Abstract
In recent years, a number of species–energy hypotheses have been developed to explain global patterns in plant and animal diversity. These hypotheses frequently fail to distinguish between fundamentally different forms of energy which influence diversity in dissimilar ways. Photosynthetically active radiation (PAR) can be utilized only by plants, though their abundance and growth rate is also greatly influenced by water. The Gibbs free energy (chemical energy) retained in the reduced organic compounds of tissue can be utilized by all heterotrophic organisms. Neither PAR nor chemical energy influences diversity directly. Both, however, influence biomass and/or abundance; diversity may then increase as a result of secondary population dynamic or evolutionary processes. Temperature is not a form of energy, though it is often used loosely by ecologists as a proxy for energy; it does, however, influence the rate of utilization of chemical energy by organisms. It may also influence diversity by allowing a greater range of energetic lifestyles at warmer temperatures (the metabolic niche hypothesis). We conclude that there is no single species/energy mechanism; fundamentally different processes link energy to abundance in plants and animals, and diversity is affected secondarily. If we are to make progress in elucidating these mechanisms, it is important to distinguish climatic effects on species' distribution and abundance from processes linking energy supply to plant and animal diversity.
Keywords: abundance, energy, photosynthetically active radiation, speciation, species richness, temperature
1. Introduction
It is clear that, as a broad generalization, species diversity on land and in the sea attains its highest values in the tropics and is lowest at the poles, with temperate regions typically intermediate. There are also longitudinal gradients in the sea, driven by the high species richness of the Indo-West Pacific and Caribbean regions, and on land across a number of continents (Rosenzweig 1995; Clarke & Crame 1997; Brown & Lomolino 1998; Gaston 2000; Gaston & Blackburn 2000). Here, we examine two broad classes of explanation for these patterns, namely those based around climate and energy availability.
The idea that favourableness of climate is a key factor promoting tropical diversity goes back to the earliest naturalists (von Humboldt 1808), and it is now widely recognized that climate exerts an important influence on plant and animal distribution (Turner et al. 1987, 1988; Currie 1991). These relationships have received increased attention through the use of statistical models (bioenvelopes) to predict the potential response of organisms to climate change (Parmesan et al. 1999; Walther et al. 2002; Parmesan & Yohe 2003; Pearson & Dawson 2003). Discussion of the effect of energy flow on diversity has its roots in the development of the trophic structure of ecosystems by Odum and Lindemann, but especially Hutchinson (1959). Wright (1983) formalized this by modifying classic island biogeography theory, replacing area with energy. Specifically, Wright argued that the diversity of one trophic level was determined by the amount of energy available from the level below, and tested this with data for plants and birds living on islands.
Although these are distinct ideas about the role of climate and energy in the regulation of diversity, they became subsumed under the umbrella term ‘species/energy hypotheses’. Originally formulated for terrestrial systems, these ideas have been extended to the sea (see, for example, Fraser & Currie 1996; Roy et al. 1998; Hunt et al. 2005; Rex et al. 2005). Recently, it has become increasingly recognized that regulation of diversity by climate and by productivity are quite separate mechanisms (Ricklefs 2004; Turner 2004). Productivity is difficult to measure in the sea and so temperature has frequently been used as a proxy measure, thereby confounding temperature and productivity effects on diversity.
The different hypotheses that are frequently grouped under the general title of ‘species/energy hypotheses’ thus differ significantly in the extent to which a physical mechanism is described, and in what is meant by energy.
2. What is meant by energy?
The literature discussing the relationship between diversity and energy availability encompasses a number of different meanings of ‘energy’. These may be summarized as follows.
Radiation energy, or more specifically photosynthetically active radiation (PAR), which is the fraction of the visible spectrum between 400 and 700 nm.
Thermal energy, frequently expressed as either temperature sensu stricto, or more loosely in combination with other factors that determine climate.
Gibbs free energy (chemical energy) released from the covalent bonds of reduced organic compounds when these are oxidized during intermediary metabolism.
These are three quite different forms of energy, and the lack of consensus or clarity in the ecological debate is caused in part by confusion between them. This confusion is not helped by our inability to define energy other than as the ability to do work (Haynie 2001). Nevertheless, clear distinction needs to be maintained between radiation energy, thermal energy and chemical energy when discussing patterns of diversity, because they differ in the way they influence diversity.
Recent discussions of species–energy relationships have distinguished between solar or ambient (thermal) energy and productive (chemical) energy (Evans et al. 2004; Rodríguez et al. 2005). Allen et al. (in press) recognize all three forms of energy identified above, but by analogy with the terminology developed to describe the physics of moving bodies, they classify them as either kinetic or potential energy. Under this scheme kinetic energy includes both radiation and thermal energy, whereas potential energy equates to Gibbs free energy (Haynie 2001). The combination of radiation and thermal energy into a single class obscures an important distinction between two forms of energy that influence organisms in quite different ways.
In this short review, we seek to distinguish the various forms of energy and identify the different ways in which they might influence biological diversity. Our aim is to clarify the mechanistic links between the various forms of energy and diversity, and specifically to distinguish rigorously between the effects of climate and chemical energy on diversity. First, we discuss the links between solar radiation (PAR) and plant diversity, for it is at this lowest level of the food web that the important distinction between the effects of energy supply on biomass and on diversity are clearest. We then discuss the links between the chemical energy retained by those plants in their tissues and the diversity of herbivores and higher levels in the food web. Finally, we explore the role of thermal energy in these mechanisms, and introduce a previously unrecognized mechanism linking temperature to diversity.
(a) Radiation (photon flux)
For plants, a fundamental resource is light. In this sense, the photons in sunlight are the primary driver of all biodiversity, for they are the source of almost all energy used in the biosphere. The major exceptions are those systems where the energy for synthesis of organic tissue is obtained chemically. Most discussions of the relationship between PAR and diversity have concerned non-microbial organisms, and for the rest of this review we will limit our discussion to higher plants and animals. We also limit ourselves primarily to terrestrial habitats, for this is where most data are available.
