Abstract
Two reproductive isolated morphs of Arctic charr (Salvelinus alpinus), termed profundal and littoral charr according to their different spawning habitats, co-occur in the postglacial lake Fjellfrøsvatn in North Norway. All profundal charr live in deep water their entire life and have a maximum size of 14 cm, while the littoral charr grow to 40 cm. Some small and young littoral charr move to the profundal zone in an ontogenetic habitat shift in the ice-free season and the rest of the population remains in epilimnic waters. The two morphs had different diet niches in the profundal zone: the profundal charr ate typical soft-bottom prey (chironomid larvae, pea mussels and benthic copepods), while the young littoral charr mainly consumed crustacean zooplankton. In four other lakes without a profundal morph (i.e. monomorphic populations), young charr also performed ontogenetic habitat shifts to the profundal zone and fed on zooplankton. The profundal morph of Fjellfrøsvatn therefore utilize a food resource niche that neither the littoral morph nor comparable monomorphic populations exploit. This suggests that intraspecific resource competition has driven incipient ecological speciation of the profundal charr of Fjellfrøsvatn. The exploitation of the soft-bottom resources by the profundal charr supports earlier experimental findings that the profundal morph is genetically different in trophic behaviour and morphology. The sympatric ecological divergence within the profundal habitat is possible because unexploited food resources (soft-bottom profundal prey) are available. Apparently, this represents a case of incipient segregation by expansion to new resource types (niche invasion), and not by subdivision of one broad ancestral niche.
Keywords: niche expansion, insipient speciation, Salvelinus alpinus, profundal feeding
1. Introduction
Ecological speciation occurs when divergent natural selection between populations that exploit distinct ecological niches leads to the evolution of reproductive isolation (Schluter 2001). The initial process of ecological speciation involves disruptive selection on resource or habitat use, where ecological processes such as resource competition and predation are central (Johnson & Gullberg 1998; Kondrasov & Kondrasov 1999; Dieckmann & Doebeli 1999; Schluter 2001). Resource divergence between sympatric populations may occur in two ways (Schluter 2000): the first is by a subdivision of resources from one broad niche to two narrow niches (niche specialization), and the second is by an expansion of the ancestral niche to new resource types or environments (niche invasion). Model studies of sympatric speciation indicate that both scenarios are possible (Wilson & Turelli 1986; Johnson & Gullberg 1998; Dieckmann & Doebeli 1999; Kondrasov & Kondrasov 1999; Kawata 2001; Gavrilets 2003; Ackermann & Doebeli 2004; Doebeli et al. 2005; Polechova & Barton 2005). Disruptive selection on trophic behaviour, morphology and/or physiology within a single population towards exploitation of different trophic niches can occur rapidly if the environment has unoccupied resources (Johnson & Gullberg 1998; Schluter 2000). So far, however, there are few examples of invasions of vacant niches and subsequent sympatric speciation within the same habitat of vertebrate populations (Kawata 2001).
Resource polymorphisms have repeatedly been found for postglacial fishes, including Arctic charr Salvelinus alpinus (Skúlason & Smith 1995; Taylor 1999; Schluter 2001; Robinson & Parsons 2002). In many cases, a generalist ancestor is assumed to have segregated into resource specialists, with subsequent morph formation (reviewed for Arctic charr by Jonsson & Jonsson 2001; Klemetsen et al. 2003a). The most common divergence is found along the epipelagic–littoral (often termed limnetic–benthic) resource axis. The demonstration of a separate charr morph that is completely confined to the profundal zone of Fjellfrøsvatn north Norway (Klemetsen et al. 1997) was therefore unusual. The profundal zones of oligotrophic postglacial lakes have restricted food resources for fish because of low diversities and densities of benthos (Mousavi 2002) that are often buried in the sediment. The physical habitat is very different by being uniformly cool and dark, mostly flat and muddy and without vegetation.
The morphs are completely isolated in their time (September versus February) and place (littoral versus profundal) of reproduction (Klemetsen et al. 1997). We term them littoral charr and profundal charr according to their spawning habitats. The profundal charr are always small (less than 15 cm, both sexes mature from 7 cm) and remain in the profundal zone for their entire lifespan (Klemetsen et al. 1997, 2003a). The littoral charr grow larger (40 cm) and perform ontogenetic habitat shifts between all habitats of the lake, with some young and small fish moving to the profundal zone in the ice-free season. The morph pair differs in their food-transmitted parasites (Knudsen 1995; Knudsen et al. 1997). This suggests divergent diet niches, but a detailed study of their diets has not previously been published. Rearing experiments demonstrated genetically based differences in morphology and behaviour between the two morphs. Naive offspring of the profundal charr were less aggressive, rested more on the bottom and were more effective predators on typical profundal benthos prey (Klemetsen et al. 2002b). Littoral charr were more effective on surface prey, plankton and littoral benthos (Klemetsen et al. in press).
