Abstract
‘Back-to-the-future’ (BTF) is an integrative approach to a restoration ecology of the oceans that attempts to solve the fisheries crisis. To this end, it harnesses the latest understanding of ecosystem processes, developments in whole ecosystem simulation modelling, and insight into the human dimension of fisheries management. BTF includes new methods for describing past ecosystems, designing fisheries that meet criteria for sustainability and responsibility, and evaluating the costs and benefits of fisheries in restored ecosystems. Evaluation of alternative policy choices, involving trade-offs between conservation and economic values, employs a range of economic, social and ecological measures. Automated searches maximize values of objective functions, and the methodology includes analyses of model parameter uncertainty. Participatory workshops attempt to maximize compliance by fostering a sense of ownership among all stakeholders. Some challenges that have still to be met include improving methods for quantitatively describing the past, reducing uncertainty in ecosystem simulation techniques and in making policy choices robust against climate change. Critical issues include whether past ecosystems make viable policy goals, and whether desirable goals may be reached from today’s ecosystem. Examples from case studies in British Columbia, Newfoundland and elsewhere are presented.
Keywords: fisheries policy, marine ecosystems, ecosystem simulation, restoration ecology
1. Introduction: why Back-to-the-future is needed
Ten years ago most fisheries scientists would have reacted to news of a global crisis in fisheries with disbelief. Today few dispute the matter (Hilborn et al. 2003), as evidenced by most of the other papers in this issue. The shift in view has been accompanied, if not partly caused by, a plethora of publications that document the present status of fisheries worldwide (e.g. Pauly et al. 2002). Indeed, some have begun to present evidence that depletions and collapses are even worse than we had thought (e.g. large fish, Myers & Worm 2003; fish biomass, Christensen et al. 2003; whales, Roman & Palumbi 2003; sharks, Baum et al. 2002; Schindler et al. 2002; turtles, Hays et al. 2003). A historical review, including Roman and pre-Columbian examples, has characterized the essential processes at work (Pitcher 2001).
Many changes in the sea parallel those well known in terrestrial ecology, such as: (i) destruction and fragmentation of habitat critical to small and juvenile animals; (ii) local extinctions (‘extirpation’) and even some global extinctions of species; (iii) huge depletions of large grazing animals (with consequent loss of the fertilizing effect of their waste products); and (iv) reductions in top predators and consequent increases of prey species leading, in some cases, to trophic cascades. Moreover, in terrestrial ecology, changes to the original wild habitat have been so obvious and far reaching that they are scarcely ever noted as such. Refuges from predators for small animals are reduced, breeding areas shifted and food supplies for herbivores altered. Over long periods of time, human agriculture and the use of wood for building and war has brought about immense shifts in habitat (Diamond 1997). For example, at the time of expansion of the Roman empire in the first century AD, it is said that, without touching the ground, a squirrel could travel in continuous woodland from north of the Alps to the Baltic. However, today most terrestrial landscapes throughout the world are human-made as a consequence of agriculture. Freshwaters have evidently seen similar large changes. Over the past 200 years, wetland habitats in North America have been so drastically altered as to decimate natural populations of salmon and almost eliminate several of the most widespread herbivores (beaver, moose, wood buffalo; Callenbach 1995; Lichatowich 2001). Hence, in terrestrial and freshwater environments, there is much effort to try to conserve what is left of these ancient wild ecosystems by creating parks, severely limiting hunting, restoring habitats and even reintroducing lost species (wolves). Accompanying these practical efforts, an entirely new field of restoration ecology has arisen (e.g. Morrison 2002) that attempts to optimize how these things are done.
In marine environments, however, this perspective has been neglected until recently, and thus, restoration ecology in terrestrial environments seems further advanced than its aquatic equivalent (Dobson et al. 1997). Indeed, terrestrial restoration ecology has developed a powerful set of analytical tools to aid recovery of degraded systems. Terrestrial reserves provide baselines against which to judge human impacts (Arcese & Sinclair 1997), and restoration is also viewed as a necessary hedge against loss from natural causes (Sinclair et al. 1995). Habitat is regarded as the essential template upon which species conservation must be founded: ‘habitats can only be preserved if they are treated as a renewable resource; otherwise all habitat will decay to zero’ (Sinclair et al. 1995, p. 585). Hunting of wild animals has to be strictly regulated or extirpations and extinctions surely occur (e.g. Ward 1997). Moreover, the economic benefits of conservation and restoration have recently been estimated as outweighing further depletion (Balmford et al. 2002).
