may even cause the largely unexplained pattern in avian ecology that young underweight at fledging are more likely to die before reaching independence (Perrins 1965; Magrath 1991), assuming that hungrier young call more conspicuously (Naef-Daenzer et al. 2001; Götmark 2002).
The adaptive timing of acquisition of the response to alarm calls by scrubwrens is consistent with studies of other bird species. Great tit nestlings continued calling after playback of an aerial ‘seeet’ alarm call when 10 days old (Rydén 1982), yet suppressed calling at 16–18 days old, just before they would normally fledge at 17–21 days old (Rydén 1978). However, there was no playback to fledglings or replication of playback stimuli. The late development of a response to aerial alarm calls in great tits contrasts with the immediate response of hatchling precocial birds, which are exposed to predators upon hatching (Impekoven 1976; Miller & Blaich 1986). The freezing response to alarm calls of hatchling mallard ducklings (Anas platyrhynchos) is an adaptation specifically when the young are still in the nest during their first 24 h, but wanes by 72 h, after the young have left the nest (Miller & Hicinbothom 1991). Despite the decline of the freezing response, ducklings one week old still show an increase in heart beat rate after alarm calls (Evans & Gaioni 1990), suggesting adaptive timing of response to alarm calls, not simply reduced attention to alarm calls.
The mechanism of development of a response to alarm calls in altricial birds is largely unknown, but both maturation and learning have roles. Cross-fostering experiments suggest that adaptive response to alarm calls does not necessarily require prior exposure. Dunnock (Prunella modularis), robin (Erithacus rubecula) and red-winged blackbird (Agelaius phoeniceus) nestlings responded to their own species' alarm call regardless of whether they were raised by another species (Davies et al. 2004; Madden et al. 2005a,b). Nonetheless, nestlings responded less to conspecific alarms when raised by foster species, so experience modifies the response. Distinguishing the relative importance of maturation, experience and learning in the alarm call response remains an exciting challenge.
Another challenge is to examine the sensory or neurological basis for any innate ontogenetic changes in response to alarm calls. One possibility is that the sensory system is tuned to specific frequencies or features of sound and this could change adaptively with age. For example, some passerines are able to hear only low frequencies when very young (Khayutin 1985), so sensory development may cause a change in response to high frequency calls. In this case, there could be adaptive variation in the timing of development of sensory ability and parental use of specific alarm calls. Given that aerial alarm calls are often of higher frequency than mobbing calls (Bradbury & Vehrencamp 1998), this may be a mechanism in some species causing young to respond to aerial alarm calls at the time of fledgling. Another possibility, which appears likely in scrubwrens, is that higher-order neural processing controls the appropriate response to specific calls. Scrubwren nestlings paused during playback of aerial alarm calls, showing that they can hear these calls before they start to respond by suppressing calling. Thus scrubwrens, like mallard young, appear to change their response independently of sensory ability.
We are unable to predict the precise time course in the development of the response to aerial alarms in scrubwrens. The timing of response might be affected by developmental age and experience as a nestling or fledging itself might ‘activate’ the response. The shape of the response curve is therefore likely to depend on underlying mechanisms, as well costs and benefits of responding at different ages. By contrast, one can predict that regardless of mechanism an adaptive change in response should develop by or around fledging, so our result that recent fledglings respond with silence supports the clearest prediction about timing. Further progress on mechanisms would require playbacks at different ages, probably on many different broods to avoid habituation, together with experimental ‘fledging’ and physiological experiments.
Although it is beneficial for fledglings to heed aerial alarm calls, why do nestlings ignore them? Clearly there is no benefit to responding to irrelevant alarms, such as may be the case with aerial alarms in species with enclosed nests, but there also may selection against responding with silence if it reduces success in competition for food with siblings (Leonard et al. 2005). This could occur either because a parent can continue to feed a brood when the other is giving an irrelevant alarm, or because young mistakenly respond to similar calls associated with feeding. The problem of recognition error is exacerbated in nestling birds because speed of response can be important in gaining food from parents (Leonard et al. 2005). In scrubwrens, the ‘chip’ contact call is often given when approaching the nest and is of similar frequency to the aerial alarm call (Platzen & Magrath 2005), and provisioning calls given by adults at the nest can contain high-frequency trill-like elements (Platzen 2004). Thus young may be selected to ignore trill-like calls until fledging, when such disregard could be lethal.