Differences in behaviour and morphology of sympatric pairs of Arctic charr are usually linked to divergence in the feeding ecology (Snorrason et al. 1994; Adams et al. 1998; Alekseyev & Pichugin 1998; Fraser et al. 1999). Here, we studied the feeding ecology of the two Arctic charr morphs in Fjellfrøsvatn in order to explore mechanisms of morph formation and possible incipient speciation. Our first hypothesis was that profundal charr and young littoral charr caught in the profundal zone have divergent diet niches related to their different parasite communities and the differences in morphology and behaviour found by experiments. We expected that the diet of the profundal morph consisted of profundal benthos prey types, while the profundal diet of young littoral charr was broader but dominated by zooplankton. Our second hypothesis was that the diet divergence of the two morphs was a result of a niche invasion of the profundal morph by expansion of the niche of the ancestral population. To test this hypothesis, we compared the diets of the sympatric morphs of Fjellfrøsvatn with the profundal diet niches of young charr from four other north Norwegian lakes that had no separate profundal morphs (monomorphic populations). If the diet of young charr caught in the profundal zone of these lakes included amounts of typical profundal soft-bottom benthos, the niche invasion hypothesis would be refuted as this would indicate that profundal benthos is a constituent of the ancestral niche of Arctic charr in northern lakes. We therefore expected that young charr from these monomorphic populations had diets similar to the young littoral morph in Fjellfrøsvatn when caught in the profundal zone. Takvatn was of special interest among these control lakes because its charr population was founded by littoral charr from Fjellfrøsvatn in the early 1930s (Svenning & Grotnes 1991). Thus, we could compare the profundal diets of two genetically close populations in two contrasting settings: first, in sympatry with the profundal morph in Fjellfrøsvatn, and second, in allopatry in Takvatn.
2. Material and methods
Fjellfrøsvatn is an oligotrophic and dimictic lake, 6.5 km2 in area and 88 m deep, situated at 125 m a.s.l. and 69° N in north Norway. The lake is ice-covered for about six months (November to May/June). For more details of the lake's characteristics, see Klemetsen et al. (1997). Arctic charr and brown trout (Salmo trutta) are the only fish species in the lake. The ontogenetic habitat shifts of the littoral morph take place in the ice-free season. Almost the entire population aggregates in shallow water, close to the ice, during the winter (Klemetsen et al. 2003b), but a few young fish may remain in deep water during the winter. We were therefore also able to get diet data from young littoral charr caught in the profundal zone from some winter months.
Takvatn and the three other reference lakes used in the study are also oligotrophic and dimictic and located in north Norway, but differ in their geographical settings and fish community compositions (table 1). All have monomorphic Arctic charr populations with young fish performing ontogenetic habitat shifts to the profundal zone.
Table 1.
Size, altitude (m a.s.l.) and fish species present (with decreasing population biomass) in the different study lakes from north Norway.
| lake | size (km2) | elevation (m a.s.l.) | max. depth (m) | fish species | location |
|---|---|---|---|---|---|
| Fjellfrøsvatn | 6.5 | 125 | 88 | S. alpinus, S. trutta | 69°05′ N, 19°20′ E |
| Takvatn | 15.0 | 214 | 80 | S. alpinus, S. trutta, G. aculeatus | 69°07′ N, 19°05′ E |
| Lille Rostavatn | 12.9 | 102 | 90 | S. alpinus, Lota lota, S. trutta, Thymallus thymallus, Salmo salar, Phoxinus phoxinus | 69°00′ N, 19°37′ E |
| Carajavri | 7.3 | 611 | 28 | S. alpinus, L. lota | 69°25′ N, 22°50′ E |
| Storvatn | 0.23 | 8 | 17 | S. alpinus, S. trutta, G. aculeatus | 70°39′ N, 23°43′ E |
The fish sampling in Fjellfrøsvatn was done monthly in both the littoral zone (0–10 m depth) and the profundal zone (25–40 m depth) from July to December 1992 and in March and May 1993. The lake was re-sampled every autumn (September/October) for 7 years from 1993 to 2003. In Takvatn, the profundal zone was sampled once each month from July to November 1985 and in March and May 1988. Takvatn was also sampled in 1989, 1999 and 2001 (August/October). In the three other lakes with monomorphic population of Arctic charr, the profundal zones were sampled at different times in the ice-free season: Lille Rostavatn (June, August, October 1997), Storvatn (October 1997) and Carajavri (August 1997). In all lakes, the fish were sampled using multi-mesh gillnets with mesh sizes from 10 to 45 mm (knot to knot). The wild phenotypes of the Fjellfrøsvatn charrs differ in a range of head, fin and body measurements. Of 14 morphological variables, 11 were significantly different between the morphs, and most of the differences persisted in hatchery raised offspring (see Klemetsen et al. 2002b for details). The morphs were separated by external morphology and colour in the field (see electronic supplementary material). Profundal charr are a faint yellow–brown with a brass tinge, with deep bodies and large eyes. There are no, or very faint, parr marks (Klemetsen et al. 1997). Juvenile littoral charr are silvery and slender, with small eyes and they often have parr marks. The DNA analyses performed by Westgaard et al. (2004) confirmed that the field separation is correct. Very few immature specimens of the profundal morph were caught in the smallest meshes (10 mm). We took a conservative approach and only included mature profundal charr in the diet comparisons. This excluded the few cases where it was difficult to separate the morphs in the field.
