Abstract
Heterogeneity in host populations and communities can have large effects on the transmission and control of a pathogen. In extreme cases, a few individuals give rise to the majority of secondary infections, which have been termed super spreading events. Here, we show that transmission of West Nile virus (WNV) is dominated by extreme heterogeneity in the host community, resulting in highly inflated reproductive ratios. A single relatively uncommon avian species, American robin (Turdus migratorius), appeared to be responsible for the majority of WNV-infectious mosquitoes and acted as the species equivalent of a super spreader for this multi-host pathogen. Crows were also highly preferred by mosquitoes at some sites, while house sparrows were significantly avoided. Nonetheless, due to their relative rarity, corvids (crows and jays) were relatively unimportant in WNV amplification. These results challenge current beliefs about the role of certain avian species in WNV amplification and demonstrate the importance of determining contact rates between vectors and host species to understand pathogen transmission dynamics.
Keywords: reservoir host, reproductive ratio, R0, infectiousness, host–vector contact rate, super spreading events
1. Introduction
Understanding the epidemiology of zoonotic pathogens requires identification of the animal species that are the key reservoir hosts (Haydon et al. 2002; Leroy et al. 2005; Li et al. 2005). This is a complex problem for multi-host vector-borne pathogens because some hosts may be able to transmit the pathogen, while others are fed on by vectors but rarely infect them with the pathogen (Hudson et al. 1995). The former hosts facilitate epidemics and may be termed amplification or reservoir hosts (Haydon et al. 2002), while the latter dampen or prevent epidemics and have been called dilution hosts (Ostfeld & Keesing 2000; LoGiudice et al. 2003). Determining the degree of host heterogeneity in pathogen transmission is especially important because for many pathogens a small fraction of infected individuals is responsible for the majority of transmission (Woolhouse et al. 1997; Ostfeld & LoGiudice 2003; Lloyd-Smith et al. 2005b). In these cases, heterogeneity greatly increases the reproductive ratio of a pathogen, R0, and the explosiveness of epidemics, if the introduced pathogen does not become extinct (Woolhouse et al. 1997; Lloyd-Smith et al. 2005a).
Heterogeneity in pathogen transmission arises primarily from variability in contact rates and variability in infectiousness of hosts (Woolhouse et al. 1997; Dye & Gay 2003). Both of these factors are likely to generate heterogeneity in the transmission of multi-host pathogens, because contact rates between hosts (or between hosts and vectors) vary significantly between species (Dobson 2004), and because species differ substantially in immunologic and infectious response to pathogen infections (Komar et al. 2003; LoGiudice et al. 2003). The composition of host communities and the feeding patterns of vectors thus play key roles in determining whether or not a vector-borne pathogen will successfully invade, persist and cause epidemics.
West Nile virus (WNV; Flaviviridae: flavivirus) is a zoonotic pathogen that is primarily transmitted between birds and mosquitoes, but is also sometimes transmitted to mammals, including horses and humans (Kramer & Bernard 2001). It has caused yearly epidemics in North America since 1999, with approximately 22 000 reported human cases, 826 deaths and an estimated 225 000 illnesses (Petersen & Hayes 2004; Centers for Disease Control & Prevention 2006a; Health Canada 2006). Although WNV has infected over 300 species of birds, 30 mammals and several reptiles in North America (Centers for Disease Control & Prevention 2006b; Marra et al. 2004), the vertebrate species that infect the majority of mosquitoes have yet to be determined. Although large numbers of corvids (birds in the family Corvidae, including jays and crows) have been found dead and tested positive for WNV (Bernard et al. 2001; Garvin et al. 2004; Reisen et al. 2004), corvids rarely make up more than ten percent of the individuals in most communities, except near roosts (Husak & Linder 2004; Sauer et al. 2005). House sparrows (Passer domesticus), a widespread and abundant species, have been hypothesized to be important in WNV transmission because of their abundance and evidence of their exposure to WNV (Komar et al. 2001). However, neither corvids nor house sparrows have been important hosts in previous studies of mosquito feeding patterns (Apperson et al. 2002; Apperson et al. 2004; Molaei et al. 2006).
