Abstract
The flowers and inflorescences of animal-pollinated dioecious plants are generally small and inconspicuous in comparison with outcrossing cosexual species. The net benefits of an attractive floral display may be different for dioecious compared to cosexual populations because dioecious species experience a more severe reduction in pollen delivery when pollinators forage longer on fewer individuals. Here, we develop a model that predicts the decrease in pollen delivery in dioecious relative to cosexual populations from female–female, female–male and male–male visit sequences as the number of individuals visited varies. To evaluate the predictions of our model we conducted a common garden experiment with dioecious and monoecious (cosexual) arrays of the insect-pollinated herb Sagittaria latifolia. We find that, although increasing the advertisements of floral rewards (i.e. increasing floral display) attracts more pollinators to individuals, the probability that these pollinators subsequently deliver pollen to neighbouring plants depends on sexual system. Because the number of individual plants visited per foraging trip did not increase significantly with floral display, the relative pollination success of dioecious versus monoecious populations decreases with increased floral display. We propose that this could explain why dioecy is strongly correlated with reduced floral display among angiosperm species.
Keywords: dioecy, flower size, floral display, monoecy, pollen delivery, pollination
1. Introduction
Dioecy is a rare sexual system in flowering plants occurring in only 6–10% of species (Renner & Ricklefs 1995). The separation of sexes evolves to reduce the harmful effects of inbreeding and/or to provide accelerating fitness gains to either or both sexes (Charnov et al. 1976; Charlesworth & Charlesworth 1987). The rarity of dioecy may be linked to the spatial separation of the sexes and the complete dependence of this sexual system on effective pollen vectors (e.g. insects, wind, water) for successful cross-fertilization (Bawa & Opler 1975; Givnish 1982; Bierzychudek 1987; Bawa et al. 1990; Charlesworth 1993). Dioecious species may experience a reduction in the proportion of pollen that is dispersed from male to female plants compared to cosexual (hermaphroditic and monoecious) species and this may affect how dioecious species allocate resources to attracting animal pollinators.
In cosexual species, large floral displays attract pollinators to individuals but may also result in increased geitonogamous pollination (pollen dispersal between flowers on the same plant) and pollen discounting (a reduction in outcrossed siring success resulting from self-pollination; e.g. Klinkhamer et al. 1994; Harder & Barrett 1995). Although pollen discounting can occur in dioecious species, because pollen can be lost in subsequent visits to other flowers on the same plant rather than exported (Harder & Wilson 1998), dioecy often evolves from monoecy in part due the reduced costs associated with geitonogamous pollination (de Jong & Geritz 2001; Reusch 2001; Barrett 2003). Pollinators usually visit more flowers per plant as daily floral display size increases (Schemske & Ågren 1995; Elle & Carney 2003), without necessarily increasing the amount of pollen actually exported. There is considerable evidence indicating that the advantage of increasing an individual's attractiveness diminishes as the benefit of additional visits is counterbalanced by increased geitonogamous selfing (e.g. Geber 1985; Klinkhamer et al. 1994; Cartar & Abrahams 1996). Therefore, the expectation might be that dioecious populations released from the constraints of geitonogamy may be free to evolve larger inflorescence sizes. However, dioecious species have reduced flower sizes (Bawa 1980; Charlesworth 1993) and equivalent flowers per inflorescence (Vamosi et al. 2003) compared with cosexual species. At present, there is no simple evolutionary explanation for these associations between floral display size and dioecy.
Theoretical models indicate that dioecious individuals can invade populations more easily when investment in floral display is low (Charlesworth & Charlesworth 1987), perhaps because reducing allocation to attraction may also reduce reproductive variance, which is thought to limit the evolution of dioecy (Wilson & Harder 2003). Consistent with these models, a phylogenetic analysis found that dioecy evolves more readily in clades with low allocation to floral display (Vamosi et al. 2003). Furthermore, when dioecy evolves in clades that have reduced investment in floral display, they may experience greater diversification than when they evolve in clades that invest more in floral display (Vamosi & Vamosi 2004). One reason for reduced diversification among showy (described as having flowers more than 1 cm in diameter) dioecious clades may be that when a dioecious species is showy, the evolution of increased sexual dimorphism becomes more likely, placing the lineage at a greater risk of extinction should pollinator service decline (Pannell & Barrett 1998; Vamosi & Otto 2002).
Models of the extinction of dioecious species often hinge on the idea that the separation of males and females in dioecious species results in greater antagonistic selection in males between attracting pollinators and having pollinators leave to visit females, making pollination more stochastic over evolutionary time scales. In plants with hermaphroditic flowers, pollen removal and pollen delivery usually occur during a single pollinator visit. In monoecious plants, pollen delivery and pollen removal do not occur during a single pollinator visit, but may occur in close sequence due to the proximity of male and female flowers. Large floral displays in these cosexual species can then result in more pollinator visits to an individual resulting in increased pollen removal and pollen delivery. However, in dioecious species, an increase in floral display may not necessarily increase pollination rates because pollen delivery and pollen removal are more spatially separated. Increased floral display may result in more visits per individual and actually reduce the proportion of pollen delivered to other individuals as the frequency of between-plant trips declines.