At first sight, it may be difficult to see how this relates to aquatic species. With major exceptions such as coral reefs (e.g. McClanahan 2002), rocky shores, oyster reefs (Lenihan & Peterson 1998) and kelp forests (Steneck et al. 2002), most marine organisms lack a physically tangible habitat made of plant architecture like most terrestrial animals. But in fact, not only are most of the structured marine habitats listed above suffering considerable losses, but also the structural habitat concept itself needs extending only a little to encompass the oceanographic structure of the ecosystem. For example, the great marine populations of fishes are bounded by tangible ocean structures (Bakun 1996). Most terrestrial systems are highly modified agricultural habitats in which food organisms are grown, so restoration or conservation of wild areas usually represents some form of loss to food production. By contrast, with the exception of aquaculture, in most marine systems habitats provide homes for the wild food that is hunted. Hence, in marine systems, the interests of both exploitation and conservation may be met by efforts to restore and preserve habitat productivity and resilience.
The fundamental issue here is only partly understood. Although we have been well aware for a long time how changes in biomass affect the population dynamics of a single species (indeed, the discipline of fisheries stock assessment is built upon the quantitative expression of that understanding), it has only recently been realized that all fisheries change the ecosystem in which they are embedded (Pitcher 2001; Walters & Martell 2004). It is now becoming clear how large these changes might be, when they may be reversible, and what their consequences are for the sustainable extraction of fisheries benefits from aquatic ecosystems.
Three ratchet-like processes have compromised fisheries and eroded aquatic biodiversity (Pitcher 2001). First, harvesting acts as a selective force on ecosystems by removing long-lived slow-growing fishes in favour of those with life histories endowed with higher turnover. This process operates both within and among species. When species become locally extinct, the past becomes hard to restore, like a ratchet. I have termed this Odum’s ratchet, after Eugene P. Odum’s concerns with human-caused extinctions (e.g. Gibbons & Odum 1993). Dulvy et al. (2003) found that most marine global extinctions were overexploited mammals or birds, or a result of habitat loss for almost-sessile invertebrates and small fishes. Rapid extinctions appear to occur especially in the early stages of exploitation of marine ecosystems (Christensen & Pauly 1997), probably because of narrow niches and k-selected life-history parameters. So far, it appears that only a few marine fishes have become globally extinct during the recent fisheries crisis, but many have suffered local extinctions due to overexploitation. However, some have argued that the risk of global extinctions may be larger than we think, especially for large slow-growing organisms, irrespective of high fecundity evolved to buffer habitat volatility (e.g. Chinese bahaba; Sadovy & Cheung 2003).
Secondly, Ludwig’s ratchet (Ludwig et al. 1993) describes the generation of overcapacity in fishing power through pressure from loans that can be repaid only by sustained catches that, because of stock and ecosystem depletion, can be generated only by ratchet-like further investment in fishing technology. It is hard to go back to using yesterday’s fishing gear. Finally, Pauly’s ratchet refers to the psychological tendency for scientists to relate changes in the system to what things were like at the time of their own professional debut, regarding earlier accounts of great abundance as anecdotal and methodologically naive (Pauly 1995). The combined effect of these three ratchets, acting over historical time, has been not only to bring about the collapse of many major commercial fisheries, but also to shift the structure of ecosystems towards lower trophic levels (Pauly et al. 1998a), favour simpler organisms and energy pathways (Parsons 1996), and compromise biodiversity (Dulvy et al. 2003; Sadovy & Cheung 2003) in ways that might be hard to reverse (Jackson et al. 2001). Many of these events have undoubtedly caught fisheries science, managers and the public by surprise (Haggan 2000).
So, we may ask, what can be done? This paper summarizes recent work supporting an idea that may provide an answer. BTF is a fresh and integrative approach to the restoration ecology of oceans. It attempts to harness the latest understanding of ecosystem processes, developments in ecosystem modelling, and insight into the human dimension of fisheries management to try to solve the ‘fisheries crisis’.