If photon flux were all that was involved in determining the abundance or diversity of higher plants, then we might expect a more even distribution of that diversity across the globe than is actually observed. There is strong latitudinal variation in the seasonality and intensity of light input, the latter being caused by the shallow angle of incidence and greater scattering in the longer atmosphere path-length towards higher latitudes. However, when averaged over the year, the difference between received energy at the tropics and poles is only about fourfold (Öpik & Rolfe 2005), whereas the variation in plant diversity is very much greater (Barthlott et al. 1996; Davies et al. 2005).
The reason that plant distribution and diversity are not linked directly to patterns of received light energy is that for a plant to make use of the light which it receives, it also needs water. As well as providing the electrons and protons needed by photosynthesis, water is the solvent for biochemistry, and is involved as reactant or product in all of the major classes of physiological reactions. Most important, however, is that movement of water is what allows the plant to absorb nutrients and to move materials. It is therefore perhaps not surprising that the most successful of the energy-related diversity hypotheses has undoubtedly been the demonstration that much of the large-scale biogeographical variation in the diversity of terrestrial vascular plants can be explained by variations in water availability (Currie 1991; O'Brien 1993, 1998; Francis & Currie 2003; Moser et al. 2005). Because water is involved, plant abundance and diversity are not dictated by light or temperature alone: hot deserts have low plant diversity. Water availability is frequently determined as potential evapo-transpiration (PET), a measure of the rate at which a saturated surface loses water to the atmosphere. O'Brien (1993, 1998) has argued that the PET–richness relationship is hump-shaped and that a combination of PET and precipitation is the best predictor of plant richness. The importance of water to plant biomass and plant richness have also been shown recently by Bjorholm et al. (2005) and Sankaran et al. (2005).
Although light is the primary energy source for plants, they use only a small fraction of the incident PAR, typically less than 1% (Öpik & Rolfe 2005). We have an excellent understanding of the molecular mechanisms by which plants utilize PAR to synthesize reduced carbon compounds for new tissue, but variations in PAR alone explain very little of the global patterns of higher plant diversity. There is thus no direct relationship between plant diversity and energy (PAR); there is, however, a fairly strong relationship with a combination of water availability and temperature. While it is widely observed that habitats with freely available water and a warmer mean temperature support a greater biomass of plants and/or a larger number of individuals, it is not immediately clear why this greater abundance should necessarily be distributed among a larger number of species. Before returning to this point, we need to examine the links between plant biomass and diversity, and the diversity of the herbivores that eat them.
(b) Chemical energy
The energy in photons trapped by plants is used to build tissue biomass. The energy retained in the covalent bonds of the reduced carbon compounds synthesized by plants is then utilized by herbivores and other heterotrophic organisms in the food web. The Gibbs free energy in the food is released by the catabolic processes of intermediary metabolism, retained within a small number of energy carrier molecules such as ATP and NADPH, and then used either to build new tissue or to perform physiological work. Organisms also store energy in the short term in the form of chemical, osmotic or electrical gradients across membranes, but these are transient and not important in terms of energy flow between organisms. For energetic relationships within food webs, the important factor is the chemical energy retained within the organic fraction of the body tissue and consumed at the next trophic level.
To a first approximation, therefore, what is important to a heterotrophic organism is simply the amount of food (and because trophic relationships are dynamic, it is the rate of production rather than standing crop biomass that is critical). Clearly, there are subtle physiological complications, such as the need for vitamins, essential amino acids (Mevi-Schütz & Erhardt 2005), specific fatty acids (Sargent et al. 1999), or stoichiometric relationships (Sterner & Elser 2002). Overall, however, it is the amount and nature of food resources that are important to a heterotroph, and not the range of species which comprise that food. This might imply that some measure of the chemical energy available as biomass should be the best predictor of animal richness, but this does not seem to be the case (e.g. Kaspari et al. 2000, 2004; Rodríguez et al. 2005). The difficulty here is the extent to which traditional ecological measures of food biomass reflect the chemical energy available to the next trophic level; for example, the presence of lignin and cellulose in plants means that many herbivores can utilize only a small fraction of their food.
Although the existence of greater food resources would obviously support a larger biomass of heterotrophic consumers, it is not at all obvious how or why this should necessarily equate to a higher heterotrophic diversity (Blackburn & Gaston 1996). This is clearly an analogous problem to that posed in the previous section for plants: why does a higher abundance of resources result in greater diversity of organisms utilizing that resource, and not simply a higher biomass?
(c) Abundance and diversity
We have established above that fairly simple and well-understood mechanisms link the availability of resources to the abundance or biomass of organisms utilizing those resources. In the case of plants, the resources are light and water (and inorganic nutrients); in the case of herbivores, the resource is their plant food, and for carnivores it is other animals. One mechanism by which the increase in plant, herbivore or carnivore abundance leads to higher diversity is that larger populations buffer species against extinction, the risk of which is an inverse and nonlinear function of population size (Lande 1993). Greater overall abundance enables more species to attain viable population sizes. This is the more-individuals hypothesis (MIH) used to explain why areas with greater resources support higher diversities (Wright 1983; Srivastava & Lawton 1998; Evans et al. 2005).
A second important factor is that a greater diversity of food plants allows for the evolution of a wider range of specialist herbivores, which in turn allows for a wider range of predators, parasites and pathogens (e.g. Gaston 1996). However, this depends on the different plant species being present in sufficient abundance to enable specialization. There is actually rather little evidence for geographic covariation between numbers of plant species and numbers of animal species, once environmental covariation has been accounted for (see, for example, Hawkins & Porter 2003a; Hawkins & Pausas 2004). Where the greater species richness of plants also translates into enhanced structural complexity, this greater structural diversity may allow a greater diversity of organisms utilizing these habitats (Hutchinson 1959; Pielou 1975; Lee & Rotenberry 2005), although caution is needed to distinguish between species diversity and habitat diversity (Rosenzweig 1995). These three mechanisms, the MIH, prey specialization and habitat complexity, allow for greater diversity at one level in the food web to promote higher diversity at higher levels. They are, however, secondary processes which build on the enhanced biomass or abundance driven by the higher levels of available energy. For a recent review of these mechanisms, see Evans et al. (2004).