Here, we develop a theoretical model that illustrates how these simple pollen dispersal dynamics can result in constraints on effective pollen delivery for dioecious species that are not experienced by cosexual species. We then compare the net benefits of floral display in dioecious and monoecious arrays of the insect-pollinated clonal herb Sagittaria latifolia to determine whether variation in floral display results in patterns of pollen delivery that are dependent upon sexual system. Specifically, we examine pollinator visits to patches of dioecious and monoecious S. latifolia and ask: (i) does variation in floral display influence pollen delivery to other individuals? and (ii) do dioecious arrays suffer or benefit in terms of successful cross-pollination when increasing their allocation to floral display?
2. Modelling pollen delivery
A pollinator that visits V individuals in a dioecious array will (assuming an equal sex ratio and no pollinator preference for male or female individuals) visit males and females randomly making the probability of any single visit sequence equal to (1/2)V. We assume that, extensive pollen carryover is possible, allowing all females visited subsequent to the first male (i) in the sequence to be pollinated. Therefore, in (V−i) visits (i.e. visits subsequent to the first male), pollen is delivered to j females regardless of where in the sequence those females are visited (so long as they occur after a male is visited). The number of sequences where j females are pollinated then equals,
| 2.1 |
Summing over all the possible different values of j and i, the pollination probability for dioecy (illustrated in figure 1) then becomes:
| 2.2 |
To incorporate the potential effects of reduced inbreeding and/or resource compensation, we allow that, although females receive fewer pollinating visits, each pollinating visit to a dioecious female flower may produce more seeds compared to cosexual flowers. We set the dioecious seed output to twice that of a cosexual and equation (2.1) then becomes:
| 2.3 |
Figure 1.
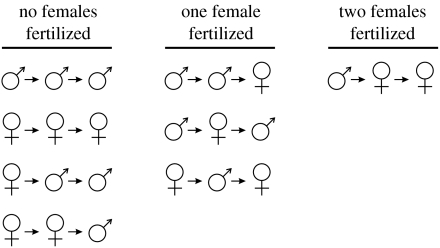
Number of fertilization events in a dioecious population, as a function of the sequence in which pollinators visit female (f) and male (m) plants. In this example, V=3 plants visited per pollinator foraging trip, which can result in either zero, one or two female plants being fertilized. The average number of pollen delivery events for this particular example is [x] [4(0)+3(1)+1(2)]/8=0.625x, where x is the advantage females experience over hermaphrodites (e.g. due to reduced inbreeding depression). Setting x to 2 (as in figure 2), then an average of 1.25 female plants are fertilized when pollinators visit three plants per trip. In contrast, all such trips in a hermaphroditic population fertilize two plants, producing a 2/1.25=1.6×advantage to hermaphroditism.
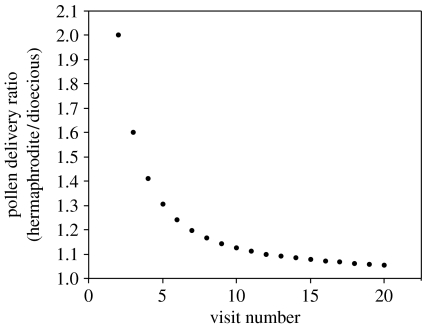
We can now compare the seed output in dioecious and cosexual arrays where, in the cosexual array, the number of pollen delivery events is simply V−1 (i.e. all but the first visit results in pollen delivery). The ratio of cosexual to dioecious seed output (R) depends largely on the value of V. As the number of individual plants visited per foraging trip, V, increases, the disadvantage experienced by dioecious arrays from male–male, female–male and female–female pollinator visits, decreases rapidly (figure 2). Thus, dioecious populations employing pollinators that make short foraging trips (in terms of individuals visited) are predicted to experience a disadvantage in seed output compared to cosexual populations.
Figure 2.
The disadvantage of dioecy in terms of pollen delivery decreases as the foraging trips of pollinators (V) includes pollen transfer between more individuals.
The number of individuals visited in a pollinator's foraging trip (V), and pollen carryover, has been considered previously in the pollination of animal-pollinated dioecious species (Barrett & Thomson 1982). Pollen carryover (and presumably the number of individuals visited per foraging trip) can be affected by floral display traits such as the number of open flowers per plant. As the number of flowers per individual increases, pollinators visit more flowers per individual, without increasing the amount of pollen exported (Harder & Barrett 1995). Outstanding empirical questions then include: (i) how is floral display related to pollen delivery to other individuals? and (ii) is the relationship between floral display and pollen delivery different between dioecious and monoecious populations?