The fish were weighed (g), measured (mm fork length) and later aged by otoliths. Stomach contents were preserved in 70% ethanol, the prey items were identified and sorted into seven prey groups including: (i) littoral prey (Lymnaea peregra, Gammarus lacustris, mayfly, caddisfly and stonefly larvae); (ii) zooplankton prey (limnetic cladocerans and copepods); (iii) insect (mainly chironomid pupae); (iv) the semi-benthic chydorid cladoceran Eurycercus lamellatus and three prey types frequently occurring on the soft profundal substratum, (v) chironomid larvae; (vi) the benthic copepod Acanthocyclops gigas and (vii) pea mussels Pisidium sp. A few littoral charr stomachs had terrestrial insects along with chironomid pupae. These were included in group (iii). The abundances of prey were estimated according to the relative volume method of Amundsen (1995).
3. Results
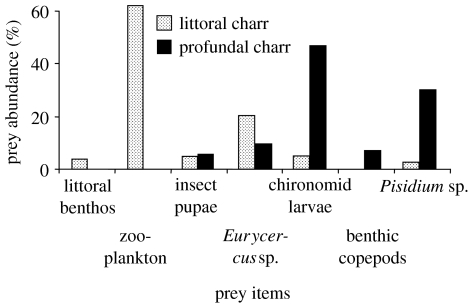
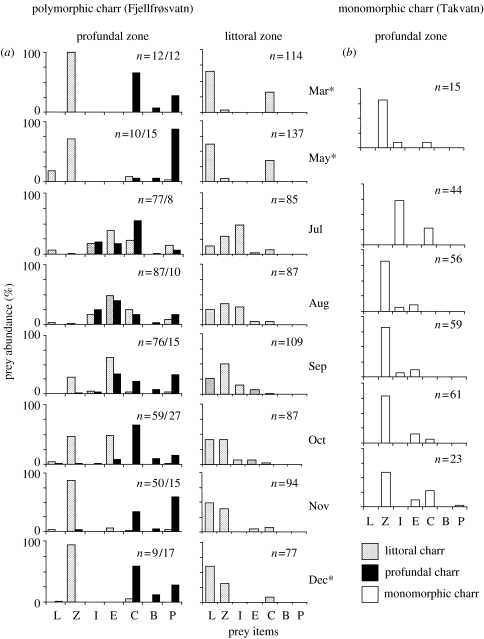
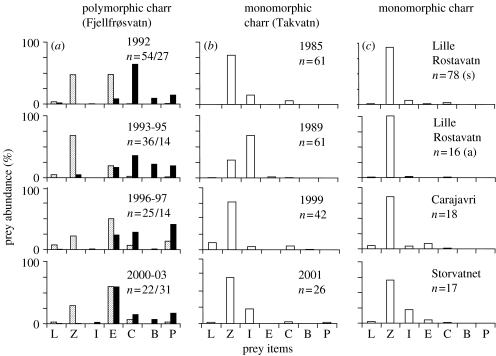
The sympatric juveniles of the littoral morph (n=380; length: =119.8 mm±18.5 s.d., range: 73–149 mm; age: =2.7 years ±0.9 s.d., range: 1–4) and mature specimens of the profundal morph (n=119; length: =109.5 mm ±18.2 s.d., range: 74–142 mm; age: =4.4 years ±1.4 s.d., range: 3–8) of Arctic charr in Fjellfrøsvatn fed on different prey items in the profundal zone when all seasons of 1992–1993 were combined (figure 1). The main prey for the profundal morph was soft-bottom benthos, including chironomid larvae, pea mussels and benthic copepods. Zooplankton was usually absent from the diet. The diet of the littoral morph was, in contrast, dominated by zooplankton. Profundal soft-bottom benthic prey was hardly eaten. These strongly divergent trophic niches were consistent throughout most of the year (figure 2a). Some minor diet overlap between the two morphs occurred only in the summer months (July and August), when chironomid pupae and larvae were eaten to some extent by both morphs. Chydorids (E. lamellatus) were also eaten by both morphs from July to October, but were always more dominant in the diet of the littoral morph. From September and throughout the winter and spring, the morphs were completely segregated in diet niches (figure 2a). The divergence in the profundal diet niches of the two Arctic charr morphs was consistent over a period of more than a decade (figure 3a). Within the littoral zone, the littoral morph fed mainly on littoral benthos and zooplankton throughout the year (figure 2a). The chironomid larvae that were occasionally taken by the charr in the littoral zone were different species than those taken by the profundal charr in the profundal zone.
Figure 1.
Annual diet composition (all months combined) of polymorphic littoral and profundal Arctic charr caught in the profundal habitat in Fjellfrøsvatn.
Figure 2.
Monthly diet composition of: (a) polymorphic Arctic charr from Fjellfrøsvatn from the profundal and littoral habitats and (b) monomorphic Arctic charr from Takvatn caught in the profundal habitat throughout the year (asterisks denotes sampling month with ice). n, number of fish; prey items: L, littoral benthos; Z, zooplankton; I, insect pupae; E, Eurycercus; C, chironomid larvae; B, benthic copepods; P, Pisidium.
Figure 3.