Acknowledgments
We thank Bob Phillips, Jim Bishop and Alan Muir for help with audio equipment, Adam Leavesley for help recording alarm calls and Andy Horn, Michael Jennions, Naomi Langmore, Ben Pitcher and anonymous referees for comments on a draft of this manuscript. The research was funded by a grant from the Australian Research Council and carried out under permits from the Australian National Botanic Gardens, Environment ACT and the Ethics Committee of the Australian National University.
References
- Beecher M.D, Beecher I.M, Hahn S. Parent–offspring recognition in bank swallows (Riparia riparia): II. Development and acoustic basis. Anim. Behav. 1981a;29:95–101. doi:10.1016/S0003-3472(81)80156-X [Google Scholar]
- Beecher M.D, Beecher I.M, Lumpkin S. Parent–offspring recognition in bank swallows (Riparia riparia): I. Natural history. Anim. Behav. 1981b;29:86–94. doi:10.1016/S0003-3472(81)80155-8 [Google Scholar]
- Blumstein D.T, Verneyre L, Daniel J.C. Reliability and the adaptive utility of discrimination among alarm callers. Proc. R. Soc. B. 2004;271:1851–1857. doi: 10.1098/rspb.2004.2808. doi:10.1098/rspb.2004.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J.W, Vehrencamp S.L. Sinauer; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Charif R.A, Mitchell S, Clark C.W. Canary 1.2 user's manual. Cornell Laboratory of Ornithology; Ithaca, NY: 1995. [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. B. 2004;271:2297–2304. doi: 10.1098/rspb.2004.2835. doi:10.1098/rspb.2004.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.S, Gaioni S.J. Conspecific calls evoke characteristic cardiac responses in mallard ducklings. Anim. Behav. 1990;39:785–796. doi:10.1016/S0003-3472(05)80390-2 [Google Scholar]
- Gosler A. Reed International; London, UK: 1993. The great tit. [Google Scholar]
- Götmark F. Predation by sparrowhawks favours early breeding and small broods in great tits. Oecologia. 2002;130:25–32. doi: 10.1007/s004420100769. [DOI] [PubMed] [Google Scholar]
- Griffin A.S. Social learning about predators: a review and prospectus. Learn. Behav. 2004;32:131–140. doi: 10.3758/bf03196014. [DOI] [PubMed] [Google Scholar]
- Hanson M.T, Coss R.G. Age differences in the response of California ground squirrels (Spermophilus beecheyi) to conspecific alarm calls. Ethology. 2001;107:259–275. doi:10.1046/j.1439-0310.2001.00659.x [Google Scholar]
- Haskell D.G. Experimental evidence that nestling begging behaviour incurs a cost due to nest predation. Proc. R. Soc. B. 1994;257:161–164. [Google Scholar]
- Hauser M.D. How infant vervet monkeys learn to recognize starling alarm calls: the role of experience. Behaviour. 1988;105:187–201. [Google Scholar]
- Herzog M, Hopf S. Behavioral responses to species-specific warning calls in infant squirrel monkeys reared in social isolation. Am. J. Psychol. 1984;7:99–106. doi: 10.1002/ajp.1350070204. [DOI] [PubMed] [Google Scholar]
- Higgins P.J. Oxford University Press; Melbourne, Australia: 1999. Handbook of Australian, New Zealand and Antarctic birds. Volume 4: parrots to dollarbird. [Google Scholar]
- Higgins P.J, Peter J.M. Oxford University Press; Melbourne, Australia: 2002. Handbook of Australian, New Zealand and Antarctic birds. Volume 6: pardalotes to shrike-thrushes. [Google Scholar]
- Impekoven M. Responses of laughing gull chicks (Larus atricilla) to parental attraction- and alarm-calls, and effects of parental auditory experience on the responsiveness to such calls. Behavior. 1976;56:250–278. [Google Scholar]