3. Experimental investigation
Using S. latifolia, we can address how pollen delivery changes with floral display. Sagittaria latifolia is composed of clonal dioecious and monoecious populations and floral display is nearly equivalent between the two sexual types for individual ramets of the same size (Dorken & Barrett 2004a). The one-day flowers are 1.5–4 cm in diameter with 3–12 flowers open simultaneously in an inflorescence (M. E. Dorken & S. C. H. Barrett 2002, unpublished data). In dioecious populations, there are often fewer open flowers per inflorescence than in monoecious inflorescences but, on average, both male and female flowers are larger in dioecious populations (Dorken & Barrett 2004a). In monoecious populations, inflorescences are effectively unisexual at any one time because they are strongly protogynous, but male and female inflorescences grow in closer proximity than in dioecious populations as a result of clonal growth. Thus, mating opportunities in populations of the two sexual systems depend critically on the spatial arrangement of flowering inflorescences.
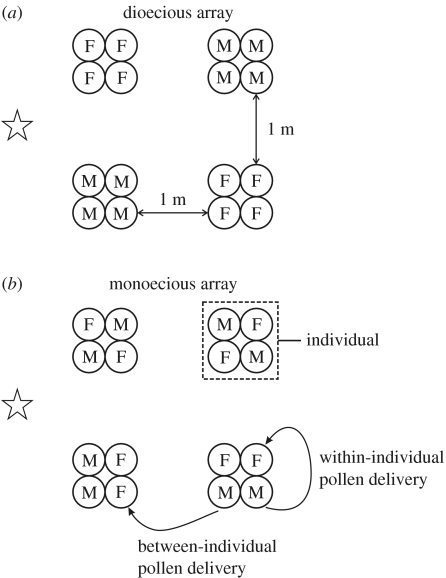
Source S. latifolia plants used in this experiment were greenhouse-raised offspring of crosses between monoecious and dioecious individuals randomly chosen from natural populations in southern Ontario in 2000 (Dorken & Barrett 2004b). To simulate monoecious and dioecious sexual systems, 16 potted plants were placed in an experimental garden at the University of Toronto between June and August 2002 in four clusters of four pots each, representing different ‘individuals’ (figure 3). In a monoecious array each individual has two pots with male inflorescences in bloom and two with female inflorescences in bloom, while in a dioecious array each individual includes either four female or four male inflorescences.
Figure 3.
Diagrammatic representation of the experiment with S. latifolia plants of contrasting sexual system. On a given day, either (a) a dioecious or (b) a monoecious array was presented to pollinators. Within each array, there were four sets of four potted plants (each with a single inflorescence) laid out in a square (= one individual), with the nearest distance between individuals being 1 m. Pollinator visits were recorded by an observer (star symbol) sitting just outside the array.
To increase variation in floral display, the plants in each trial were chosen haphazardly from a large number of possible plants in the greenhouse based on their perceived ‘showiness’ and either the 16 most ‘showy’ or the 16 most non-showy plants were selected. Showy and non-showy trial dates were chosen prior to the start of the experiment. Before each pollinator observation trial, we converted our subjective showiness score into a quantitative measure of floral display, as follows. We recorded maximum diameter of each flower using callipers and counted the number of flowers per inflorescence (as in Sarkissian et al. 2001). Thus, the total number of flowers per inflorescence and the size of flowers of each individual represent the important floral display components. Because there may be a trade-off between flower number and flower size, we also combined the two to create a composite floral display index (i.e. flower number × flower size). From these data, we also calculated the degree of sexual dimorphism (male : female) in floral display of each array.
Pollinator visitation within experimental arrays was observed for 1 h during calm and sunny days. During each 1 h trial, we recorded the identity of all insects that visited the patch and the locations visited (i.e. a particular inflorescence on a given individual; figure 3) by each insect within every foraging trip. All trials were conducted between 11:00 and 15:00 and only one trial (i.e. either a monoecious or dioecious array) was conducted per day. The order of trials was determined randomly at the beginning of the season, with a total of 10 replicates each of dioecious and monoecious arrays. We calculated the proportion of movements of pollinators (i.e. transition rates) that were between male–male, male–female, female–male and female–female flowers/individuals, as well as the ratio of male : female flowers/individuals visited. Pollen delivery events were, for the purposes of this experiment, defined as visits to female flowers that occurred subsequent to a male flower and the amount of pollen delivered was not directly measured. For the purpose of analyses, it is important to note that we disregard ‘within-individual’ pollen delivery events (see figure 3). Any visits to female flowers within monoecious arrays that occur after a visit to a male flower of a different clone are counted as a ‘between-individual’ pollen delivery event, even if there were male flowers from the same individual visited in between. We recognize that, in cases where a male flower from the same individual is visited right before a female flower, there will be delivery of geitonogamous pollen delivery and the potential for pollen and ovule discounting (Dorken et al. 2002). However, the purpose of our experiment was to compare the number of opportunities for between-individual pollen delivery in dioecious versus monoecious arrays with changes in floral display rather than to measure the relative proportions of within- and between-individual pollen delivery within monoecious arrays.