The stability of the diet composition in the profundal habitat of: (a) polymorphic Arctic charr from Fjellfrøsvatn, (b) monomorphic Arctic charr from Takvatn over several years and (c) three monomorphic Arctic charr populations (s, summer; a, autumn). n, number of fish; for prey items: see figure 2.
Young monomorphic charr in Takvatn (descendants of the littoral charr in Fjellfrøsvatn) had a profundal diet niche dominated by zooplankton in all seasons, similar to the littoral charr morph in Fjellfrøsvatn (figure 2a,b). The chydorid E. lamellatus, however, was found more frequently in charr stomachs from Fjellfrøsvatn than in Takvatn. The diet niche of the young monomorphic charr in Takvatn was stable between years (figure 3b). Also, the profundal diets of young monomorphic Arctic charr caught at different times of the ice-free season in the three other lakes were dominated by zooplankton and remarkably similar to the profundal diets of Takvatn charr and young littoral charr in Fjellfrøsvatn (figure 3c). Soft-bottom prey types from the profundal zone (chironomid larvae, Pisidium and benthic copepods) were absent or had very low abundances in the diets. The low abundances of surface and littoral prey indicate that the fish had been in deep water for a considerable time prior to being caught in the profundal nets.
4. Discussion
The juveniles of the littoral morph in Fjellfrøsvatn had mainly a zooplanktivorous diet niche in the profundal habitat, and consumed mainly zooplankton and littoral benthos in the littoral zone of the lake. The diets of the littoral charr were therefore similar in the littoral and the profundal habitats. In contrast, the profundal morph mainly utilized soft-bottom prey types. The diets of the two morphs were almost entirely different except for some overlap in the utilization of semi-benthic chydorids and chironomid pupae during a few summer months when these prey types are usually abundant. The results support the hypothesis that the two sympatric Arctic charr morphs had highly divergent trophic niches when co-occurring within the profundal habitat.
The persistent diet segregation between the two morphs both through the year and between years suggests a high stability in their foraging niches in Fjellfrøsvatn. This conclusion supported studies using food-transmitted parasites as indicators of the diet niches of the morph pair in the lake (Knudsen 1995; Knudsen et al. 1997, 2004). These field results mirror observations from experimental studies where naive offspring of the profundal morph are more successful in acquiring typical profundal prey (chironomid larvae) than the littoral morph (Klemetsen et al. 2002b), while littoral charr offspring are more effective predators on surface prey (Gerris), zooplankton (Daphnia) and littoral benthos (Gammarus; Klemetsen et al. in press). Field studies have repeatedly found littoral–pelagic resource diversifications in Arctic charr (see reviews by Jonsson & Jonsson 2001 and Klemetsen et al. 2003a) and specific diet preferences have been observed between offspring of littoral–pelagic morphs (Skúlason et al. 1993; Adams & Huntingford 2002a,b), but similar segregations between littoral and profundal morphs are rare. Distinct profundal charr morphs, often recognized as separate species (see Freyhof & Kottelat 2005), are known from several pre-alpine lakes. Salvelinus profundus from Lake Konstanz (now extinct because of eutrophication) fed entirely on profundal benthos (cocoons of Turbellaria, Pisidium, copepods, chironomids) during winter (Dörfel 1974; Freyhof & Kottelat 2005). Likewise, Quartier (1951) found that the profundal charr of Lake Neuchâtel (also extinct) had eaten profundal benthos. These early records indicate that segregations of charr morphs, by expansion to a profundal benthivore niche like in Fjellfrøsvatn, may have taken place in the pre-alpine lakes of Europe.
The four control lakes with monomorphic populations are spread over a wide geographic region and represent typical fish communities of subarctic charr lakes. We found that ontogenetic niche shifts with young fish moving to the profundal zone were common in monomorphic Arctic charr populations irrespective of the fish community of their lake and that their food niches never included typical profundal benthos. The charr population in Takvatn was founded by littoral charr from Fjellfrøsvatn about 70 years ago (Svenning & Grotnes 1991), and exhibits typical ontogenenetic niche shifts (Klemetsen et al. 1989, 1992, 2002a). The charr did not expand their feeding niche to include soft-bottom prey despite a high intraspecific competition (Amundsen 1989, 1995) and high interspecific competition from three-spined sticklebacks (Gasterosteus aceuleatus) in the littoral zone (Jørgensen & Klemetsen 1995). This indicates that the ancestral niche of the Arctic charr does not include soft-bottom profundal prey, as this resource is not utilized by introduction to a new lake with very high inter and intraspecific competition for food.
Our results support the hypothesis that a niche expansion (niche invasion sensu Schluter 2000), and not a subdivision of the ancestral niche, has taken place in Fjellfrøsvatn. Thus, the profundal morph of Fjellfrøsvatn has evolved from the ancestral littoral morph to become an effective soft-bottom prey feeder by invading a novel profundal food niche. The morph pair in Fjellfrøsvatn has strong reproductive isolation and genetic differences in behaviour and morphology (Klemetsen et al. 1997, 2002b, in press), in addition to differences in microsatellite DNA (Westgaard et al. 2004; Wilson et al. 2004). Arctic charr probably immigrated to Fjellfrøsvatn in early postglacial times when the sea-level was high and the geography of the area makes a later, second invasion unlikely (Klemetsen et al. 1997). The two morphs therefore probably diverged in sympatry.