We studied mosquito feeding patterns and epidemiology of WNV in urban and residential areas to determine the primary reservoir host species of WNV and the impact of host heterogeneity on pathogen amplification. We found that WNV transmission was dominated by extreme heterogeneity in the community of avian host species, with a single relatively uncommon species accounting for the majority of WNV-infectious mosquitoes.
2. Material and methods
We collected data on avian host abundance and serology, vector feeding, mosquito abundance and WNV infection prevalence at five sites in Maryland and Washington, DC from May to September 2004. All sampling at each site was done within ca 1 km radius circle, which had relatively homogenous percentage forest cover and land use. The sites included three urban areas, the National Mall in Washington, DC (including the National Gallery of Art, the Hirshhorn Museum and the National Museum of Natural History); Foggy Bottom, DC (500 m northeast of the Watergate hotel); Baltimore, MD, (300 m west of Camden Yards); and two residential areas, Takoma Park, MD; and Bethesda, MD.
We estimated the abundance of birds using between four and six unlimited distance point transects, 6 min in duration, performed at least 150 m apart at each site monthly from May to September (20–30 point counts/site totalling ca 200 observations and ca 400 individuals/site). Censuses were performed during times of peak activity (generally within 30 min of dawn). We used program Distance, which accounts for species differences in observability (Thomas et al. 2004), to estimate the density of each species at each site.
We trapped birds throughout each site approximately monthly using mist nets and obtained 0.1 ml of blood by brachial or jugular venipuncture. Blood was tested for flavivirus antibodies using an enzyme-linked immunosorbent assay (ELISA; Ebel et al. 2002). We tested 346 flavivirus positive samples from birds trapped in 2003 by plaque-reduction neutralization test (PRNT; Calisher et al. 1989) to distinguish between WNV and St Louis encephalitis virus (SLEV), and found no evidence of SLEV exposure. Thus, we interpreted our ELISA positive 2004 samples as evidence of WNV infection and recovery.
We collected mosquitoes from throughout each of the same sites using eight CDC light traps (four at 1.5 m height and four in tree canopies at 8–20 m; pairs of traps were separated by at least 100 m), and four CDC gravid traps for two nights twice per month from May to September and by aspirating mosquitoes from vegetation with a large backpack mounted aspirator. Engorged mosquitoes were primarily obtained from light (61%) and gravid (38%) traps. We identified all non-engorged mosquitoes (approx. 23 000) to the species level, where possible, and tested them for WNV RNA using real time RT–PCR (Kauffman et al. 2003) in groups (pools) of 20–50 individuals. The temporal pattern of abundance and WNV prevalence in mosquitoes at these sites has been described elsewhere (Kilpatrick et al. 2006).
We used PCR to identify the species composition of morphologically similar Culex mosquitoes (Crabtree et al. 1995). We identified 40 mosquitoes/site as well as all engorged Culex and found that more than 90% were Culex pipiens. We identified the sources of blood meals using PCR amplification of the cytochrome b gene (Ngo & Kramer 2003) and nucleotide sequencing of the amplified product. We found 181 individual blood meals that yielded a PCR product and obtained DNA sequences from 163 of these; 11 blood meals contained DNA from both mammal and avian hosts, which were subsequently analysed as separate feedings. We calculated feeding preference indices of Cx. pipiens and Culex restuans mosquitoes (the primary enzootic vectors of WNV in this region (Kilpatrick et al. 2005)), Pi, on each avian host i,
| 2.1 |
If mosquitoes feed on host species in proportion to their abundance, the fraction of blood meals from each species, fi, will be the same as the fraction of the community made up by that species, ai, and Pi will be 1. We tested whether Pi for each species at each site was significantly different from 1 by performing 10 000 multinomial simulations comparing the observed distribution of blood meals between species with those expected under the null hypothesis that mosquitoes fed on birds in proportion to their abundance (Hassan et al. 2003). Culex mosquitoes obtain blood meals every 6–21 days and can live for 10–65 days in captivity, depending on temperature (Oda et al. 1999; Spielman & D'Antonio 2001).
Several avian species that were present at a site were not found in any of the blood meals at that site. We determined whether this was due to avoidance by mosquitoes or insufficient sample sizes by performing multinomial simulations and calculating the probability of observing at least 0.5 blood meals from unrepresented species, given our sample size of blood meals from that site. If the probability was less than 0.05, we conservatively reported the feeding index with 0.5 blood meals from that host as a minimum avoidance estimate.