The existence of mechanisms linking plant diversity to herbivore and higher trophic level diversity means that these may also be correlated with factors that drive plant diversity. Thus many studies have shown that animal diversity is frequently correlated with PET, actual evapo-transpiration or remotely sensed measures of plant production such as the normalized difference vegetation index (NDVI; Currie 1991; Hawkins et al. 2003; Bailey et al. 2004; Seto et al. 2004; Bellocq & Gómez-Insausti 2005; Pautasso & Gaston 2005). Although the availability of water may affect the ability of animals to exploit their food resources, these correlations are generally indirect, mediated through the effects of water availability and temperature on plant diversity.
We thus have two quite different forms of energy influencing diversity at different levels of the food web, namely light energy in plants and chemical energy in heterotrophs (herbivores and carnivores). In both cases, however, the link to diversity is indirect, and is mediated through population processes linked to abundance. We now explore how these two relationships are influenced by a third form of energy, namely thermal energy (enthalpy), and to what extent thermal energy is validly approximated by temperature.
3. Temperature and diversity
Temperature is not energy; it is a measure of the tendency of a body to gain or lose heat. Two bodies are said to be at the same temperature if there is no net heat flow between them; this is the definition of temperature and is sometimes formalized as the zeroth law of thermodynamics (Haynie 2001). Temperature can be expressed either as an interval scale such as degrees Celsius, or a ratio scale such as Kelvin (Stevens 1946).
Neither is temperature a direct measure of heat content; two bodies at the same temperature can contain very different quantities of heat energy, this being expressed as thermal capacity (specific heat capacity in the older literature). The very different thermal capacities of air and water mean that a given volume of water contains very much more heat (by a factor of roughly ×4) than the same mass of air at the same temperature, a difference which is fundamental to the global climate system and to thermal ecology.
Because temperature is not energy, it cannot be used as such by organisms (Huston 2003). Despite this, many discussions of the species–energy hypothesis equate temperature with energy supply, and the ecological literature is replete with graphs of diversity as a function of temperature. Most often, temperature here is being used as a surrogate for some measure of chemical energy (for example, net primary production), but sometimes temperature is viewed (incorrectly) as a source of energy in itself.
Despite this lack of clarity in the literature, it is possible to discern two separate themes in the profusion of proposed links between diversity and temperature: (i) temperature affects diversity directly and (ii) temperature affects diversity by determining rates of speciation and extinction. We will discuss these in turn.
(a) Direct effects of temperature on diversity
A direct effect of temperature on diversity occurs when there is no intermediate link between the two, whereby a change in temperature leads inevitably and directly to a change in diversity. Any hypothesis that temperature controls diversity directly has the simple consequential prediction that diversity will be greater where temperatures are higher. This explanation has its origin in the intuitive feeling that warmer habitats are easier places to make a living than colder ones, a view that goes back to the earliest naturalists and which has been summarized succinctly by Currie (1991) as ‘benign conditions permit more species’. It appears in a number of guises, including the ‘physiological tolerance hypothesis’ (Currie et al. 2004), the ‘range limitation hypothesis’ (Evans et al. 2005) and the ‘thermoregulatory loads’ hypothesis (Lennon et al. 2000). Turner (2004) calls it the ‘ambient energy hypothesis’ to distinguish it from the productivity-based hypothesis of Wright (1983). These are all slightly different hypotheses, but have a common theme in that warmer places are seen as more amenable, either by allowing greater activity, more species to exist (though it is not clear quite how), or in the case of endotherms by reducing the physiological costs in keeping warm and thereby releasing energy to allow larger populations. Although the precise physical mechanisms are not always specified, these ideas are given strength by the widely observed correlation between latitude (which is frequently taken as a proxy for climate or environmental temperature) and diversity. These correlations are widespread, and involve plants, ectotherms and endotherms (Gaston 2000).
The problem with this hypothesis comes with envisaging a mechanism by which temperature could regulate diversity (or at least set a maximum value: Currie 1991). One can recognize that particular habitats could set specific physiological challenges that only certain groups of organisms could meet. Examples might be polar regions with shallow permafrost and winter temperatures below the freezing point of body fluids, temperate regions with regular winter frosts, or tropical areas with low humidity. In a classic contribution to ecology, Hutchinson (1959) suggested that as one moves from tropics to poles habitats become somehow tougher, with fewer and fewer organisms being able to tolerate the conditions. But in the same essay, Hutchinson himself posed the counter question: if some species of a group of organisms can evolve to live in tougher places, why cannot others? This perceptive question is centred on the subjective view of some habitats or regions being in some way tougher for organisms. While this might seem intuitively reasonable it is not easy to envisage a mechanism by which temperature alone could regulate diversity.
Although temperature is not energy, confusion has arisen by grouping what are essentially hypotheses of regulation by an abiotic factor (temperature) within the class of ‘species/energy hypotheses’. Temperature undoubtedly affects the activity and distribution of many organisms (Turner et al. 1987, 1988) and also has a powerful influence on energetics (Clarke 2003); nevertheless, a cow, lizard or fish can bask forever in warm temperatures, but without food it will die. At present, therefore, we are left with the intuitive (but dangerously anthropocentric) feeling that somehow warmer places are more amenable, and hence can support more species, but with no viable mechanistic explanation as to how this might work. In the case of plants, it is possible that temperature might limit overall abundance in polar regions through its control of nutrient availability and transpiration, and through the MIH mechanism thereby limit diversity. This, of course, is limitation by temperature as an abiotic environmental factor, not as a source of energy, and the same is true of ectothermic animals, where temperature influences the rate of physiological processes. In the absence of a convincing mechanism, it is difficult to move the debate beyond the current phase of simply correlating diversity with temperature.
A second possibility is that diversity is controlled not by mean temperature, but by variability in temperature. Although a number of authors have considered the possibility of a link between diversity and climate variability, the modern debate was opened by Stevens (1989). Stevens suggested that organisms living at high latitudes required a more generalist physiology and ecology because of the strong seasonal variation in climate, and thus species were able to become more widespread. In contrast, at low latitudes the low climatic variability resulted in more localized species ranges, and hence higher richness. What keeps diversity low at high latitudes is thus not the low mean temperature, but the large seasonal differences between summer and winter. This mechanism is thus quite independent of any postulated effect of mean temperature on diversity; the two processes could of course act in parallel or even synergistically. Gaston & Chown (1999) have recently shown that the critical factor is the relationship between the variability and the mean value, which suggests that many species occurring at intermediate latitudes can also occur across much of the tropics.