Resource competition seems important as a driving force in ecological speciation (Schluter 1993, 2000, 2001; Swanson et al. 2003; Bolnick 2004; Rundle & Nosil 2005). Intraspecific competition is diversifying in the sense that any individual able to use an exclusive resource efficiently will experience reduced intraspecific competition and fitness advantages (Roughgarden 1972; Swanson et al. 2003). High intraspecific resource competition within the ancestral Arctic charr population in Fjellfrøsvatn may have promoted some individuals to utilize a vacant food niche while the ancestral population continued to use the original resources. In model studies of incipient sympatric speciation, disruptive selection is central to initiate divergence (Johnson & Gullberg 1998; Ackermann & Doebeli 2004), and may involve a period of individual specialization (Bolnick et al. 2003). Individual charr are able to specialize on a few prey types over long time intervals (Amundsen 1995; Knudsen 1995; Knudsen et al. 2004). Thus, the divergent dietary niches observed in the profundal habitat of Fjellfrøsvatn may reflect that resource competition drove the initial stage of the morph formation process in the lake. Expansion to new resources or environments is an important issue in adaptive radiation, and the process of speciation can occur rapidly if an unoccupied niche is invaded (Schluter 2000). Resource-dependent selection of individuals is central in sympatric speciation, especially with respect to adaptations for consuming untapped resources (Wilson & Turelli 1986; Johnson & Gullberg 1998; Schluter 2000; Kawata 2001). In order to utilize a new resource type effectively, strong selective pressure on the consumer to improve advantageous traits (adaptive behaviour and phenotypic plasticity) is expected (Pritchard & Schluter 2001). Evidently, the profundal morph is able to locate and feed on prey items partly buried in the soft bottom substrate in the dim light intensities at 25 m depths or more (which become extremely dim in the winter, with thick ice, snow and polar night darkness). This suggests that specialized foraging traits (i.e. in behaviour, senses and morphology) that improve the exploitation of benthic prey types have been selected, possibly by disruptive selection. Individuals that use a narrow diet niche are under strong selection to evolve novel traits in behaviour and morphology (Futuyama & Moreno 1988; Schluter 2000; Albertson et al. 2003; Streelman & Danley 2003). Trade-offs in functional morphology and foraging behaviour are often found between pelagic and littoral morphs among postglacial fishes (Schluter 1993, 1996, 2000; Robinson & Wilson 1994; Skúlason et al. 1999; Prolux & Magnan 2002). Genetically based differences in morphology (fins, head, body shape) and behaviour (prey handling, aggressive behaviour, microhabitat selection) were found between the morphs from Fjellfrøsvatn (Klemetsen et al. 2002b, in press). These findings suggest that ecomorphological traits have evolved in the profundal morph to efficiently exploit alternative food resources.
The profundal habitat provides marginal food resources for fish growth in subarctic lakes (Klemetsen et al. 1989, 1992). The profundal morph of Fjellfrøsvatn grows slowly, and both sexes become sexually mature at the extremely small size of 7 cm (weight 3–5 g). The maximum length observed is 14 cm (Klemetsen et al. 1997). In Arctic charr, heterochronous adaptations that occur early in the ontogeny (Eiriksson et al. 1999; Adams & Huntingford 2002a) may be important driving mechanisms in the evolution of trophic polymorphism (Skúlasson et al. 1999; Klemetsen et al. 2003a). Small morphotypes are often paedomorphs, i.e. they mature sexually while their somatic phenotypes are still juvenile. In most theoretical studies of sympatric speciation, ecological specialization is the driving force with assortative mating as an important by-product to fulfil reproductive isolation (Schluter 2000; Dieckmann & Doebeli 2004; Rundle & Schluter 2004). Body size is often an important trait in assortative mating of sympatric pairs of postglacial fishes (Nagel & Schluter 1998; Rundle & Schluter 2004; Boughman et al. 2005). The very small-sized profundal phenotype of Fjellfrøsvatn is most likely under selection for heterochronic differences, notably paedomorphosis, which could produce traits that are important for assortative mating. The evolution of assortative mating may thus be based directly on ecological traits induced by a profundal lifestyle.
The profundal zone in Fjellfrøsvatn is a refuge almost devoid of fish predators. Only a few piscivorous charr and brown trout have been caught here in spite of extensive sampling through many years and fish-eating birds, mainly red-breasted mergansers Mergus serrator, rarely dive that deep. Predation is suggested to be an important cause of the commonly observed ontogenetic niche shift of young monomorphic Arctic charr to the profundal zone (Klemetsen et al. 1989, 2003a; Langeland et al. 1991). Absence of predators probably contributed importantly to the development of a viable life-history strategy of the profundal charr morph of Fjellfrøsvatn.