We compared floral display, pollinator behaviour and pollen delivery between dioecious and monoecious arrays. Floral display, sexual dimorphism, number of individuals visited per foraging trip and transition rates between flower types were analysed with one-way ANOVAs, with sexual system being the main effect in all cases. We used analysis of covariance (ANCOVA) to test for interactions between: (i) sexual system and sexual dimorphism on their effects on preferences of pollinators for male flowers and (ii) sexual system and composite floral display on their effects on between-individual pollination. In our analyses of pollinator behaviour (i.e. activity and flower preference), we treated individual foraging trips as independent observations. For our consideration of the effects of flower visitation on pollen delivery, we pooled observations collected in a trial, treating the array as a single replicate. Although doing so reduces our sample sizes, pollen delivery among dioecious individuals is a neighbourhood-level phenomenon (figure 3), thus, this level of resolution allows us to examine whether there is a relationship between floral display and the number of individuals visited (see model; V).
4. Results
(a) Floral display and pollinator preferences
Floral display did not differ between dioecious and monoecious arrays in any of our three metrics (flower size: F1,18=0.003; p=0.96; flower number: F1,18=0.006; p=0.94; flower size × flower number: F1,18=0.04; p=0.85). Flowers of S. latifolia used in this experiment were 2.52±0.35 (mean ± s.e.) cm in diameter with 3.78±1.95 open flowers per inflorescence. The monoecious and dioecious arrays also had equivalent sexual dimorphism, with dioecious arrays having a composite showiness (flower size × number) index ratio (male : female) of 1.12 and monoecious arrays a ratio of 1.00 (F1,18=0.62; p=0.44). Flower visitors to the experimental arrays of S. latifolia included bees, wasps and flies. Specifically, we observed members of Apidae (Apis mellifera, Bombus, Xylocopa), Halictidae, Vespidae, Megachilidae (Osmia) and Syrphidae visiting experimental plants. In total, 3143 visits were observed to the 80 individuals in the experimental arrays (20 trials with four individuals in each) in 872 foraging trips. Overall, pollinators exhibited preference for male flowers over female flowers in both dioecious and monoecious arrays. However, the degree of preference did not differ between the sexual systems, with pollinators visiting male flowers approximately 1.58× as often as female flowers in dioecious arrays and 1.49× in monoecious arrays (F1,18=0.96; p=0.34). Pollinator preference for male flowers increased with sexual dimorphism (male : female showiness) of the array (F1,17=7.67; p=0.01) but was not affected by sexual system of the array (F1,17=0.39; p=0.54). Furthermore, the interaction between sexual dimorphism and sexual system on the preference of pollinators for males was not significant (ANCOVA: F1,16=2.81; p=0.11). These results indicate that monoecious and dioecious arrays were similar in all respects except their spatial arrangement.
(b) Flower visitation and pollen delivery
Although pollinators visited an average of 3.61 flowers per foraging trip, many of these visits were between flowers on the same individual. Averaging over all observed visitation sequences (872 foraging trips) for both sexual systems, a single foraging trip by a pollinator within an array of four individuals included an average of 1.47 individuals, indicating that interplant transfers were relatively rare even when the distance between individuals was quite small (1 m). The number of S. latifolia individuals visited per foraging trip did not differ between dioecious and monoecious arrays (foraging trips included an average of 1.41 and 1.53 individuals in dioecious and monoecious arrays respectively; F1,870=2.11; p=0.15). The mean number of individuals visited per foraging trip differed significantly between the three main groups of pollinators (i.e. social bees, solitary bees and other (flies and wasps): F2,869=87.57; p<0.001). Tukey's HSD test revealed that social bees visited more individuals per foraging trip than solitary bees and other pollinators, with no differences between the latter two groups (see electronic supplementary material). There was also a significant difference among the three groups in their preference for male flowers (F2,868=6.45; p=0.002). Tukey's HSD test again revealed a stronger preference in social bees than in solitary bees and other pollinators, with no differences between the latter two groups. Finally, transition rates between flower types (i.e. male–male, male–female, female–male and female–female) in foraging trips that included at least two flowers were observed to include fewer transitions between individuals in dioecious arrays compared to monoecious arrays (F1,463=44.59; p<0.0001; electronic supplementary material). These results indicate that V in our model or the number of individuals (greater than or equal to 2) visited, may vary with sexual system if they employ the same pollinators.