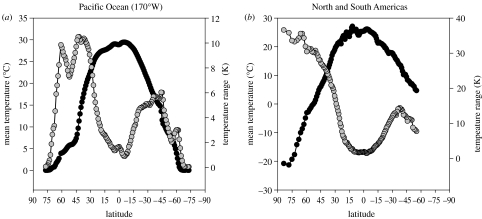
As is often the case, Stevens (1989) tested his hypothesis only with data from one hemisphere and one continent (in this case North America). Most of the examples used were terrestrial, although Stevens did extend the argument to the sea. The marine taxa used (fishes and molluscs) do show a strong diversity cline from tropics to poles, at least in shallow water, but this is not matched by the pattern of temperature variability in the seas, which is very different from that on land. Polar marine environments are typically much less variable seasonally than cold or warm temperate latitudes, with tropical regions also being relatively stable (figure 1). These differences allow for a simple test of the role of climate variability in governing broad patterns of diversity. If temperature variability were the key determinant of diversity, we would expect marine diversity to peak in polar and tropical latitudes, with reduced diversity in the highly seasonal temperature latitudes. This is not what is observed (Clarke & Crame 1997). However, there are some indications that strong climate seasonality is associated with reduced marine diversity, both in benthos and plankton. The first is that the diversity of benthic molluscs on both the Atlantic and Pacific continental shelves of North America drops steeply at the point where marine temperatures switch from the relatively aseasonal tropics to the seasonal temperature zones (Roy et al. 1998). The second is that the diversity of epipelagic marine copepods is lower in more seasonal provinces (Woodd-Walker et al. 2002). These are, however, simply correlations and it is not at all clear what the mechanism underpinning a reduction in diversity in climatically seasonal habitats might be (although one possibility might be if an obligately eurythermal physiology were necessarily more energetically expensive than one adapted to a narrow range of temperatures).
Figure 1.
(a) Mean (black) and annual (grey) range of sea-surface temperature (SST) along 170° W in the Pacific Ocean. Note how the largest seasonal variations are in the temperate latitudes. Data are averages for the period 1983–2003. The AVHRR Pathfinder v. 5.0 SST data were obtained from the Physical Oceanography Distributed Active Archive Centre (PO.DAAC) at the NASA Jet Propulsion Laboratory, Pasadena, California (http://podaac.jpl.nasa.gov). (b) Mean (black) and annual (grey) range of synoptic air temperature for terrestrial habitats. Mean annual temperature data (°C) for the period 1961–1990 at 10 min resolution interpolated from station means (New et al. 2002), and resampled to an equal-area grid using a Behrmann projection at a resolution of 96486.2 m at the standard parallels of 30° N and 30° S (provided by R. G. Davies). Data are for North and South America (available at http://www.cru.uea.ac.uk/cru/data/tmc.htm), and plotted after pooling into bins of 1° of latitude, with the range calculated from seasonal average maximum and minimum in each bin.
(b) Temperature, speciation and extinction
The previous two classes of explanation involving temperature concern an equilibrium world: temperature sets limits to maximum diversity either through its absolute value or its seasonal variability, and organisms have diversified until those limits are reached and the habitats saturated. Currie (1991) recognized that in North America many higher latitude areas have only recently recovered from the last glacial maximum, but felt that there has been sufficient time for the new habitats to become saturated; the observed patterns were thus the result of limits set by climate and not slow and incomplete recolonization. There is, however, some evidence for a historical recolonization signal in North American species richness (Hawkins et al. 2003; Hawkins & Porter 2003b; Hawkins 2004).
Another class of explanation is that rates of speciation and extinction are determined by temperature, and as a result tropical areas have achieved higher diversities than temperate or polar regions. This could operate both as a non-equilibrium mechanism (if the process of diversification in colder regions is simply slower and has not yet proceeded so far as in the tropics) and as an equilibrium mechanism (if rates of speciation and extinction are at steady state, but differ between tropical and polar regions).
The most recent contribution to this debate is that of Allen et al. (2002), who linked the widely observed correlation between diversity (both plant and animal) and temperature to the metabolic theory of ecology (Brown et al. 2004), and to the principle of energetic equivalence (Damuth 1987). They argue that diversity is linked directly and mechanistically to temperature through ‘the generally faster biological rates observed at higher temperatures’ (Brown et al. 2003). If temperature did govern diversity through its effect on speciation rate then this mechanism could not explain the observed diversity patterns in birds and mammals, whose body temperatures are maintained at a relatively high and more or less constant level (Storch 2003). In fact, both birds and mammals show strong diversity gradients, so this mechanism linking diversity directly to temperature fails to provide a convincing general explanation.
Allen et al. (in press) have subsequently developed the idea further, abandoning the link to the energetic equivalence of populations (which is itself controversial: Gaston & Blackburn 2000) but combining the metabolic theory of ecology and the neutral model of biodiversity (Hubbell 2001). They propose that temperature dictates the evolutionary rate of a population, whereas the availability of free energy governs the number of populations; these two processes in concert dictate the overall speciation rate and thus standing diversity. Turner (2004) has also proposed that large-scale patterns in diversity can be explained by an interaction between temperature and processes formalized in Hubbell's neutral theory of biodiversity.
A central tenet of these proposals is that temperature affects the evolutionary rate of populations. This evolutionary rates hypothesis (Rohde 1992) makes several key assumptions (Evans & Gaston 2005). Most importantly, it assumes that higher temperatures promote mutation. This can be either through direct pathways, or through indirect pathways such as an effect of temperature on generation time, metabolic rate or population size. Analysing the relationship between temperature and speciation rates thus involves the disentangling of two separate effects: the influence of temperature on mutation rate, and on generation time. The first process generates mutations (although these can be corrected), but it is meiosis that determines whether these mutations are transmitted to the next generation. Recently, Gillooly et al. (2005) have tackled this in a formal manner, starting with the premise that mutation rate is driven directly by metabolic rate, through the production of reactive oxygen species and free radicals (an idea proposed over a decade earlier by Martin (1995)). Using the metabolic theory of ecology, they predict the relationship to be expected between mutation rate and temperature, after correction for body mass. Data for four different sections of the mitochondrial genome encompassed the predicted value, whereas data for the nuclear genome were equivocal (Gillooly et al. 2005). In contrast, Held (2001) could detect no decrease in the rate of molecular substitutions in polar crustaceans compared with those from warmer waters. Any evolutionary rates hypothesis for a direct link between temperature and diversity implies that adaptation to new ecological circumstances would not be mutation-limited, and that mutation rates limit speciation rates. There is rather little evidence for this (Evans & Gaston 2005).