If species have equal initial seroprevalence and infection rates, one can estimate the fraction, Fi, of WNV-infectious mosquitoes resulting from feeding on each avian species i as the product of the relative abundance, ai, the host reservoir competence, Ci, and feeding index, Pi. The host reservoir competence is a measure of the sum of the probability that an infected host will transmit virus to a biting mosquito on each of the 7 days following infection (viremic periods were 1–7 days in length; Komar et al. 2003). We assumed Pi =1 (no preference) for species that were not detected in mosquito blood meals and were not significantly avoided (including many of the ‘other birds’ in figure 1a). We estimated the host reservoir competence for birds using data from laboratory infections (Komar et al. 2003; Komar et al. 2005). For unstudied species we used values for birds in the same family because there is more variation between taxonomic families of birds than within them (data for 22 species from Komar et al. (2003); ANOVA, F6,15=8.01, p=0.002). For mammals, we used a reservoir competence value of 0, based on experimental infections in several mammals (Komar 2003) and peak viremias seen in humans (Biggerstaff & Petersen 2002). We assessed the role of each species in amplifying WNV by calculating the change in the community reservoir competence (the sum of the Fi values) if the species was removed from the community (Schmidt & Ostfeld 2001).
Figure 1.
(a) Relative abundance of birds at two residential sites and three urban sites. For scientific names see AOU (2005). (b) Percent of avian feedings from each host species based on identification of Culex mosquito blood meals by PCR amplification of the cytochrome b gene followed by DNA sequencing. Sample size of mosquito feedings in parentheses. (c) Feeding indices of Culex mosquitoes and 95% CI. Positive values are preferences; negative values designate avoidance and are calculated as (−1/Pi). Columns with an asterisk are minimum avoidance estimates (see §2). All preferences, except hatched columns, are significantly different from 1 (two-tailed p<0.05; all robin preferences p<0.0001). (d) Amplification fraction (proportion of abundance×feeding preference×reservoir competence) of each species, a surrogate for the fraction of West Nile virus infectious mosquitoes resulting from feeding on that avian host.
We calculated the relative number of infectious mosquitoes produced by a single infected host of each species by multiplying the feeding index, Pi by the reservoir competence, Ci, of that species and used this number to compare the heterogeneity in secondary infections between species to that for super spreading events (Lloyd-Smith et al. 2005b). Super spreading events have been defined as individuals that infect more than the 99th percentile of a Poisson distribution with mean equal to R0 (Lloyd-Smith et al. 2005b). We considered the number of secondary infections from individuals of each host species and compared them to the null hypothesis of host homogeneity assuming R0=1.
Finally, we determined the quantitative impact of host heterogeneity on the reproductive ratio of the virus by calculating the relative reproductive ratio (Woolhouse et al. 1997)
where Ĉ is the average host competence at that site, fi is the fraction of blood meals from host i and ai is the fraction of the community made up by host i. This expression is based on the assumptions that: (i) mosquitoes form a homogenous group that feeds on avian host i with probability fi (corresponding to equation 23 and the m/1 model in Hasibeder & Dye (1988); see also Dye & Hasibeder (1986)) and (ii) that all hosts bitten by an infectious mosquito will become infected (Komar et al. 2003; otherwise R0,rel should be multiplied by this probability divided by the site average). This expression accounts for species differences in host competence, which are normalized by the site average competence. Thus, R0,rel=1 for a homogenous host community with randomly feeding mosquitoes. The relative reproductive ratio, R0,rel, measures the increase in the pathogen reproductive ratio, R0 (which also depends on vector biting rate, vector and host competence, host and vector death rates and host recovery period; Aron & May 1982), due to heterogeneity in feeding and host competence. We tested the hypothesis that increasing R0,rel would increase virus transmission (Anderson & May 1991) and lead to earlier detection of WNV-infected mosquitoes.