2. What is Back-To-the-Future?
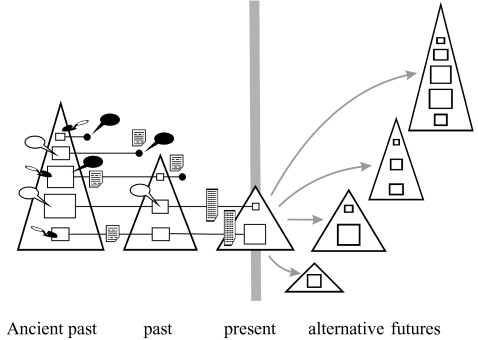
BTF is a science-based restoration ecology aimed at the creation of truly sustainable food and wealth from capture fisheries and aquatic ecosystems (Pitcher et al. 1999). These future fisheries are designed to be responsible according to set criteria, and are forecast to be sustainable using simulations that take account, as far as is possible, of risk and uncertainty. The fisheries are embedded in aquatic ecosystems that, by quantitative analysis and with the consent of stakeholders, trade off wealth and food with a specified degree of retention of their unexploited biodiversity, trophic structure and resilience against change. Hence, BTF uses past ecosystem states as candidates for adoption as policy goals for the future (Pitcher 1998a; figure 1). In practice, the policy goals are subject to several practical constraints from species, habitat, climate changes and the human dimension of management. The logical choice of policy goal is the one that maximizes benefits set against the costs of restoration and management. Other considerations, however, complicate this task. For example, the way in which expected future benefits are calculated greatly affects the outcome, an issue that is discussed in more detail below (see § 7).
Figure 1.
Diagram illustrating the BTF concept for the restoration of past ecosystems. Triangles at left represent a series of ecosystem models, constructed at appropriate past times, where the vertex angle is inversely related and height directly related to biodiversity and internal connectance. Time-lines of some representative species in the models are indicated, where box size represents relative abundance and filled circles represent local extinctions. Sources of information for constructing and tuning the ecosystem models are illustrated by symbols for historical documents (paper sheet symbol), data archives (tall data table symbol), archaeological data (trowel), the traditional environmental knowledge of indigenous peoples (open balloons) and local environmental knowledge (filled balloons). Alternative future ecosystems, restored ‘Lost Valleys’, taken as alternative policy goals, are drawn to the right (modified from Pitcher et al. 1999 and Pitcher 2001).
The BTF process starts with the construction of descriptive models of past ecosystems; it goes on to devise sustainable and responsible fisheries that might be operated within each of these ecosystems if they were to be restored; it compares forecast benefits among these systems; it selects candidate policy goals by setting them against the likely costs of restoration using suitable instruments of restoration; and finally, it attempts to achieve consensus on agreed restoration goals through a sense of ownership of the process among all principal stakeholders. Once in place, adaptive management procedures are set up using the quantitative BTF procedures, to insure against unexpected developments. These six logical steps in the development of a BTF policy are summarized in table 1 (Pitcher 1998a; Pitcher et al. 2004).
Table 1.
Stages in the BTF process for the restoration of fisheries and aquatic ecosystems. (Modified from tables in Pitcher et al. (1998, 2004).)
| stage | goals | steps |
|---|---|---|
| 1 | model construction of past and present aquatic ecosystems |
|
| 2 | evaluation of ecological, economic and social benefits that could be gained from each system |
|
| 3 | choice of system that maximizes benefits to society |
|
| 4 | design of instruments to achieve this policy goal |
|
| 5 | participatory choice of instruments |
|
| 6 | adaptive management: implementation and monitoring |
|
3. Methodological challenges posed by Back-to-the-future
Many new concepts have been developed as a part of the BTF research, and so it is not surprising that existing methods have not been adequate to express them. This section briefly describes new methods that have been developed. They can be divided into four groups: (i) methods required to describe and model past ecosystems; (ii) ecosystem-based methods to determine sustainable fisheries; (iii) methods that set out a rational basis for choosing appropriate ecosystem restoration goals; and finally (iv) practical techniques that attempt to secure compliance and consent through participation (Pitcher 2004a).
4. Modelling past ecosystems
At the start of the BTF process, the present-day ecosystem is represented by mass-balance and dynamic simulation modelling (at present using Ecopath with Ecosim; Walters et al. 1997, 2000) using techniques that have received a degree of approval by marine ecologists (e.g. Whipple et al. 2000). This in itself is a far from trivial task, especially if fitting to time-series of fisheries and survey data is undertaken.
Models for past ecosystems may be assembled using scientific archival data, archaeological data, historical information, and local and traditional environmental knowledge (Christensen 2002). Scientific data derive mainly from published scientific papers, although material from unpublished reports and archives can often be valuable. Archaeological data have a similar set of sources. Historical information is gathered mainly from relevant books, letters, trade accounts and other historical documents, although, unlike science and archaeology, where searchable databases are the norm, finding and locating historical material can be quite difficult. In some cases, translations are required. Local and traditional environmental knowledge, however, is rarely published and often has to be derived largely from oral sources through interviews and discussion held in coastal communities. An integrated example for a species in a heavily depleted system is presented by Martell & Wallace (1998). A history of inshore habitat changes can be valuable (e.g. McLoughlin 2002).