The evolutionary rate hypothesis also assumes implicitly that there is a strong correlation between current and historic energy levels; this is probably reasonable. Although it is now recognized that variations in the Earth's orbit will result in changes in the amount and distribution of solar energy received on the surface of the planet, these changes are too small, and plants utilize such a small fraction of that solar energy, that they do not influence diversity directly. These Milankovitch climate cycles do, however, have a profound influence on the distribution and timing of the seasons, together with other climate factors, and these climate cycles in turn are a major driver of speciation and extinction (Clarke & Crame 1997, 2003; Dynesius & Jansson 2000; Jansson & Dynesius 2002). Finally, the evolutionary rates hypothesis assumes that changes in species' ranges following speciation do not sufficiently weaken the correlation between the rate of speciation in an area and species richness. This seems unlikely.
Speciation is, however, only one aspect of diversity; extinction is also important. Unfortunately, studies of variation in extinction with latitude or temperature are almost non-existent.
4. Temperature and metabolic niches
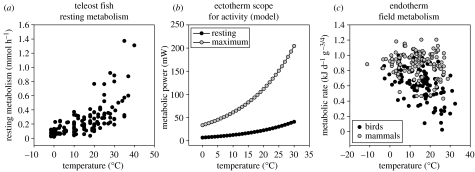
Recent studies have revealed a previously unrecognized relationship between environmental temperature and the diversity of metabolic niches, which is that the absolute scope for activity (the difference between maximal and resting metabolic rates) in ectotherms increases with environmental temperature. This increased absolute metabolic scope follows because of two factors; the first is that resting metabolic rate increases with temperature (figure 2a), and the second is that relative metabolic scope (the ratio between resting and maximal metabolic rate) remains more or less constant across the physiological temperature range (Clarke 2003). This implies that there are more energetic ways of making a living at warm temperatures than at cold temperatures, and hence a potential opportunity for higher diversity (Clarke 1993, 2003; Clarke & Johnston 1999). The mechanisms underpinning these relationships are not yet clear, though they undoubtedly involve a balance between the costs of maintenance processes and of a particular ecological lifestyle (Clarke 1993, 2004; Clarke & Fraser 2004). Data for resting metabolism in teleost fish suggest that there is indeed a wider range of metabolic rates in warmer than in colder fish (figure 2b), and this is coupled with a wider range of lifestyles; in particular, highly active predatory lifestyles are found only in warmer water fish (Clarke & Johnston 1996).
Figure 2.
Temperature and metabolic niches. (a) Variation in resting metabolic rate in teleost fish (Clarke & Johnston 1999). Note the wider range of values at higher temperatures. (b) Diagram showing how absolute aerobic scope also increases with temperature. This arises because resting metabolic rate increases positively with temperature and relative aerobic scope (the ratio of active to resting metabolism) is temperature invariant. Although the model was based on data for teleost fish, it also applies to other ectotherms (Clarke 2003). (c) Energetic niches in mammals (black) and birds (grey). Data are for field metabolic rate, and have been corrected for body mass assuming a mass exponent of 0.75 (redrawn from Anderson & Jetz 2005). Note the wider range of values at higher temperatures.
Recently, Anderson & Jetz (2005) have shown a related pattern for birds and mammals (figure 2c), whereby the range of metabolic rates (in this case field metabolic rates) is wider at lower latitudes. They link this pattern to temperature, and their explanation is thus a version of the benign environment hypothesis. Lovegrove (2000, 2003) has shown that resting metabolic rate tends to be lower in mammals from tropical habitats, and this may be linked to lower thermoregulatory costs. The mechanism thus differs between ectotherms and endotherms, though both are related directly to the correlation between environmental temperature and resting metabolic rate. Nevertheless, there are strong parallels in that environmental temperature is affecting diversity through its influence on metabolic rate and the consequent range of lifestyles that can be supported. We term this the metabolic niche hypothesis, and propose that this affords a mechanism by which diversity may be linked positively to environmental temperature. We emphasize that this is only one mechanism, and we do not suggest it is the complete explanation for the widely observed correlation between diversity and environmental temperature; we do, however, suggest that it is worthy of further exploration.
5. Summary
The range of species–energy hypotheses in the literature encompasses three different meanings of energy, namely PAR, Gibbs free energy retained in the reduced carbon compounds that comprise tissue (chemical energy), and thermal energy. Temperature is not energy, and only PAR and chemical energy can be utilized by organisms and thereby affect their abundance and biomass; temperature does, however, affect the rate at which organisms make use of PAR and chemical energy, and it may influence the rate of molecular evolution.
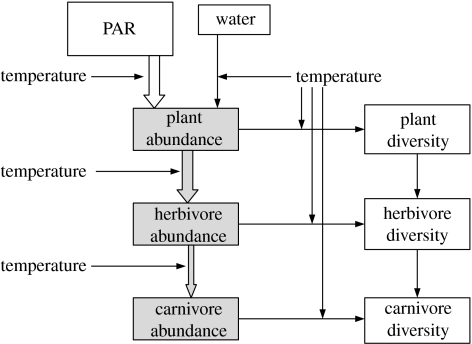
Only plants can utilize PAR and this step is the source of almost all fixed biological energy on the planet. However, the diversity of land plants is correlated not with energy, but with a combination of energy and water availability. The energy extracted from PAR by plants is used to reduce CO2 to the organic compounds comprising tissue, and this chemical energy fuels all other levels of the food web. In neither case does the amount of energy, nor the rate of its utilization, affect diversity directly. Rather it increases biomass and/or abundance, and diversity may increase as a second-order effect through population dynamics and speciation processes. In all cases, temperature plays a key role in influencing rates, though it is not a source of energy in itself. This is shown schematically in figure 3.