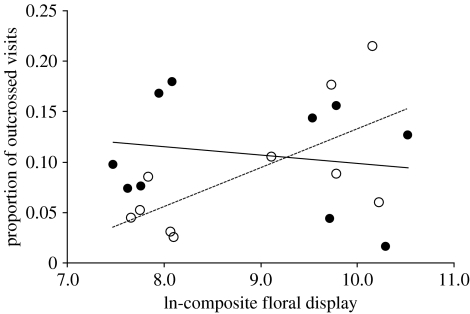
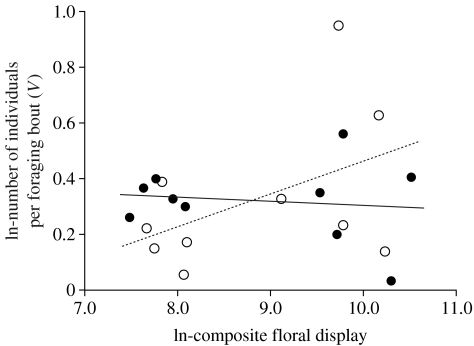
More pollinators visited the arrays as the season progressed (i.e. total visits increased with trial date: F1,18=7.26; p=0.01), despite the fact that floral display did not change as a function of trial date (F1,18=1.95; p=0.19). Therefore, all pollen delivery events were analysed as the proportion of the number of total visits received on a given day to control for variation among days in the absolute number of flower visits. Although the residual total number of visits (corrected for seasonal changes in pollinator activity) increased with composite floral display of arrays (F1,18=16.85; p=0.0007), the proportion of visits that involved between-individual pollination increased as a function of composite floral display in monoecious (F1,8=6.75; p=0.032) but not dioecious (F1,8=0.37; p=0.56) arrays. The difference between the slopes for the two sexual systems was significant (ANCOVA: F1,16=4.91; p=0.041; figure 4). The latter pattern was repeated for the other floral display metrics (flower number: F1,16=3.93; p=0.065; flower size: F1,16=4.74; p=0.045). Hence, when floral display was low, dioecious arrays achieved more effective pollen delivery than monoecious arrays. Conversely, when floral display was high, the opposite was true and monoecious arrays achieved more effective pollen delivery. Finally, because our model revealed the importance of the number of individuals included in foraging trips (V, in our model) in determining the disadvantage of dioecy from male–male, male–female and female–female foraging visits, we examined how V varied with floral display in the two sexual systems. This post hoc analysis revealed a non-significant tendency for V to increase with floral display in monoecious (F1,8=2.10; p=0.19) but not in dioecious (F1,8=0.12; p=0.74) arrays (figure 5). These results may warrant further attention as they indicate that selection for increased versus decreased floral display may depend upon sexual system.
Figure 4.
The relationship between ln-transformed composite floral display (i.e. flower number × flower size) and the proportion of total number of visits resulting in cross-pollination for dioecious (filled symbols) and monoecious (open symbols) arrays.
Figure 5.
The relationship between ln-transformed composite floral display (i.e. flower number × flower size) and the length of foraging trips of pollinators (in terms of number of individual plants visited or V in the model) for dioecious (filled symbols) and monoecious (open symbols) arrays. Power to detect significant differences between monoecious and dioecious arrays was low due to the high variance in V (number of individuals visited per foraging trip) observed in monoecious trials.
5. Discussion
(a) Pollen delivery, pollinator behaviour and the correlates of dioecy
Our model and experimental results indicate that investment in floral display can have different consequences for dioecious and cosexual plant populations. The model predicts that, all else being equal, populations of animal-pollinated dioecious species will experience proportionally less pollen delivery than cosexual species as pollinators visit fewer plants before leaving a patch. This finding suggests that divergent selection for different floral traits and, presumably, different pollinators are likely to occur after the origin of dioecy. A simple experimental manipulation of the spatial arrangement of flowering ramets of S. latifolia revealed that the clustered spatial arrangement of males and females in dioecious species reduced the advantages of increased floral display size.
Pollinator foraging studies have shown that pollinators perform interplant movements more often (e.g. Free 1960; Frankie et al. 1976; Beach 1981), or become erratic (Biernaskie et al. 2002; Biernaskie & Cartar 2004) as floral rewards diminish. However, as floral rewards decline, a given plant will probably receive fewer visitors overall, with many pollinators preferentially revisiting rewarding individuals (Beach 1981). Interplant movements decreased as floral display increased in dioecious, but not monoecious arrays. Where the balance is struck between selection for increased visitation compared to other individuals in the population and selection for decreased pollen discounting thus depends upon the sexual system. The close proximity of male and female flowers in monoecious arrays may reduce a pollinator's ability to develop constancy for a single floral type in a particular foraging trip (e.g. Ishii 2005). Such a spatial arrangement of male and female flowers may cause pollinators to leave monoecious individuals more readily than dioecious individuals, resulting in: (i) more pollinator visits per individual in dioecious arrays and (ii) less geitonogamous pollination in monoecious arrays compared to that expected in plants with hermaphroditic flowers. We acknowledge that the arrays in our experiment were necessarily small (16 plants) in order to facilitate observing all pollinator visits and the potential exists that small arrays affect the behaviour of pollinators. Though it is hard to imagine why small array size would lead to our observations that pollinators behaved differently in dioecious and monoecious arrays, how floral inconstancy may reduce geitonogamous pollination in monoecious populations warrants further attention.
Dioecious species typically have small, inconspicuous flowers (Bawa 1980; Fox 1985; Muenchow 1987; Sakai & Weller 1999), and are thought to be visited by opportunistic pollinators such as small bees, flies and beetles (Opler et al. 1980; but see Renner & Feil 1993). We predict that dioecious species should evolve to employ these pollinators if they have long foraging trips, thus increasing the number of male–female interplant pollen transfers. Common pollinators of dioecious species, such as beetles, flies and moths often forage to feed themselves and are less likely to return to a central location (i.e. nest), potentially increasing the number of different individuals visited. Also, these pollinators tend not to exhibit as much grooming behaviour as do hymenopteran pollinators (e.g. Mitchell et al. 2004), which may increase the likelihood of pollen reaching more subsequent individuals. In a comparison of the effectiveness of various pollinators of dioecious Silene alba, moths were indeed found to deliver larger amounts of pollen to more females and to females located farther away than bees, despite their greater abundance (Young 2002).