3. Results
A large fraction of the 174 hosts identified from blood meals by PCR and DNA sequencing came from a single, relatively uncommon species. American robins (Turdus migratorius; hereafter, robins) made up an among-site average of 3.7%±1, s.e.=1.2 (range among sites 1.0–7.5%), of the total avian abundance (figure 1a), but accounted for 43.4%±8.9 (range 24–71%) of mosquito feedings (figure 1b). Mosquitoes thus fed on robins 16.7±4.4 (range 6.4–30.6) times more often than would be expected if mosquitoes showed no feeding preferences (figure 1c). The fraction of blood meals that were identified as robins did not differ between 1.5 m height and canopy mosquito traps (χ2=0.76; n=103; p=0.38), suggesting that collection height did not bias estimates of feeding preferences towards this species. The feeding index for robins showed a slightly non-significant decrease with the abundance of robins at the site (r=−0.83; n=5; p=0.08).
Integrating these data with reservoir competence values from experimental infections (Komar et al. 2003, 2005) showed that if initial seroprevalence and infection rates were similar for each species then approximately 59.3%±9.1 (range 35–88%) of the WNV-infectious Culex mosquitoes likely became infected from feeding on viraemic robins (figure 1d). Between May and July 2004, 42.8%±15% of WNV-antibody-negative adult robins seroconverted (assuming no mortality) and became WNV-antibody-positive at these sites. Similarly, over a single month between July and August, 20%±10% of hatch-year (young of the year) robins seroconverted. In comparison, house sparrows, which were significantly avoided by mosquitoes (figure 1c; see below), had seroconversions rates (assuming 18% mortality; L Kramer et al. 2005, unpublished data) of only 11.8%±7.8% of adults for May–July and 7.2%±3.5% of hatch-year birds from July to August. Initial (May) seroprevalences were 56.3%±12.4% for adult American robins and 19.8%±0.2% for adult house sparrows, and 0% for hatch-year birds of both species, which were 3–6 times as numerous as adults by September. Thus our estimates of the fraction of WNV-infectious mosquitoes resulting from feeding on each host underestimate the importance of robins compared to house sparrows (figure 1d).
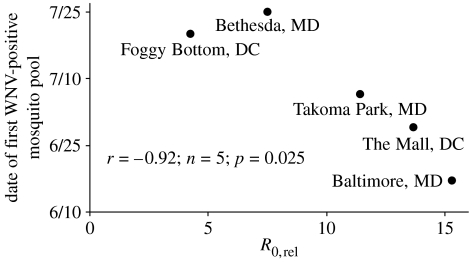
This host heterogeneity in mosquito feeding and reservoir competence increased the relative reproductive ratio, R0,rel, by a factor of 10.4±2.0 (range 4.3–15.3) relative to a homogenous host community. As a possible consequence, WNV-infected mosquitoes were first detected earlier at sites with larger R0,rel (figure 2).
Figure 2.
Relative reproductive ratios, R0,rel, resulting from host heterogeneity in mosquito feeding and reservoir competence and the date in 2004 that WNV was first detected in mosquitoes.
If a single WNV-infected robin was placed into one of these communities where on average each host infected a single mosquito, the robin would infect 23.9±6.6 (range 8.9–40.1) mosquitoes. These estimated numbers of secondary mosquito infections from a single robin relative to an average individual host was far greater than the 99th percentile of a Poisson distribution with mean 1, which is 4.0, and suggests that robins functioned as the species equivalent of super spreaders at all sites for this multi-host pathogen (Lloyd-Smith et al. 2005b).
The fraction of mosquito feedings from robins varied significantly over time (Kilpatrick et al. 2006). They were strongly preferred from May to August when they were found in 47.2%±7.6 (range 30.4–71.4%) of blood meals, but declined significantly in abundance in September when they were not found in any of 19 blood meals collected across the five sites. Thus, robins were even more important in WNV amplification from June to August than the season-average calculations above suggest. Mammals (15/23 mammalian blood meals were humans; others included eastern gray squirrels, Sciurus carolinensis (2 blood meals), cow (2), cat (1), dog (1) and opossum (1)) became an important host for Culex blood meals in August and September as Cx. pipiens shifted feeding away from robins (Kilpatrick et al. 2006).