- Insley S.J, Paredes R, Jones I.L. Sex differences in razorbill Alca torda parent-offspring vocal recognition. J. Exp. Biol. 2003;206:25–31. doi: 10.1242/jeb.00072. doi:10.1242/jeb.00072 [DOI] [PubMed] [Google Scholar]
- Khayutin S.N. Sensory factors in the behavioral ontogeny of altricial birds. Adv. Study Behav. 1985;15:105–152. [Google Scholar]
- Klump G.M, Curio E. Reactions of blue tits Parus caeruleus to hawk models of different sizes. Bird Behav. 1983;4:78–81. [Google Scholar]
- Kroodsma D.E, Bayers B.E, Goodale E, Johnson S, Liu W. Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 2001;61:1029–1033. doi:10.1006/anbe.2000.1676 [Google Scholar]
- Kullberg C, Lind J. An experimental study of predator recognition in great tit fledglings. Ethology. 2002;108:429–441. doi:10.1046/j.1439-0310.2002.00786.x [Google Scholar]
- Leavesley, A. 2003 The information conveyed in the alarm calls of the white-browed scrubwren (Sericornis frontalis). Honours thesis, Australian National University, Canberra.
- Leavesley A, Magrath R.D. Communicating about danger: urgency alarm calling in a bird. Anim. Behav. 2005;70:365–373. doi:10.1016/j.anbehav.2004.10.017 [Google Scholar]
- Leech S.M, Leonard M.L. Begging and the risk of predation in nestling birds. Behav. Ecol. 1997;8:644–646. [Google Scholar]
- Leedman A.W, Magrath R.D. Long-term brood division and exclusive parental care in a cooperatively breeding passerine. Anim. Behav. 2003;65:1093–1108. doi:10.1006/anbe.2003.2164 [Google Scholar]
- Leonard M.L, Horn A.G, Mukhida A. False alarms and begging in nestling birds. Anim. Behav. 2005;69:701–708. doi:10.1016/j.anbehav.2004.05.022 [Google Scholar]
- Loesche P, Stoddard P.K, Higgins B.J, Beecher M.D. Signature versus perceptual adaptations for individual vocal recognition in swallows. Behavior. 1991;118:15–25. [Google Scholar]
- Madden J.R, Kilner R.M, Davies N.B. Nestling responses to adult food and alarm calls: 1. Species specific responses in two cowbird hosts. Anim. Behav. 2005a;70:619–627. doi:10.1016/j.anbehav.2004.11.019 [Google Scholar]
- Madden J.R, Kilner R.M, Davies N.B. Nestling responses to adult food and alarm calls: 2. Cowbirds and re-winged blackbirds raised by eastern phoebe hosts. Anim. Behav. 2005b;70:629–637. doi:10.1016/j.anbehav.2004.11.020 [Google Scholar]
- Magrath R.D. Nestling weight and juvenile survival in the blackbird, Turdus merula. J. Anim. Ecol. 1991;60:335–351. [Google Scholar]
- Magrath R.D. Group breeding dramatically increases reproductive success of yearling but not older female scrubwrens: a model for cooperatively breeding birds? J. Anim. Ecol. 2001;70:370–385. doi:10.1046/j.1365-2656.2001.00498.x [Google Scholar]
- Magrath R.D, Yezerinac S.M. Facultative helping does not influence reproductive success or survival in cooperatively-breeding white-browed scrubwrens. J. Anim. Ecol. 1997;66:658–670. [Google Scholar]
- Magrath R.D, Leedman A.W, Gardner J.L, Giannasca A, Nathan A.C, Yezerinac S.M, Nicholls J.A. Life in the slow lane: reproductive life history of the white-browed scrubwren, an Australian endemic. Auk. 2000;117:479–489. [Google Scholar]
- Marler P. Characteristics of some animal calls. Nature. 1955;176:6–8. [Google Scholar]
- Mateo J.M. The development of alarm-call response behaviour in free-living juvenile Belding's ground squirrels. Anim. Behav. 1996;52:489–505. doi:10.1006/anbe.1996.0192 [Google Scholar]