Figure 3.
A conceptual diagram showing the complexity of processes that link energy supply to organism diversity. Solid arrows illustrate the transfer of energy, with photosynthetically active radiation (PAR) shown as an open arrow and chemical energy shown as grey arrows. The influence of temperature is all-pervasive and complex; it influences the rate of utilization of energy (left-hand side of the diagram) and also the availability of water, and rate of mutation and population processes linking abundance to diversity (right-hand side of the diagram). This diagram emphasizes that there is no single species/energy hypothesis, that different processes underpin the evolution of plant diversity and animal diversity, and that temperature has a complex influence.
There is thus no single species–energy hypothesis, but rather there is a suite of mechanisms linking diversity to energy. In particular, there is a fundamental distinction between plants and animals in the nature of the energy they use, and the role played by temperature. To make further progress, we need to distinguish clearly between those processes that influence plant diversity and those affecting animal diversity. We now have an outline working understanding of the control of plant diversity by a combination of energy supply (PAR) and water dynamics, sufficient to be codified in an empirical model (O'Brien 1998; Field et al. 2005). The influence of plant diversity on herbivore, and thereby on carnivore diversity means that there are also statistical associations between the same environmental factors and animal diversity. Together these provide the structure for the productivity hypothesis advanced originally by Wright (1983), with the more individuals hypothesis providing the mechanistic link from abundance to diversity. Associations between herbivore or carnivore diversity and abiotic environmental variations are thus best viewed as epiphenomena, and not separate hypotheses or mechanisms in themselves. We should abandon the use of environmental temperature as a surrogate for radiation or chemical energy, especially in the sea, using instead direct measures of the form of energy that is hypothesized to influence diversity. This is not always easy, especially in the sea, but only then we will be in a position to make progress in understanding the true relationship(s) between species diversity and energy.
Acknowledgments
The ideas in this paper were first outlined at a workshop organized by the Santa Fe Institute and the Centre for Theoretical Studies in Prague, October 2004. We thank Drew Allen and colleagues for allowing us sight of their unpublished paper on a related theme, and support from NERC NER/O/S/2001/01257. We thank two referees for constructive comments which significantly improved the paper.
References
- Allen A.P, Brown J.H, Gillooly J.F. Global biodiversity, biochemical kinetics, and the energy-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. doi:10.1126/science.1072380 [DOI] [PubMed] [Google Scholar]
- Allen, A. P., Gillooly, J. F. & Brown, J. H. In press. Recasting the species–energy hypothesis: the different roles of kinetic and potential energy in regulating biodiversity. In Scaling biodiversity (ed. D. Storch). Princeton, NJ: Princeton University Press.
- Anderson K.J, Jetz W. The broad-scale ecology of energy expenditure of endotherms. Ecol. Lett. 2005;8:310–318. doi:10.1111/j.1461-0248.2005.00723.x [Google Scholar]
- Bailey S.A, Horner-Devine M.C, Luck G, Moore L.A, Carney K.M, Anderson S, Betrus C.J, Fleishman E. Primary productivity and species richness: relationships among functional guilds, residency groups and vagility classes at multiple spatial scales. Ecography. 2004;27:207–217. doi:10.1111/j.0906-7590.2004.03631.x [Google Scholar]
- Barthlott W, Lauer W, Placke A. Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde. 1996;50:317–327. [Google Scholar]
- Bellocq M.I, Gómez-Insausti R. Raptorial birds and environmental gradients in the southern Neotropics: a test of species-richness hypotheses. Austral Ecol. 2005;30:900–906. doi:10.1111/j.1442-9993.2005.01533.x [Google Scholar]
- Bjorholm S, Svenning J.-C, Skov F, Balslev H. Environmental and spatial controls of palm (Arecaceae) species richness across the Americas. Global Ecol. Biogeogr. 2005;14:423–429. doi:10.1111/j.1466-822x.2005.00167.x [Google Scholar]
- Blackburn T.M, Gaston K.J. A sideways look at patterns in species richness, or why there are so few species outside the tropics. Biodivers. Lett. 1996;3:44–53. [Google Scholar]
- Brown J.H, Lomolino M.V. Sinauer; Sunderland, MA: 1998. Biogeography. [Google Scholar]
- Brown J.H, Allen A.P, Gillooly J.F. Response to heat and biodiversity (Huston) Science. 2003;299:512–513. doi: 10.1126/science.299.5606.512. doi:10.1126/science.299.5606.512 [DOI] [PubMed] [Google Scholar]
- Brown J.H, Gillooly J.F, Allen A.P, Savage V.M, West G.B. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Clarke A. Seasonal acclimatization and latitudinal compensation in metabolism: do they exist? Funct. Ecol. 1993;7:139–149. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003;18:573–581. doi:10.1016/j.tree.2003.08.007 [Google Scholar]
- Clarke A. Is there a universal temperature dependence of metabolism? Funct. Ecol. 2004;18:252–256. doi:10.1111/j.0269-8463.2004.00842.x [Google Scholar]
- Clarke A, Crame J.A. Diversity, latitude and time: patterns in the shallow sea. In: Ormond R.F.G, Gage J.D, Angel M.V, editors. Marine biodiversity: causes and consequences. Cambridge University Press; Cambridge, UK: 1997. pp. 122–147. [Google Scholar]
- Clarke A, Crame J.A. The importance of historical processes in global patterns of diversity. In: Blackburn T.M, Gaston K.J, editors. Macroecology: concepts and consequences. vol. 43. Blackwell; Oxford, UK: 2003. pp. 130–151. [Google Scholar]