Fish crows also were highly selected by mosquitoes at two sites, The Mall and Foggy Bottom (figure 1c), where they were fed on 24.6 and 10.4 times more often than expected from their low relative abundance. However, their rarity made them relatively unimportant in WNV amplification. We estimated that they accounted for only 2.0%±1.2 (range 0.02–6.0%) of WNV infected mosquitoes across the sites (figure 1d). The most abundant species at these sites, house sparrows, made up 55.7%±5.1 of the total abundance (figure 1a). However, they were fed on 7.9±2.5 (range 2.2–15.4) times less often than would be expected by chance (figure 1c) and they accounted for only 10.6%±4.0 (range 0–21.0%) of mosquito feedings (figure 1b), and an estimated 23.9%±7.2 (range 4.7–40.2%) of WNV-infectious mosquitoes (figure 1d). If a single infected house sparrow were placed into one of these communities where on average each host infected a single mosquito it would infect only 0.43±0.13 (range 0.11–0.83) mosquitoes.
We assessed the role of each avian species in amplifying or dampening WNV transmission by calculating the change in the community reservoir competence or average host quality after removing a species from the community. Removing robins resulted in the largest decrease in community reservoir competence, −29.7%±10.6 (range −8.9 to −64.5%) followed by house sparrows −14.4%±5.0, (range −2.1 to −28.9%). In contrast, removing poorly competent hosts resulted in increased community reservoir competence and increased likelihood of WNV amplification. The most important hosts for dampening WNV transmission were mammals (including humans), +17.1%±6.6 (range 3.0–38.1%), mourning doves, +7.2%±2.3 (range 1.3–14.0%), European starlings +5.5%±1.6 (range 0.5–9.7%), rock doves, +5.9%±5.3 (range 0.0–27.1%) and gray catbirds, +2.3%±1.1 (range 0.5–6.5%).
4. Discussion
Our data show extreme heterogeneity in mosquito feeding and as a result, strong heterogeneity in the transmission of WNV. Transmission of many other multi-host pathogens is also likely to be influenced by heterogeneity due to differences in host–vector contact rates, differences in infectiousness among hosts (St Louis encephalitis virus; Reisen et al. 2003) or both (eastern equine encephalitis virus (Komar et al. 1999; Hassan et al. 2003); Lyme disease: variation in relative tick burdens (LoGiudice et al. 2003)). Host heterogeneity is also likely to impact the transmission of directly transmitted multi-host pathogens such as avian influenza (Alexander 2000; Guan et al. 2004). As a result, it is crucial to quantify the heterogeneity in the transmission of a pathogen to avoid greatly underestimating R0 and the dynamics of epidemics (Lloyd-Smith et al. 2005b).
The extreme heterogeneity we documented in WNV transmission among hosts resulted in higher values of R0,rel than have been reported for any human vector-borne pathogens (Woolhouse et al. 1997), and at our most well studied site, the National Mall, the R0,rel of 15.3 exceeded the highest R0,rel measured for sexually transmitted diseases, including HIV (Woolhouse et al. 1997). To compare our estimates of R0,rel to other multi-host pathogens we estimated R0,rel for Lyme disease in the north east USA (Ixodes scapularis ticks transmitting Borrelia burgdorferi among mammal hosts; data from LoGiudice et al. 2003) and Chagas disease from Argentina (Triatoma infestans transmitting Trypanosoma cruzi between humans, chickens, and dogs; data from Gurtler et al. 1997; Cohen & Gurtler 2001). We found that R0,rel for Lyme disease varied from 11.1 to 16.5 depending on the densities of white-footed mice (1–100 ha) and chipmunks (1–50 ha). We found that R0,rel for Chagas varied in a complex fashion, depending on the abundance of hosts (each ranging from 1 to 5), from R0,rel=0.64 with humans, 1; chickens, 5; dogs, 1 to R0,rel=11.1 with humans, 1; chickens, 1; dogs, 2 and R0,rel was 3.4 for base scenario modelled in Cohen & Gurtler (2001). However, for both of these pathogens the major source of heterogeneity that resulted in high values of R0,rel was species differences in host competence (values of R0,rel were 1.9–2.5 for Lyme disease and 0.79–2.8 for Chagas disease with no variability in host competence) whereas for WNV, highly preferential feeding by mosquitoes resulted in the high values of R0,rel (R0,rel were nearly as high, 4.2–13.2, without variability in host competence; figure 2).
Our results show that transmission of WNV was dominated by heterogeneity in both urban and residential areas in the eastern USA where most human cases occur (Andreadis et al. 2004; Centers for Disease Control & Prevention 2006b). This suggests that WNV transmission in these areas is likely to be extremely intense in subgroups of hosts (particularly robins), but much less in others, resulting in large differences in WNV exposure of different host species. This has important implications for the impacts of WNV on bird populations (Marra et al. 2004). It also shows that avian abundance is a poor indicator of the relative importance of each species in WNV transmission and care must be taken when estimating the competence of a host community (Ezenwa et al. 2005).