(b) Implications for pollen limitation and persistence of dioecy
The problems associated with animal-pollination of dioecious species may prohibit the persistence of dioecious populations except in areas where pollinator abundances are high (Vamosi & Otto 2002). We suggest that unfavourable pollen receipt may restrict the ranges of dioecious species and could have implications for the pollen limitation observed in some dioecious species (House 1992; Knight et al. 2005; Voigt et al. 2005) and the increased extinction observed in dioecious lineages (Heilbuth 2000; Heilbuth et al. 2001; Vamosi & Vamosi 2004, 2005). Pollen limitation may select for traits that encourage wind pollination within dioecious lineages. Indeed dioecy is strongly correlated with wind pollination (especially in temperate zones), probably because wind provides more reliable pollen transport in situations where pollinators are not abundant (Vamosi & Otto 2002; Vamosi et al. 2003; Culley et al. 2005).
Empirical data from gynodioecious species suggest that seed production of females is ca 1–2× that of hermaphrodites (Richards 1997; Asikainen & Mutikainen 2003) and a community-wide comparison found that dioecious species had, on average, ca 1.6× seeds per fruit of cosexual species (Ibarra-Manriquez & Oyama 1992). In our model, we examined pollination dynamics when dioecious females produce twice the number of seeds for every successful pollen delivery event and this did not adequately compensate for the combinatorial disadvantages of dioecy. If females in dioecious populations rarely produce more than twice the number of seeds of individuals in related cosexual populations, they must experience increased fitness at some other life stage (e.g. seedling survival through outbreeding advantage) in order to compensate for the 50% loss in fitness from male function. Although the life stage at which fitness advantages commonly manifest themselves is clear for many gender dimorphic species (e.g. Hebe subalpina (Delph & Lloyd 1996) and Schiedea adamantis (Sakai et al. 1997)) it has remained elusive in others (e.g. Geranium sylvaticum (Asikainen & Mutikainen 2003) and Daphne laureola (Medrano et al. 2005)). Our results concerning the spatial constraints to pollen delivery should further disadvantage dioecious species. The question regarding dioecy and its rarity is perhaps not why does dioecy represent only 6–10% of all angiosperms, but rather how do extant animal-pollinated dioecious plant species manage to persist at all?
Acknowledgments
We are grateful to M. Dorken and S. Renner for advice and comments on the manuscript, B. Hall and A. Petrie for logistic support and the Natural Sciences and Engineering Research Council of Canada for postdoctoral fellowships to J.C.V. and S.M.V. and a Discovery grant to S.C.H.B. that funded this work.
Supplementary Material
Summary statistics of the behaviour of pollinator groups visiting experimental dioecious and monoecious arrays of S. latifolia.
References
- Asikainen E, Mutikainen P. Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae) Am. J. Bot. 2003;90:226–234. doi: 10.3732/ajb.90.2.226. [DOI] [PubMed] [Google Scholar]
- Barrett S.C.H. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Phil. Trans. R. Soc. B. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. doi:10.1098/rstb.2003.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S.C.H, Thomson J.D. Spatial pattern, floral sex ratios, and fecundity in dioecious Aralia nudicaulis (Araliaceae) Can. J. Bot. 1982;60:1662–1670. [Google Scholar]
- Bawa K.S. Evolution of dioecy in flowering plants. Annu. Rev. Ecol. Syst. 1980;11:15–39. doi:10.1146/annurev.es.11.110180.000311 [Google Scholar]
- Bawa K.S, Opler P.A. Dioecism in tropical forest trees. Evolution. 1975;29:167–179. doi: 10.1111/j.1558-5646.1975.tb00824.x. doi:10.2307/2407150 [DOI] [PubMed] [Google Scholar]
- Bawa K.S, Ashton P.S, Nor S.M. Man and the biosphere. UNESCO; Paris, France: 1990. Reproductive ecology of tropical forest plants. [Google Scholar]
- Beach J.H. Pollinator foraging and the evolution of dioecy. Am. Nat. 1981;118:572–577. doi:10.1086/283851 [Google Scholar]
- Biernaskie J.M, Cartar R.V. Variation in rate of nectar production depends on floral display size: a pollinator manipulation hypothesis. Funct. Ecol. 2004;18:125–129. doi:10.1111/j.1365-2435.2004.00815.x [Google Scholar]