Information about the local fisheries, with analyses and surveys, and about local aquatic fauna and flora is relatively easily found, especially as an output of ‘science workshops’ comprising research partners and local scientists with expert knowledge of the area and the taxonomic groups (see table 1). One of the principal problems here is data that have been gathered on either a very small or a very large scale compared with the area of focus. Another issue often requiring a lot of work is the concordance of measurement units, because specialists on different taxa often work in very different fields. Scientists who generously make the relevant information available, often from a lifetime’s work on a group of organisms, are encouraged to publish a paper in one of the project reports, so that they retain a recognized ownership of material that otherwise could easily vanish into model parameters. For example, in four BTF projects in Canada, the output from this process of consultation with the science community has been presented in detail in a series of reports (Haggan & Beattie 1999; Pauly et al. 1998b; Ainsworth et al. 2002; Pitcher et al. 2002a, b; Heymans 2003a), where information essential to the modelling process, such as biomass, relative fishing mortalities and diets, is assembled together with bibliographies.
In the absence of relevant local publications, as is often the case, interviews, conducted under suitable partnership agreements, have been used to gather traditional and local ecological knowledge for use in the modelling (e.g. Haggan et al. 1998; Salas et al. 1998; Ainsworth & Pitcher 2005a).
Once found, the information is assembled into a relational database together with evaluations of its scope and quality, to ease retrieval of relevant information for the models (e.g. Erfan 2004). Even so, a significant task is systematizing the way in which information is collated for use in the models. The reason is that, once documented, all this information has to be expressed in a form that can be used in building ecosystem model structure, in setting parameters, or in shaping dynamic responses to changes. Although presence and absence of a species is easily dealt with, the models require us to know actual biomasses, size and growth parameters, and items in the diet.
For ease of comparison, the structure of the past and present ecosystem models should be similar, although of course biomasses and fluxes can be vastly different. Global extinctions of species, such as the great auk in the North Atlantic (over half a million birds before 1830; Montevecchi & Kirk 1996) or Steller’s sea cow in the North Pacific (Andersen 1995), mean that there is little choice but to eliminate these species from future restoration goals.
When species have gone locally extinct (‘extirpation’), one has the choice of eliminating them from models of the future ecosystem altogether, or allowing the possibility of them returning, either through natural migration or through active reintroduction (Pitcher 2004b). Examples of the former are the more than 200 humpback whales resident until the 1920s in the Strait of Georgia (Merilees 1985; Winship 1998), or almost a quarter of a million walrus resident in Newfoundland before 1800 (Mercer 1967). An example of the latter is sea otters reintroduced to Vancouver Island in British Columbia. To accommodate dynamic ecosystem modelling, biomasses of groups have to be set to very low levels rather than zero, and this creates some technical problems as they may undergo unexpected resurgence. Simulating changes to habitat can be tricky, moreover, when they are keystone species that cause large changes in habitat structure, such as the sea otter (Simenstad et al. 1978; Pitcher 1998b). Sea otters alter the type of kelp cover available to a suite of juvenile fishes and invertebrates by foraging on kelp-eating invertebrates that themselves graze selectively (Reidman & Estes 1990).
Another frequent problem is that reconstructions of the ancient past may suggest the presence of top predators in such large numbers that they are unable to be supported by what are thought to be realistic levels of forage organisms (e.g. reconstruction of the North Sea as it was in 1880; Mackinson 2001). We can probably rule out the answer that the apparent high abundance of top predators was a false impression, but perhaps such a high abundance of prey is actually acceptable. For example, the diet of abundant old predators would have been broader in the ancient past because of intense competition. Moreover, it is also likely that there were more forage fish species filling a diversity of niches in the ancient past (Pitcher 2004c). To answer these questions, we might look for evidence of ancient diets using archaeology and stable isotope analysis, and look for archaeological evidence of more forage fish species.