Our results also suggest that robins, which occur across much of North America (Sallabanks & James 1999; Sauer et al. 2005), may be the most important amplification host for WNV in urban and residential areas in the eastern USA during the amplification of the virus in May–August and possibly in other regions of the USA as well (Apperson et al. 2004; Molaei et al. 2006). We found that mosquito-feeding preferences for robins were highly significant at all five sites. This finding challenges current beliefs about the primary role of corvids and house sparrows (Komar et al. 2001; Garvin et al. 2004) in WNV amplification. Although corvids were preferred by Culex mosquitoes, and thus can act as early indicators of WNV transmission, their rarity made them relatively unimportant in WNV amplification. In contrast, the avoidance of house sparrows by mosquitoes made them much less important than their abundance suggests (Komar et al. 2001).
Extreme heterogeneity, as we have demonstrated here, has important implications for disease control and prevention and for predicting which areas will be hotspots for pathogen transmission. The extremely high values of R0,rel of WNV and other pathogens in communities dominated by heterogeneity in the host community will make it extremely difficult to control epidemics without highly focused efforts (Woolhouse et al. 1997; Lloyd-Smith et al. 2005b). More broadly, our results demonstrate the importance of determining contact rates between vectors and host species in understanding pathogen transmission (Hasibeder & Dye 1988). Finally, we have shown that WNV transmission is dominated by host heterogeneity with a single species appearing to act as the equivalent of a community super spreader in both urban and residential areas of the eastern USA.
Acknowledgments
We thank the field crew of 2004 (especially H. Brightman and R. Peters); the Smithsonian's Neighborhood Nestwatch program and residents, the staff of Rock Creek Park (Meadowside Nature Center), Fort Dupont Park, the Smithsonian National Museum of Natural History, the National Gallery of Art and the Hirshhorn museum for permission to use their property; A. Dupuis for performing the 2003 PRNTs; the Wadsworth Center Molecular Genetics core for DNA sequencing and primer preparation; and J. Lloyd-Smith, R. Ostfeld, A. Dobson and three anonymous reviewers for helpful comments and discussion. This work was funded by NIAID contract no. NO1-AI-25490, the National Fish and Wildlife Foundation grant no. 2003-0209-000, and core funding to the Consortium for Conservation Medicine from the V. Kann Rasmussen Foundation.
Footnotes
Present address: Smithsonian Migratory Bird Center, National Zoological Park, 3001 Connecticut Ave, Washington, DC 20008, USA
References
- Alexander D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000;74:3–13. doi: 10.1016/s0378-1135(00)00160-7. doi:10.1016/S0378-1135(00)00160-7 [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Dynamics and control. Oxford University Press; London, UK: 1991. Infectious diseases of humans. [Google Scholar]
- Andreadis T.G, Anderson J.F, Vossbrinck C.R, Main A.J. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector-Borne and zoonotic diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. doi:10.1089/vbz.2004.4.360 [DOI] [PubMed] [Google Scholar]
- AOU. The A.O.U. check-list of North American birds. 7th edn. vol. 2005. American Ornithologists Union; Washington, DC: 2005. [Google Scholar]
- Apperson C.S, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York city, with characters and techniques for identification of Culex mosquitoes. J. Med. Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Apperson C.S, et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. doi:10.1089/153036604773083013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron J.L, May R.M. The population biology of malaria. In: Anderson R.M, editor. Population dynamics of infectious diseases: theory and applications. Chapman & Hall; London, UK: 1982. pp. 139–179. [Google Scholar]
- Bernard K.A, et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2001;7:679–685. doi: 10.3201/eid0704.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff B, Petersen L. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion. 2002;42:1019–1026. doi: 10.1046/j.1537-2995.2002.00167.x. doi:10.1046/j.1537-2995.2002.00167.x [DOI] [PubMed] [Google Scholar]