- Biernaskie J.M, Cartar R.V, Hurly T.A. Risk-averse inflorescence departure in hummingbirds and bumble bees: could plants benefit from variable nectar volumes? Oikos. 2002;98:104. doi:10.1034/j.1600-0706.2002.980110.x [Google Scholar]
- Bierzychudek P. Pollinators increase the cost of sex by avoiding female flowers. Ecology. 1987;68:444–447. doi:10.2307/1939276 [Google Scholar]
- Cartar R.V, Abrahams M.V. Risk-sensitive foraging in a patch departure context: a test with worker bumble bees. Am. Zool. 1996;36:447–458. [Google Scholar]
- Charlesworth D. Why are unisexual flowers associated with wind pollination and unspecialized pollinators? Am. Nat. 1993;141:481–490. doi:10.1086/285485 [Google Scholar]
- Charlesworth D, Charlesworth B. The effect of investment in attractive structures on allocation to male and female functions in plants. Evolution. 1987;41:948–968. doi: 10.1111/j.1558-5646.1987.tb05869.x. doi:10.2307/2409184 [DOI] [PubMed] [Google Scholar]
- Charnov E.L, Maynard Smith J, Bull J.J. Why be an hermaphrodite? Nature. 1976;263:125–126. doi:10.1038/263125a0 [Google Scholar]
- Culley T.M, Weller S.G, Sakai A.K. The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 2005;17:361–369. doi:10.1016/S0169-5347(02)02540-5 [Google Scholar]
- de Jong T.J, Geritz S.A.H. The role of geitonogamy in the gradual evolution towards dioecy in cosexual plants. Selection. 2001;2:133–146. doi:10.1556/Select.2.2001.1-2.10 [Google Scholar]
- Delph L.F, Lloyd D.G. Inbreeding depression in the gynodioecious shrub Hebe subalpina (Scrophulariaceae) New Zeal. J. Bot. 1996;34:241–247. [Google Scholar]
- Dorken M.E, Barrett S.C.H. Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): a clonal aquatic plant. J. Ecol. 2004a;92:32–44. doi: 10.1111/j.1365-294X.2004.02246.x. doi:10.1111/j.1365-2745.2004.00857.x [DOI] [PubMed] [Google Scholar]
- Dorken M.E, Barrett S.C.H. Sex determination and the evolution of dioecy from monecy in Sagittaria latifolia (Alismataceae) Proc. R. Soc. B. 2004b;271:213–219. doi: 10.1098/rspb.2003.2580. doi:10.1098/rspb.2003.2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken M.E, Friedman J, Barrett S.C.H. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae) Evolution. 2002;56:31–41. doi: 10.1111/j.0014-3820.2002.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Elle E, Carney R. Reproductive assurance varies with flower size in Collinsia parviflora (Scrophulariaceae) Am. J. Bot. 2003;90:888–896. doi: 10.3732/ajb.90.6.888. [DOI] [PubMed] [Google Scholar]
- Fox J.F. Incidence of dioecy in relation to growth form, pollination, and dispersal. Oecologia. 1985;67:244–249. doi: 10.1007/BF00384293. doi:10.1007/BF00384293 [DOI] [PubMed] [Google Scholar]
- Frankie G, Opler P.A, Bawa K.S. Foraging behaviour of solitary bees: implications for outcrossing of a neotropical forest tree species. J. Ecol. 1976;64:1049–1057. [Google Scholar]
- Free J.B. The behaviour of honeybees visiting the flowers of fruit trees. J. Anim. Ecol. 1960;29:385–395. [Google Scholar]
- Geber M.A. The relationship of plant size to self-pollination in Mertensia ciliata. Ecology. 1985;66:762–772. doi:10.2307/1940537 [Google Scholar]
- Givnish T.J. Outcrossing versus ecological constraints in the evolution of dioecy. Am. Nat. 1982;119:849–865. doi:10.1086/283959 [Google Scholar]
- Harder L.D, Barrett S.C.H. Mating cost of large floral displays in hermaphrodite plants. Nature. 1995;373:512–515. doi:10.1038/373512a0 [Google Scholar]
- Harder L, Wilson W.G. A clarification of pollen discounting and its joint effects with inbreeding depression on mating-system evolution. Am. Nat. 1998;152:684–695. doi: 10.1086/286199. doi:10.1086/286199 [DOI] [PubMed] [Google Scholar]
- Heilbuth J.C. Lower species richness in dioecious clades. Am. Nat. 2000;156:221–241. doi: 10.1086/303389. doi:10.1086/303389 [DOI] [PubMed] [Google Scholar]
- Heilbuth J.C, Ilves K.L, Otto S.P. The consequences of dioecy for seed dispersal: modeling the seed-shadow handicap. Evolution. 2001;55:880–888. doi: 10.1554/0014-3820(2001)055[0880:tcodfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- House S.M. Population density and fruit set in three dioecious tree species in Australian tropical rain forest. J. Ecol. 1992;80:57–69. [Google Scholar]
- Ibarra-Manriquez G, Oyama K. Ecological correlates of reproductive traits of Mexican rain forest trees. Am. J. Bot. 1992;79:383–394. doi:10.2307/2445150 [Google Scholar]
- Ishii H.S. Analysis of bumblebee visitation sequences within single bouts: implication of the overstrike effect on short-term memory. Behav. Ecol. Sociobiol. 2005;57:599–610. doi:10.1007/s00265-004-0889-z [Google Scholar]