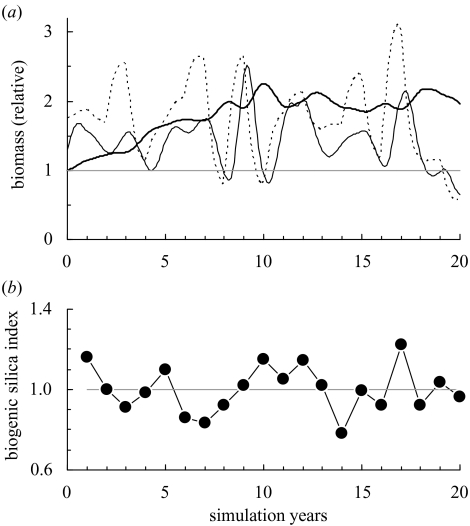
Representing changes in ecosystem structure over long periods represents a major challenge. Clearly, the effect of climate change has to be accommodated in the forecasts as much as possible. Primary production, and other parameters of the ecosystem model, such as stock–recruitment relationships, may be driven by a variety of forcing functions. In the major oceans, inter-annual variation may seek to accommodate El Niña/La Niña alternation, or proxies such as a local upwelling index, decadal oscillations or temperature anomalies (e.g. in the English Channel; Stanford & Pitcher 2004). Longer-term climate cycles may be included in the forcing function, such as the 62-year ‘la vieja/el viejo’ (‘old man/old woman’) alternation between warm/cold eastern boundary current sardine/anchovy regimes (Chavez et al. 2003). In some cases, silica deposits may accurately reveal the past annual abundance of diatoms (e.g. Johnson et al. 2001). Although precise forecasts of inter-annual climate changes are not possible, randomized selections of such data may be used to drive forecasts on the basis of likely scenarios. Figure 2 illustrates an ecosystem simulation forecast driven in this way.
Figure 2.
Example of output from an ecosystem model driven for 20 years by a random selection of recent annual primary production values taken from historical series of biogenic silica in bottom deposits ((b) scaled and normalized to a mean of 1, indicated by grey bar; data from Johnson et al. (2001)). (a) Relative biomasses of three of the 36 groups in an ecosystem simulation model of Lake Malawi (T. J. Pitcher, unpublished data). The thick solid line is small pelagic zooplanktivorous fishes (Engraulicypris sardella), the dashed line is a water-column insect larva (Chaoborus edulis), the thin solid line is a large benthic catfish (Bagrus meridionalis). The starting values for this model came from a ‘Lost Valley’ optimization for ecological values.
To emulate changes in species composition, the modelling system could perhaps be modified to use a ‘cast of players’, members of which might be brought on- and off-stage when conditions are appropriate (Pitcher & Forrest 2004). For example, in the Cueva de Nerja, Andalusia, human middens reveal the fishes that early Mediterranean people were eating over a 9000-year sequence (Rosello-Izquierdo & Morales-Muniz 2001; Morales et al. 1994). Early in the sequence from ca. 14000 BP, the human diet consisted of a sparid fauna similar to the present, but during a pluvial period between 11000 and 9000 BP humans were eating large cod and haddock, fauna typical of Norway today. By 8000 BP, a typical Mediterranean fauna had returned.
Early periods of depletion by human exploitation also had significant impacts on ecosystem structure and function (e.g. Wallace 1998). Recent reconstruction work on North American inshore ecosystems by Jackson et al. (2001) shows what may be possible in this respect.
Ideally, the timing of the series of snapshot ecosystem models for BTF may depend on the locality, the dawn of quantitative documentary evidence, and major shifts in resource and ecosystem history such as the introduction of new fishing gears, damming of rivers and collapses of fish stocks. However, because of the large amount of work involved in drawing up each ecosystem model, the gaps in time between a series of BTF models may become quite large. Thus, an ideal choice of the time snapshots to use is generally constrained by the resources available for the research. This raises a significant methodological problem in that failure to cover important changes that occurred within these time-gaps can prejudice the choice of appropriate policy goals at the end of the BTF process. In the event, the choice of the time periods to model in a BTF analysis is something of a compromise (Pitcher 2004a), and, in case studies so far, has entailed four or five ecosystem models spread over a few hundred years.
In many cases, additional informative models might be drawn up for pre-modern humans in the late Pleistocene post-glacial era (Neolithic). Although such ancient ecosystems would be unlikely to ever become practical policy goals, they have the advantage of providing a ‘pristine’ baseline against which all more recent changes might be assessed. In fact, for some areas of the world only recently colonized by Europeans, such as Australia, New Zealand and the Pacific coast of America (Diamond 1997), models of ‘pre-contact’ ecosystems may serve this ‘baseline’ purpose well.