In conclusion, we found highly divergent trophic niches between a reproductive isolated pair of polymorphic Arctic charr when co-occurring in a profundal habitat that has marginal diet resources. Four monomorphic Arctic charr populations had planktivorous diets in the profundal zone highly similar to the diet of the littoral morph, but distinctly different from the profundal morph, in Fjellfrøsvatn. This suggests that the profundal morph has evolved to become an effective consumer of a novel and previously untapped resource type (the profundal, soft-bottom prey). This niche invasion is most likely a result of disruptive selection that has promoted behavioural and ecomorphological adaptations to the new resource type. The existence of such adaptations in the profundal morph is supported by experimental studies with the morph pair. High intraspecific resource competition and low predation have most likely been fundamental in the process of incipient divergence from the ancestral population. The polymorphism of charr in Fjellfrøsvatn could represent a case of incipient speciation by niche expansion, where a separate morph has invaded a vacant resource niche in a habitat concurrently occupied by the ancestral population.
Acknowledgments
Many people have been involved in these studies, but we want especially to thank Laina Dalsbø for her skilled field and laboratory assistance over many years.
Supplementary Material
From top to bottom: littoral morph male; littoral morph female; profundal morph male; profundal morph female. Photograph, Rune Knudsen
References
- Ackermann M, Doebeli M. Evolution of niche width and adaptive diversification. Evolution. 2004;58:2599–2612. doi: 10.1111/j.0014-3820.2004.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Adams C.E, Huntingford F.A. The functional significance of inherited differences in feeding morphology in sympatric polymorphic population of Arctic charr. Evol. Ecol. 2002a;16:15–25. doi:10.1023/A:1016014124038 [Google Scholar]
- Adams C.E, Huntingford F.A. Inherited differences in head allometry in polymorphic Arctic charr from Loch Rannoch, Scotland. J. Fish Biol. 2002b;60:515–520. doi:10.1006/jfbi.2002.1867 [Google Scholar]
- Adams C.E, Huntingford F.A, Fraser D, Greer R.G, Askew C.M, Walker A.F. Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. J. Fish Biol. 1998;52:1259–1271. doi:10.1111/j.1095-8649.1998.tb00970.x [Google Scholar]
- Albertson R.C, Streelman J.T, Kocher T.D. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl Acad. Sci. USA. 2003;100:5252–5257. doi: 10.1073/pnas.0930235100. doi:10.1073/pnas.0930235100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyev S.S, Pichugin M.Y. A new form of charr Salvelinus alpinus from lake Davatchan in Transbaikalia and its morphological differences from sympatric forms. J. Ichol. 1998;38:328–337. [Google Scholar]
- Amundsen P.-A. Effects of intensive fishing on food consumption and growth of stunted Arctic charr (Salvelinus alpinus L.) in Takvatn, northern Norway. Physiol. Ecol. Japan. 1989;1(Suppl.):265–278. [Google Scholar]
- Amundsen P.-A. Feeding strategy of Arctic charr (Salvelinus alpinus): general opportunist, but individual specialist. Nordic J. Freshw. Res. 1995;71:150–156. [Google Scholar]
- Bolnick D. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004;58:608–618. [PubMed] [Google Scholar]
- Bolnick D.I, Svanbäck R, Fordyce J.A, Yang L.H, Davis J.M, Hulsey C.D, Forister M.L. The ecology of individuals: incidence and implications of individual specialisation. Am. Nat. 2003;161:1–28. doi: 10.1086/343878. doi:10.1086/343878 [DOI] [PubMed] [Google Scholar]
- Boughman J.W, Rundle H.D, Schluter D. Parallel evolution of sexual isolation in sticklebacks. Evolution. 2005;59:361–373. [PubMed] [Google Scholar]
- Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. doi:10.1038/22521 [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Doebeli M. Adaptive dynamics of speciation: sexual populations. In: Dieckmann U, Doebeli M, Metz J.A.J, Tautz D, editors. Adaptive speciation. Cambridge University Press; Cambridge, UK: 2004. pp. 76–111. [Google Scholar]
- Doebeli M, Dieckmann U, Metz J.A.J, Tautz D. What have we also learned: adaptive speciation is theoretically plausible. Evolution. 2005;59:691–695. [PubMed] [Google Scholar]
- Dörfel H.-J. Untersuchungen zur Problematik der Saiblingpopulationen (Salvelinus alpinus L.) im Überlinger See (Bodensee) Arch. Hydrobiol. 1974;47(Suppl.):80–105. [Google Scholar]