- Calisher C.H, Karabatsos N, Dalrymple J.M, Shope R.E, Porterfield J.S, Westaway E.G, Brandt W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2006a West Nile virus: statistics, surveillance, and control Atlanta, GA: Centers for Disease Control and Prevention. http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm
- Centers for Disease Control and Prevention 2006b West Nile virus: vertebrate ecology Atlanta, GA: Centers for Disease Control and Prevention. http://www.cdc.gov/ncidod/dvbid/westnile/birds&mammals.htm
- Cohen J.E, Gurtler R.E. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. doi:10.1126/science.1060638 [DOI] [PubMed] [Google Scholar]
- Crabtree M.B, Savage H.M, Miller B.R. Development of a species-diagnostic polymerase chain reaction assay for the identification of Culex vectors of St. Louis encephalitis virus based on interspecies sequence variation in ribosomal DNA spacers. Am. J. Trop. Med. Hyg. 1995;53:105–109. [PubMed] [Google Scholar]
- Dobson A. Population dynamics of pathogens with multiple host species. Am. Nat. 2004;164:S64–S78. doi: 10.1086/424681. doi:10.1086/424681 [DOI] [PubMed] [Google Scholar]
- Dye C, Gay N. Modeling the SARS epidemic. Science. 2003;300:1884–1885. doi: 10.1126/science.1086925. doi:10.1126/science.1086925 [DOI] [PubMed] [Google Scholar]
- Dye C, Hasibeder G. Population-dynamics of mosquito-borne disease—effects of flies which bite some people more frequently than others. Trans. R. Soc. Trop. Med. Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. doi:10.1016/0035-9203(86)90199-9 [DOI] [PubMed] [Google Scholar]
- Ebel G.D, Dupuis Alan P, II, Nicholas D, Young D, Maffei J, Kramer L.D. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg. Infect. Dis. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa V.O, Godsey M.S, King R.J, Guptill S.C. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B. 2005;273:109–117. doi: 10.1098/rspb.2005.3284. doi:10.1098/rspb.2005.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin M.C, Tarvin K.A, Smith J, Ohajuruka O.A, Grimes S. Patterns of West Nile virus infection in Ohio blue jays: implications for initiation of the annual cycle. Am. J. Trop. Med. Hyg. 2004;70:566–570. [PubMed] [Google Scholar]
- Guan Y, et al. H5N1 influenza: a protean pandemic threat. Proc. Natl Acad. Sci. USA. 2004;101:8156–8161. doi: 10.1073/pnas.0402443101. doi:10.1073/pnas.0402443101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtler R.E, Cohen J.E, Cecere M.C, Chuit R. Shifting host choices of the vector of Chagas disease, Triatoma infestans, in relation to the availability of hosts in houses in north-west Argentina. J.Appl. Ecol. 1997;34:699–715. [Google Scholar]
- Hasibeder G, Dye C. Population-dynamics of mosquito-borne disease—persistence in a completely heterogeneous environment. Theor. Popul. Biol. 1988;33:31–53. doi: 10.1016/0040-5809(88)90003-2. doi:10.1016/0040-5809(88)90003-2 [DOI] [PubMed] [Google Scholar]
- Hassan H.K, Cupp E.W, Hill G.E, Katholi C.R, Klingler K, Unnasch T.R. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- Haydon D.T, Cleaveland S, Taylor L.H, Laurenson M.K. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. 2006 West Nile virus, vol. 2006. http://www.phac-aspc.gc.ca/wnv-vwn/mon-e.html
- Hudson P.J, et al. Persistence and transmission of tick-borne viruses: Ixodes ricinus and louping-ill virus in red grouse populations. Parasitology. 1995;111:S49–S58. doi: 10.1017/s0031182000075818. [DOI] [PubMed] [Google Scholar]
- Husak M.S, Linder E.T. Geographic locale and relative dominance patterns among North American passerine communities. Ecography. 2004;27:668–676. doi:10.1111/j.0906-7590.2004.03708.x [Google Scholar]