- Clarke A, Fraser K.P.P. Why does metabolism scale with temperature? Funct. Ecol. 2004;18:243–251. doi:10.1111/j.0269-8463.2004.00841.x [Google Scholar]
- Clarke A, Johnston I.A. Evolution and adaptive radiation of Antarctic fishes. Trends Ecol. Evol. 1996;11:212–218. doi: 10.1016/0169-5347(96)10029-x. doi:10.1016/0169-5347(96)10029-X [DOI] [PubMed] [Google Scholar]
- Clarke A, Johnston N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. doi:10.1046/j.1365-2656.1999.00337.x [Google Scholar]
- Currie D.J. Energy and large-scale patterns of animal and plant species richness. Am. Nat. 1991;137:27–49. doi:10.1086/285144 [Google Scholar]
- Currie D.J, Mittelbach G.G, Cornell H.V, Field R, Guegan J.F, Hawkins B.F, Kaufman D.M, Kerr J.T, Oberdorff T, O'Brien E, Turner J.R.G. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004;7:1121–1131. doi:10.1111/j.1461-0248.2004.00671.x [Google Scholar]
- Damuth J. Interspecific allometry of population density in mammals and other animals: the independence of body mass and population energy use. Biol. J. Linn. Soc. 1987;31:193–246. [Google Scholar]
- Davies T.J, Savolainen V, Chase M.W, Goldblatt P, Barraclough T.G. Environment, area, and diversification in the species-rich flowering plant family Iridaceae. Am. Nat. 2005;166:418–425. doi: 10.1086/432022. doi:10.1086/432022 [DOI] [PubMed] [Google Scholar]
- Dynesius M, Jansson R. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA. 2000;97:9115–9120. doi: 10.1073/pnas.97.16.9115. doi:10.1073/pnas.97.16.9115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K.L, Gaston K.J. Can the evolutionary-rates hypothesis explain species–energy relationships? Funct. Ecol. 2005;19:899–915. doi:10.1111/j.1365-2435.2005.01046.x [Google Scholar]
- Evans K.L, Warren P.H, Gaston K.J. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 2004;79:1–25. doi: 10.1017/s1464793104006517. doi:10.1017/S1464793103006183 [DOI] [PubMed] [Google Scholar]
- Evans K.L, Greenwood J.J.D, Gaston K.J. Dissecting the species–energy relationship. Proc. R. Soc. B. 2005;272:2155–2163. doi: 10.1098/rspb.2005.3209. doi:10.1098/rspb.2005.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field R, O'Brien E.M, Whittaker R.J. Global models for predicting woody plant richness from climate: development and evaluation. Ecology. 2005;86:2263–2277. [Google Scholar]
- Francis A.P, Currie D.J. A globally consistent richness–climate relationship for angiosperms. Am. Nat. 2003;161:523–536. doi: 10.1086/368223. doi:10.1086/368223 [DOI] [PubMed] [Google Scholar]
- Fraser R.H, Currie D.J. The species richness–energy hypothesis in a system where historical factors are thought to prevail. Am. Nat. 1996;148:138–159. doi:10.1086/285915 [Google Scholar]
- Gaston K.J. Spatial covariance in the species richness of higher taxa. In: Hochberg M.E, Clobert M.E, Barbault R, editors. Aspects of the genesis and maintenance of biological diversity. Oxford University Press; Oxford, UK: 1996. pp. 221–242. [Google Scholar]
- Gaston K.J. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. doi:10.1038/35012228 [DOI] [PubMed] [Google Scholar]
- Gaston K.J, Blackburn T.M. Blackwell Science; Oxford, UK: 2000. Pattern and process in macroecology. [Google Scholar]
- Gaston K.J, Chown S.L. Why Rapoport's rule does not generalise. Oikos. 1999;84:309–312. [Google Scholar]
- Gillooly J.F, Allen A.P, West G.B, Brown J.H. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc. Natl Acad. Sci. USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. doi:10.1073/pnas.0407735101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.A. Summer vegetation, degalciation and the anomalous bird diversity gradient in eastern North America. Global Ecol. Biogeogr. 2004;13:321–325. doi:10.1111/j.1466-822X.2004.00095.x [Google Scholar]
- Hawkins B.A, Pausas J.G. Does plant richness influence animal richness?: the mammals of Catalonia (NE Spain) Divers. Distrib. 2004;10:247–252. doi:10.1111/j.1366-9516.2004.00085.x [Google Scholar]
- Hawkins B.A, Porter E.E. Does herbivore diversity depend on plant diversity? The case of California butterflies. Am. Nat. 2003a;161:40–49. doi: 10.1086/345479. doi:10.1086/345479 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A, Porter E.E. Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Global Ecol. Biogeogr. 2003b;12:475–481. doi:10.1046/j.1466-822X.2003.00060.x [Google Scholar]
- Hawkins B.A, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Haynie D.T. Cambridge University Press; Cambridge, UK: 2001. Biological thermodynamics. [Google Scholar]
- Held C. No evidence for slow-down of molecular substitution rates at subzero temperatures in Antarctic serolid isopods (Crustacea, Isopoda, Serolidae) Polar Biol. 2001;24:497–501. doi:10.1007/s003000100245 [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. The unified neutral theory of biodiversity and biogeography. [DOI] [PubMed] [Google Scholar]
- Hunt G, Cronin T.M, Roy K. Species–energy relationship in the deep sea: a test using the Quaternary fossil record. Ecol. Lett. 2005;8:739–747. doi:10.1111/j.1461-0248.2005.00778.x [Google Scholar]
- Huston M.A. Heat and biodiversity. Science. 2003;299:512. doi: 10.1126/science.299.5606.512. doi:10.1126/science.299.5606.512 [DOI] [PubMed] [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Jansson R, Dynesius M. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 2002;33:741–777. doi:10.1146/annurev.ecolsys.33.010802.150520 [Google Scholar]
- Kaspari M, O'Donnell S, Kercher J.R. Energy density, and constraints to species richness: ant assemblages along a productivity gradient. Am. Nat. 2000;155:280–293. doi: 10.1086/303313. doi:10.1086/303313 [DOI] [PubMed] [Google Scholar]
- Kaspari M, Ward P.S, Yuan M. Energy gradients and the geographic distribution of local ant diversity. Oecologia. 2004;140:407–413. doi: 10.1007/s00442-004-1607-2. doi:10.1007/s00442-004-1607-2 [DOI] [PubMed] [Google Scholar]