- Eiriksson G.M, Skulason S, Snorrason S.S. Heterochrony in skeletal development and body size in progeny of two morphs of Arctic charr from Tingvallavatn, Iceland. J. Fish Biol. 1999;55(Suppl. A):175–185. [Google Scholar]
- Fraser D, Adams C.E, Huntingford F.A. Trophic polymorphism among Arctic charr Salvelinus alpinus from Loch Ericht, Scotland. Ecol. Freshw. Fish. 1999;7:184–191. [Google Scholar]
- Freyhof J, Kottelat M. Salvelinus evasus sp. n. from deep waters of Lake Ammersee, southern Germany (Teleostei: Salmonidae), with comments on two extinct species. Rev. Suisse Zool. 2005;112:253–269. [Google Scholar]
- Futuyama D.J, Moreno G. The evolution of ecological speciation. Ann. Rev. Ecol. Syst. 1988;19:16–36. [Google Scholar]
- Gavrilets S. Models of speciation: what have we learned in 40 years? Evolution. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Johnson P.A, Gullberg U. Theory and models of sympatric speciation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York, NY: 1998. pp. 79–89. [Google Scholar]
- Jonsson B, Jonsson N. Polymorphism and speciation in Arctic charr. J. Fish Biol. 2001;58:605–638. doi:10.1006/jfbi.2000.1515 [Google Scholar]
- Jørgensen L, Klemetsen A. Food resource partitioning of Arctic charr Salvelinus alpinus (L.) and the three-spined stickleback, Gasterosteus aculeatus L., in the littoral zone of lake Takvatn in northern Norway. Ecol. Freshw. Fish. 1995;4:77–84. [Google Scholar]
- Kawata M. Invasion of vacant niches and subsequent sympatric speciation. Proc. R. Soc. B. 2001;269:55–63. doi: 10.1098/rspb.2001.1846. doi:10.1098/rspb.2001.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemetsen A, Amundsen P.A, Muladal H, Rubach S, Solbakken J.I. Habitat shifts in a dense, resident Artic charr Salvelinus alpinus population. Physiol. Ecol. Japan. 1989;1(Suppl.):187–200. [Google Scholar]
- Klemetsen A, Muladal H, Amundsen P.-A. Diet and food consumption of young, profundal Takvatn charr (Salvelinus alpinus) Nordic J. Freshw. Res. 1992;67:34–43. [Google Scholar]
- Klemetsen A, Amundsen P.A, Knudsen R, Hermansen B. A profundal, winter-spawning morph of Arctic charr Salvelinus alpinus (L.) in lake Fjellfrøsvatn, northern Norway. Nordic J. Freshw. Res. 1997;73:13–23. [Google Scholar]
- Klemetsen A, Amundsen P.A, Grotnes P.E, Knudsen R, Kristoffersen R, Svenning M.A. Takvatn through 20 years: long-term effects of an experimental mass removal of Arctic charr Salvelinus alpinus, from a subarctic lake. Environ. Biol. Fish. 2002a;64:39–47. doi:10.1023/A:1016062421601 [Google Scholar]
- Klemetsen A, Elliott J.M, Knudsen R, Sørensen P. Evidence for genetic differences in the offspring of two sympatric morphs of Arctic charr. J. Fish Biol. 2002b;60:933–950. doi:10.1006/jfbi.2002.1905 [Google Scholar]
- Klemetsen A, Amundsen P.-A, Dempson B, Jonsson B, Jonsson N, O'Connell M.F, Mortensen E. Atlantic salmon Salmo salar L, brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshw. Fish. 2003a;12:1–59. doi:10.1034/j.1600-0633.2003.00010.x [Google Scholar]
- Klemetsen A, Knudsen R, Staldvik F.J, Amundsen P.A. Habitat, diet and food assimilation of Arctic charr under the winter ice in two subarctic lakes. J. Fish Biol. 2003b;62:2082–1098. doi:10.1046/j.1095-8649.2003.00101.x [Google Scholar]
- Klemetsen, A., Knudsen, R., Primicerio, R. & Amundsen, P.-A. In press. Divergent natural selection on the feeding behaviour of two sympatric Arctic charr (Salvelinus alpinus) morphs. Ecol. Fresh. Fish
- Knudsen R. Relationships between habitat, prey selection and parasite infection in Arctic char (Salvelinus alpinus) Nordic J. Freshw. Res. 1995;71:333–344. [Google Scholar]
- Knudsen R, Kristoffersen R, Amundsen P.-A. Parasite communities in two sympatric morphs of Arctic charr, Salvelinus alpinus (L.), in northern Norway. Can. J. Zool. 1997;75:2003–2009. [Google Scholar]
- Knudsen R, Curtis M.A, Kristoffersen R. Aggregation of helminths: the role of feeding behaviour of fish hosts. J. Parasitol. 2004;90:1–7. doi: 10.1645/GE-3184. [DOI] [PubMed] [Google Scholar]
- Kondrasov A.S, Kondrasov F.A. Interactions among quantitative traits in the course of sympatric speciation. Nature. 1999;400:351–354. doi: 10.1038/22514. doi:10.1038/22514 [DOI] [PubMed] [Google Scholar]
- Langeland A, L'Abée-Lund J.H, Jonsson B, Jonsson N. Resource partitioning and niche shift in Arctic charr Salvelinus alpinus and brown trout Salmo trutta. J. Anim. Ecol. 1991;60:895–912. [Google Scholar]
- Mousavi, S. K. 2002 Community structure of Chironomidae (Diptera) in subarctic lakes. Doctor scientarium thesis, University of Tromsø, Norway.