- Klinkhamer P.G.L, de Jong T.J, Metz J.A.J. Why plants can be too attractive—a discussion of measures to estimate male fitness. J. Ecol. 1994;82:191–194. [Google Scholar]
- Knight T.M, et al. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005;103:956–961. [Google Scholar]
- Medrano M, Alonso C, Herrera C.M. Mating system, sex ratio, and persistence of females in the gynodioecious shrub Daphne laureola L. (Thymelaeaceae) Heredity. 2005;94:37–43. doi: 10.1038/sj.hdy.6800550. doi:10.1038/sj.hdy.6800550 [DOI] [PubMed] [Google Scholar]
- Mitchell R.J, Karron J.D, Holmquist K.G, Bell J.M. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Funct. Ecol. 2004;18:116–124. doi:10.1111/j.1365-2435.2004.00812.x [Google Scholar]
- Muenchow G.E. Is dioecy associated with fleshy fruit? Am. J. Bot. 1987;74:287–293. doi:10.2307/2444031 [Google Scholar]
- Opler P.A, Baker H.G, Frankie G.W. Plant reproductive characteristics during secondary succession in neotropical lowland forest ecosystems. Biotropica. 1980;12:40–46. doi:10.2307/2388155 [Google Scholar]
- Pannell J.R, Barrett S.C.H. Baker's Law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. doi:10.2307/2411261 [DOI] [PubMed] [Google Scholar]
- Renner S.S, Feil J.P. Pollinators of tropical dioecious angiosperms. Am. J. Bot. 1993;80:1100–1107. doi:10.2307/2445757 [Google Scholar]
- Renner S.S, Ricklefs R.E. Dioecy and its correlates in the flowering plants. Am. J. Bot. 1995;82:596–606. doi:10.2307/2445418 [Google Scholar]
- Reusch T.B.H. Fitness-consequences of geitonogamous selfing in a clonal marine angiosperm (Zostera marina) J. Evol. Biol. 2001;14:129–138. doi: 10.1046/j.1420-9101.2001.00257.x. doi:10.1046/j.1420-9101.2001.00257.x [DOI] [PubMed] [Google Scholar]
- Richards A.J. Allen and Unwin; London, UK: 1997. Plant breeding systems. [Google Scholar]
- Sakai A.K, Weller S.G. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber M.A, Dawson T.E, Delph L.F, editors. Gender and sexual dimorphism in flowering plants. Springer; Berlin, Germany: 1999. [Google Scholar]
- Sakai A.K, Weller S.G, Chen M.-L, Chou S.-Y, Tasanont C. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae) Evolution. 1997;51:724–736. doi: 10.1111/j.1558-5646.1997.tb03656.x. doi:10.2307/2411149 [DOI] [PubMed] [Google Scholar]
- Sarkissian T.S, Barrett S.C.H, Harder L.D. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology. 2001;82:363–373. doi:10.2307/2679865 [Google Scholar]
- Schemske D.W, Ågren J. Deceit pollination and selection on female flower size in Begonia involucrata: an experimental approach. Evolution. 1995;49:207–214. doi: 10.1111/j.1558-5646.1995.tb05972.x. doi:10.2307/2410306 [DOI] [PubMed] [Google Scholar]
- Vamosi J.C, Otto S.P. When looks can kill: the evolution of sexually-dimorphic floral display and the extinction of dioecious plants. Proc. R. Soc. B. 2002;269:1187–1194. doi: 10.1098/rspb.2002.2004. doi:10.1098/rspb.2002.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi J.C, Vamosi S.M. The role of diversification in causing the correlates of dioecy. Evolution. 2004;58:723–731. doi: 10.1111/j.0014-3820.2004.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Vamosi J.C, Vamosi S.M. Present day risk of extinction may exacerbate the lower species richness of dioecious clades. Divers. Distrib. 2005;11:25–32. doi:10.1111/j.1366-9516.2005.00119.x [Google Scholar]
- Vamosi J.C, Otto S.P, Barrett S.C.H. Phylogenetic analysis of the ecological correlates of dioecy in angiosperms. J. Evol. Biol. 2003;16:1006–1018. doi: 10.1046/j.1420-9101.2003.00559.x. doi:10.1046/j.1420-9101.2003.00559.x [DOI] [PubMed] [Google Scholar]
- Voigt F.A, Jung S, Farwig N, Bohning-Gaese K. Low fruit set in a dioecious tree: pollination ecology of Commiphora harveyi in South Africa. J. Trop. Ecol. 2005;21:179–188. doi:10.1017/S026646740400210X [Google Scholar]
- Wilson W.G, Harder L.D. Reproductive uncertainty and the relative competitiveness of simultaneous hermaphroditism versus dioecy. Am. Nat. 2003;162:220–241. doi: 10.1086/376584. doi:10.1086/376584 [DOI] [PubMed] [Google Scholar]
- Young H.J. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae) Am. J. Bot. 2002;89:433–440. doi: 10.3732/ajb.89.3.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary statistics of the behaviour of pollinator groups visiting experimental dioecious and monoecious arrays of S. latifolia.