- Maurer G, Magrath R.D, Leonard M.L, Horn A.G, Donnelly C. Begging to differ: scrubwren nestlings beg to alarm calls and vocalize when parents are absent. Anim. Behav. 2003;65:1045–1055. doi:10.1006/anbe.2003.2148 [Google Scholar]
- McCowan B, Franceschini N.V, Vicino G.A. Age differences and developmental trends in alarm peep responses by Squirrel monkeys (Saimiri sciureus) Am. J. Psychol. 2001;53:19–31. doi: 10.1002/1098-2345(200101)53:1<19::AID-AJP2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Miller D.B, Blaich C.F. Alarm call responsivity of mallard ducklings: III. Acoustic features affecting behavioral inhibition. Dev. Psychobiol. 1986;19:291–301. doi: 10.1002/dev.420190402. doi:10.1002/dev.420190402 [DOI] [PubMed] [Google Scholar]
- Miller D.B, Hicinbothom G. Alarm call responsivity of mallard ducklings: X. ontogenetic adaptation or artifact of arousal? Bird Behav. 1991;9:114–120. [Google Scholar]
- Naef-Daenzer B, Widmer F, Nuber M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 2001;70:730–738. doi:10.1046/j.0021-8790.2001.00533.x [Google Scholar]
- Newton I, Marquiss M. Food, predation and breeding season in sparrowhawks (Accipiter nisus) J. Zool. Lond. 1982;197:221–240. [Google Scholar]
- Norusis M.J. SPSS Inc; Chicago, IL: 2000. SPSS 10 guide to data analysis. [Google Scholar]
- Perrins C.M. Population fluctuations and clutch size in the great tit, Parus major L. J. Anim. Ecol. 1965;34:601–647. [Google Scholar]
- Platzen, D. 2004 Parent–nestling vocal interactions in the white-browed scrubwren. PhD thesis, Australian National University, Canberra.
- Platzen D, Magrath R.D. Parental alarm calls suppress nestling vocalization. Proc. R. Soc. B. 2004;271:1271–1276. doi: 10.1098/rspb.2004.2716. doi:10.1098/rspb.2004.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzen D, Magrath R.D. Adaptive differences in response to two types of parental alarm calls in altricial nestlings. Proc. R. Soc. B. 2005;272:1101–1106. doi: 10.1098/rspb.2005.3055. doi:10.1098/rspb.2005.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawiradilaga, D. M. 1996 Foraging ecology of pied currawongs Strepera graculina in recently colonized areas of their range. Ph.D. thesis, Australian National University, Canberra.
- Ramakrishnan U, Coss R.G. Age differences in the responses to adult and juvenile alarm calls by Bonnet macques (Macaca radiata) Ethology. 2000;106:131–144. doi:10.1046/j.1439-0310.2000.00501.x [Google Scholar]
- Rydén O. Differential responsiveness of great tit nestlings, Parus major, to natural auditory stimuli. Z. Tierpsychol. 1978;47:236–253. doi: 10.1111/j.1439-0310.1978.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Rydén O. Selective resistance to approach: a precursor to fear responses to an alarm call in great tit nestlings Parus major. Dev. Psychobiol. 1982;15:113–120. doi: 10.1002/dev.420150204. doi:10.1002/dev.420150204 [DOI] [PubMed] [Google Scholar]
- Schodde R, Mason I.J. CSIRO Publishing; Melbourne, Australia: 1999. The dictionary of Australian birds: passerines. [Google Scholar]
- Seyfarth R.M, Cheney D.L. The ontogeny of vervet monkey alarm calling behaviour: a preliminary report. Z. Tierpsychol. 1980;54:37–56. [Google Scholar]
- Siegel S, Castellan N.J. McGraw Hill; New York, NY: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]