- Nagel L, Schluter D. Body size, natural selection, and speciation in sticklebacks. Evolution. 1998;52:209–218. doi: 10.1111/j.1558-5646.1998.tb05154.x. [DOI] [PubMed] [Google Scholar]
- Polechova J, Barton N.H. Speciation through competition: a critical review. Evolution. 2005;59:1194–1210. [PubMed] [Google Scholar]
- Pritchard J.R, Schluter D. Declining interspecific competition during character displacement: summoning the ghost of competition past. Evol. Ecol. Res. 2001;3:209–220. [Google Scholar]
- Prolux R, Magnan P. Physiological performance of two forms of lacustrine brook charr, Salvelinus fontinalis, in the open water habitat. Environ. Biol. Fish. 2002;64:127–136. doi:10.1023/A:1016049820846 [Google Scholar]
- Quartier A. Morphologie et biologie de Salvelinus alpinus dans le lac de Neuchâtel. Rev. Suisse Zool. 1951;58:631–637. [Google Scholar]
- Robinson B.W, Parsons K.J. Changing times, spaces, and faces: tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes. Can. J. Fish. Aquat. Sci. 2002;59:1819–1833. doi:10.1139/f02-144 [Google Scholar]
- Robinson B.W, Wilson D.S. Character release and displacement in fishes: a neglected literature. Am. Nat. 1994;144:596–627. doi:10.1086/285696 [Google Scholar]
- Roughgarden J. Evolution and niche width. Am. Nat. 1972;106:683–718. doi:10.1086/282807 [Google Scholar]
- Rundle H.D, Nosil P. Ecological speciation. Ecol. Lett. 2005;8:336–352. doi:10.1111/j.1461-0248.2004.00715.x [Google Scholar]
- Rundle H.D, Schluter D. Natural selection and ecological speciation in sticklebacks. In: Dieckmann U, Doebeli M, Metz J.A.J, Tautz D, editors. Adaptive speciation. Cambridge University Press; Cambridge, UK: 2004. pp. 192–209. [Google Scholar]
- Schluter D. Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology. 1993;74:699–709. [Google Scholar]
- Schluter D. Ecological speciation in postglacial fishes. Phil. Trans. R. Soc. B. 1996;351:807–814. [Google Scholar]
- Schluter D. Oxford University Press; Oxford, UK: 2000. The ecology of adaptive radiation. [Google Scholar]
- Schluter D. The ecology and origin of species. Trends Ecol. Evol. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. doi:10.1016/S0169-5347(01)02198-X [DOI] [PubMed] [Google Scholar]
- Skúlason S, Smith T.B. Resource polymorphism in vertebrates. Trends Ecol. Evol. 1995;10:366–370. doi: 10.1016/s0169-5347(00)89135-1. doi:10.1016/S0169-5347(00)89135-1 [DOI] [PubMed] [Google Scholar]
- Skúlason S, Snorrason S.S, Ota D, Noakes D.L.G. Genetically based differences in foraging behaviour among sympatric morphs of Arctic charr (Pisces: Salmonidae) Anim. Behav. 1993;45:1179–1192. doi:10.1006/anbe.1993.1140 [Google Scholar]
- Skúlason S, Snorrason S.S, Jonsson B. Sympatric morphs, populations and speciation in freswater fish with emphasis on Arctic charr. In: Magurran A.E, May R.M, editors. Evolution of biological diversity. Oxford University Press; Oxford, UK: 1999. pp. 70–92. [Google Scholar]
- Snorrason S.S, Skúlason S, Jonsson B, Malmquist H.J, Jonasson P.M, Sandlund O.T, Lindem T. Trophic specialization in Arctic charr Salvelinus alpinus (Pisces Salmonidae): morphological divergence and ontogenetic niche shifts. Biol. J. Linn. Soc. 1994;52:1–18. doi:10.1006/bijl.1994.1035 [Google Scholar]
- Streelman J.T, Danley P.D. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 2003;18:126–130. doi:10.1016/S0169-5347(02)00036-8 [Google Scholar]
- Svenning M.-A, Grotnes P. Stationarity and homing ability of landlocked Arctic charr. Nordic J. Freshw. Res. 1991;66:36–43. [Google Scholar]
- Swanson B.O, Gibb A, Marks J.C, Hendrickson D.A. Trophic polymorphism and behavioural differences decrease intraspecific competition in a cichlid, Herichthys minckleyi. Ecology. 2003;84:1441–1446. [Google Scholar]
- Taylor E.B. Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Rev. Fish Biol. Fish. 1999;9:299–324. doi:10.1023/A:1008955229420 [Google Scholar]
- Westgaard J.I, Klemetsen A, Knudsen R. Genetic differences between two sympatric morphs of Arctic charr Salvelinus alpinus (L.) confirmed by microsatellite DNA. J. Fish Biol. 2004;65:1185–1191. doi:10.1111/j.1095-8649.2004.00524.x. [Google Scholar]
- Wilson A.J, Gislason D, Skúlason S, Snorrason S, Adams C.E, Alexander G, Danzmann R.G, Ferguson M.M. Population genetic structure of Arctic charr, Salvelinus alpinus from northwest Europe on large and small spatial scales. Mol. Ecol. 2004;13:1129–1142. doi: 10.1111/j.1365-294X.2004.02149.x. doi:10.1111/j.1365-294X.2004.02149.x [DOI] [PubMed] [Google Scholar]
- Wilson D.S, Turelli M. Stable underdominance and the evolutionary invasion of empty niches. Am. Nat. 1986;127:835–850. doi:10.1086/284528 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
From top to bottom: littoral morph male; littoral morph female; profundal morph male; profundal morph female. Photograph, Rune Knudsen