- Lande R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 1993;142:911–927. doi: 10.1086/285580. [DOI] [PubMed] [Google Scholar]
- Lee P.-Y, Rotenberry J.T. Relationships between bird species and tree species assemblages in forested habitats of eastern North Amnerica. J. Biogeogr. 2005;32:1139–1150. doi:10.1111/j.1365-2699.2005.01254.x [Google Scholar]
- Lennon J.J, Greenwood J.J.D, Turner J.R.G. Bird diversity and environmental gradients in Britain: a test of the species–energy hypothesis. J. Anim. Ecol. 2000;69:581–598. doi:10.1046/j.1365-2656.2000.00418.x [Google Scholar]
- Lovegrove B.G. The zoogeography of mammalian basal metabolic rate. Am. Nat. 2000;156:201–219. doi: 10.1086/303383. doi:10.1086/303383 [DOI] [PubMed] [Google Scholar]
- Lovegrove B.G. The influence of climate on the basal metabolic rate of small mammals: a slow–fast metabolic continuum. J. Comp. Physiol. B. 2003;173:87–112. doi: 10.1007/s00360-002-0309-5. [DOI] [PubMed] [Google Scholar]
- Martin A.P. Metabolic rate and directional nucleotide substitution in animal mitochondrial DNA. Mol. Biol. Evol. 1995;12:1124–1131. doi: 10.1093/oxfordjournals.molbev.a040286. [DOI] [PubMed] [Google Scholar]
- Mevi-Schütz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. Am. Nat. 2005;165:411–419. doi: 10.1086/429150. [DOI] [PubMed] [Google Scholar]
- Moser D, Dullinger S, Englisch T, Niklfeld H, Plutzar C, Sauberer N, Zechmeister H.G, Grabherr G. Environmental determinants of vascular plant species richness in the Austrian Alps. J. Biogeogr. 2005;32:1117–1127. doi:10.1111/j.1365-2699.2005.01265.x [Google Scholar]
- New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim. Res. 2002;21:1–25. [Google Scholar]
- O'Brien E.M. Climatic gradients in woody plant species richness: towards an explanation based on an analysis of southern Africa's woody flora. J. Biogeogr. 1993;20:181–198. [Google Scholar]
- O'Brien E.M. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr. 1998;25:379–398. doi:10.1046/j.1365-2699.1998.252166.x [Google Scholar]
- Öpik H, Rolfe S.A. Cambridge University Press; Cambridge, UK: 2005. The physiology of flowering plants. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi:10.1038/21181 [Google Scholar]
- Pautasso M, Gaston K.J. Resources and global avian assemblage structure in forests. Ecol. Lett. 2005;8:282–289. doi:10.1111/j.1461-0248.2005.00724.x [Google Scholar]
- Pearson R.G, Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003;12:361–371. doi:10.1046/j.1466-822X.2003.00042.x [Google Scholar]
- Pielou E.C. Wiley; New York, NY: 1975. Ecological diversity. [Google Scholar]
- Rex M.A, Crame J.A, Stuart C.T, Clarke A. Large scale biogeographic patterns in marine mollusks: a confluence of history and productivity. Ecology. 2005;86:2288–2297. [Google Scholar]
- Ricklefs R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004;7:1–15. doi:10.1046/j.1461-0248.2003.00554.x [Google Scholar]
- Rodríguez M.Á, Belmontes J.A, Hawkins B.A. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecol. 2005;28:65–70. doi:10.1016/j.actao.2005.02.006 [Google Scholar]
- Rohde K. Latitudinal gradients in species-diversity—the search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Roy K, Jablonski D, Valentine J.W, Rosenberg G. Marine latitudinal diversity gradients: tests of causal hypotheses. Proc. Natl Acad. Sci. USA. 1998;95:3699–3702. doi: 10.1073/pnas.95.7.3699. doi:10.1073/pnas.95.7.3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran M, et al. Determinants of woody cover in African savannas. Nature. 2005;438:846–849. doi: 10.1038/nature04070. doi:10.1038/nature04070 [DOI] [PubMed] [Google Scholar]
- Sargent J, Bell G, McEvoy L, Tocher D, Estevez A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture. 1999;177:191–199. doi:10.1016/S0044-8486(99)00083-6 [Google Scholar]
- Seto K.C, Fleishman E, Fay J.P, Betrus C.J. Linking spatial patterns of bird and butterfly species richness with landsat TM derived NDVI. Int. J. Remote Sens. 2004;25:4309–4324. doi:10.1080/0143116042000192358 [Google Scholar]
- Srivastava D.S, Lawton J.H. Why more productive sites have more species: an experimental test of theory using tree-hole communities. Am. Nat. 1998;152:510–529. doi: 10.1086/286187. doi:10.1086/286187 [DOI] [PubMed] [Google Scholar]
- Sterner R.W, Elser J.J. Princeton University Press; Princeton, NJ: 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. [Google Scholar]
- Stevens G.C. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 1989;133:240–256. doi:10.1086/284913 [Google Scholar]
- Stevens S.S. On the theory of scales of measurement. Science. 1946;103:677–680. doi: 10.1126/science.103.2684.677. [DOI] [PubMed] [Google Scholar]
- Storch D. Comment on “global biodiversity, biochemical kinetics, and the energy-equivalence rule”. Science. 2003;299:346b. doi: 10.1126/science.1078627. doi:10.1126/science.1078627 [DOI] [PubMed] [Google Scholar]
- Turner J.R.G. Explaining the global biodiversity gradient: energy, area, history and natural selection. Basic Appl. Ecol. 2004;5:435–448. doi:10.1016/j.baae.2004.08.004 [Google Scholar]
- Turner J.R.G, Gatehouse C.M, Corey C.A. Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos. 1987;48:195–205. [Google Scholar]
- Turner J.R.G, Lennon J.J, Lawrenson J.A. British bird species and the energy theory. Nature. 1988;335:539–541. doi:10.1038/335539a0 [Google Scholar]
- von Humboldt A. J. G. Cotta; Tübingen, Germany: 1808. Ansichten der natur mit wissenschaftlichen Erlauterungen. [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Woodd-Walker R.S, Ward P, Clarke A. Large scale patterns in diversity and community structure of surface water copepods from the Atlantic Ocean. Mar. Ecol. Prog. Ser. 2002;236:189–203. [Google Scholar]
- Wright D.H. Species–energy theory: an extension of species–area theory. Oikos. 1983;41:496–506. [Google Scholar]