- Kauffman E, Jones S, Dupuis A, II, Ngo K, Bernard K, Kramer L.D. Virus detection protocols for West Nile virus in vertebrate and mosquito specimens. J. Clin. Microbiol. 2003;41:3661–3667. doi: 10.1128/JCM.41.8.3661-3667.2003. doi:10.1128/JCM.41.8.3661-3667.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A.M, Kramer L.D, Campbell S, Alleyne E.O, Dobson A.P, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A.M, Kramer L.D, Jones M.J, Marra P.P, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. doi:10.1371/journal.pbio.0040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. West Nile virus: epidemiology and ecology in North America. Adv. Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- Komar N, Dohm D.J, Turell M.J, Spielman A. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris) Am. J. Trop. Med. Hyg. 1999;60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- Komar N, Panella N.A, Burns J.E, Dusza S.W, Mascarenhas T.M, Talbot T.O. Serologic evidence for West Nile virus infection in birds in the New York city vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001;7:621–625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of north American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Panella N.A, Langevin S.A, Brault A.C, Amador M, Edwards E, Owens J. Avian hosts for West Nile virus in St. Tammany Parrish, Louisiana, 2002. Am. J. Trop. Med. Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- Kramer L.D, Bernard K.A. West Nile virus in the western hemisphere. Curr. Opin. Infect. Dis. 2001;14:519–525. doi: 10.1097/00001432-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Leroy E.M, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. doi:10.1038/438575a [DOI] [PubMed] [Google Scholar]
- Li W.D, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. doi:10.1126/science.1118391 [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O, Cross P.C, Briggs C.J, Daugherty M, Getz W.M, Latto J, Sanchez M.S, Smith A.B, Swei A. Should we expect population thresholds for wildlife disease? Trends. Ecol. Evol. 2005a;20:511–519. doi: 10.1016/j.tree.2005.07.004. doi:10.1016/j.tree.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O, Schreiber S.J, Kopp P.E, Getz W.M. Superspreading and the impact of individual variation on disease emergence. Nature. 2005b;438:355–359. doi: 10.1038/nature04153. doi:10.1038/nature04153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld R.S, Schmidt K.A, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. 2003;100:567–571. doi: 10.1073/pnas.0233733100. doi:10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P.P, et al. West Nile virus and wildlife. Bioscience. 2004;54:393–402. [Google Scholar]
- Molaei G, Andreadis T, Armstrong P, Anderson J, Vossbrinck C. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo K.A, Kramer L.D. Identification of mosquito bloodmeals using polymerase chain reaction (PCR) with order-specific primers. J. Med. Entomol. 2003;40:215–222. doi: 10.1603/0022-2585-40.2.215. [DOI] [PubMed] [Google Scholar]
- Oda T, Uchida K, Mori A, Mine M, Eshita Y, Kurokawa K, Kato K, Tahara H. Effects of high temperature on the emergence and survival of adult Culex pipiens molestus and Culex quinquefasciatus in Japan. J. Am. Mosq. Control Assoc. 1999;15:153–156. [PubMed] [Google Scholar]
- Ostfeld R.S, Keesing F. Biodiversity and disease risk: the case of lyme disease. Conserv. Biol. 2000;14:722–728. doi:10.1046/j.1523-1739.2000.99014.x [Google Scholar]
- Ostfeld R.S, LoGiudice K. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology. 2003;84:1421–1427. [Google Scholar]
- Petersen L.R, Hayes E.B. Westward ho? The spread of West Nile virus. N. Engl J. Med. 2004;351:2257–2259. doi: 10.1056/NEJMp048261. doi:10.1056/NEJMp048261 [DOI] [PubMed] [Google Scholar]
- Reisen W.K, Chiles R.E, Martinez V.M, Fang Y, Green E.N. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 2003;40:968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg. Infect. Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallabanks R, James R.C. American Robin (Turdus migratorius) In: Poole A, Gill F, editors. The birds of North America. vol. 462. Philadelphia Academy of Natural Sciences; Philadelphia, PA: 1999. pp. 1–20. [Google Scholar]
- Sauer, J. R., Hines, J. E. & Fallon, J. 2005 The North American breeding bird survey, results and analysis 1966–2004. Version 2005.2., vol. 2005. Laurel, MD: USGS Patuxent Wildlife Research Center.
- Schmidt K.A, Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Spielman A, D'Antonio M. Hyperion; New York, NY: 2001. Mosquito: a natural history of our most persistent and deadly foe. [Google Scholar]
- Thomas L, et al. University of St. Andrews; Fife, UK: 2004. Distance 4.1. Release 2.: research unit for wildlife population assessment. [Google Scholar]
- Woolhouse M.E.J, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. doi:10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]