In models of the distant past, the estimation of the size and impacts of ancient fisheries presents many problems. Although the history of fishing technology is quite well known from archaeology and from traditional knowledge, its probable fishing power may be estimated, and ancient diets may be calculated, nevertheless, the size of the human populations that engaged in fishing is often hard to assess. Estimates of ancient human population sizes are often the subject of controversy among archaeologists and anthropologists. Heymans (2003b) and Wallace (1998) present examples from Newfoundland and British Columbia, respectively. It is emphasized, however, that aboriginal fisheries in these ecosystems are described only to provide an accurate picture of the ancient ecosystem, and the same fisheries would not necessarily be chosen for a future restoration policy. (Although aboriginal fisheries in some form may well be appropriate members of a future sustainable fisheries portfolio, as outlined below in § 5(iii).)
Finally, many of these problems may be eased if we were able to run a model of the past forward to simulate its change into a more recent ecosystem. Performing this using Ecosim is very difficult and requires a great deal of data on past fisheries and climate, but has been possible for some ecosystems that have undergone rapid change, such as the Gulf of Thailand (Christensen 1998). Some practical solutions to the issues discussed here may be found, for a Canadian example, in Heymans & Pitcher (2004).
5. Methods for devising sustainable fisheries
A marine ecosystem restored to some semblance of its past state might be thought of as a ‘Lost Valley’ (we are grateful to Dr Daniel Pauly for suggesting this term in 2001): an ecosystem discovered complete with all of its former diversity and abundance of creatures (Pitcher et al. 2004). The BTF process aims to describe a series of such ‘Lost Valleys’ as a set of potential restoration goals.
Since a ‘Lost Valley’ has to be fished sustainably, we have to ask how this might be achieved? Using the same fishing fleet as today to fish a restored ecosystem is generally not a viable option, because massive depletion would soon re-emerge (e.g. Hong Kong; Pitcher et al. 2004). Nor is it realistic to expect the fishing gear and methods of former times, including those of aboriginal fisheries, to be re-employed. Of course, some former fisheries might have attractively low bycatch, operating costs or ease of construction and use, so it is evident that some rational criteria for the selection and operation of sustainable fisheries need to be devised.
Criteria for opening responsible and sustainable fisheries have been designed as part of the BTF process (table 2; Pitcher et al. 2004) and are further discussed here. The list of nine criteria is meant to be operated in a hierarchical fashion.
Minimal bycatch discards. Over the past decade, trawl, trap and purse seine fisheries have attained impressive reductions in bycatch through the use of separators, lifters, gates and excluders (review: Kennelly & Broadhurst 2002), and by altering fishing practices (e.g. dolphins released in tuna purse seine fisheries (Hall 1988); long-line setting adapted to reduce hook mortality in seabirds (Brothers et al. 1999)). These technological advances may be successfully used to greatly reduce unintended catches of non-target species of fishes, marine mammals, reptiles, birds and invertebrates. Moreover, in some jurisdictions, discards have become illegal (e.g. Norway).
No damage to habitat. Unmodified bottom trawls and dredges may do great harm to sessile benthic invertebrates (e.g. sponges, gorgonids, corals) whose architecture acts as a refuge habitat for juveniles of many commercial fish species (Hall 1988; Watling & Norse 1998). To meet this criterion, technological improvements to the fishery will have to be used to minimize damage, for example by allowing trawls that fish only above the bottom. Where some collateral damage to benthos is inevitable, such as in prawn trawls, large and progressive reductions in damage, say 10-fold, might be mandated.
Include aboriginal fisheries. Some fisheries by indigenous or aboriginal peoples were sustainable over thousands of years (e.g. eulachon, salmon and halibut in the Pacific Northwest; Richardson 1992). In terms of equity, they should be included in the candidate fisheries portfolio, provided the take is sustainable, and where such customary rights are recognized. Aboriginal fishers often have an intimate connexion with and knowledge of coastal marine ecosystems and their support for a policy such as BTF could enhance compliance with regulations.
Include traditional target species. Provided criteria (i) and (ii) above are satisfied, this category is included because there will be an understandable demand for traditional desirable fish species in local fishing communities. For example, the historic Atlantic halibut fishery has not proven sustainable, but the species would be in demand as a target in a restored ecosystem.
Minimize risk to charismatic species. While it is evident from the recorded history of seabirds, whales, seals and sirenians that many ‘charismatic’ species are sensitive to exploitation by humans (see, for example Mowat 1984; Roman & Palumbi 2003), this criterion may well be in conflict with criteria (iii) and (iv), because coastal peoples traditionally exploited seals, sea lions, whales, dugongs, turtles, ducks, gulls, petrels, auks and other seabirds (e.g. Australia, Williams & Baines 1993; British Columbia, Brown et al. 1997). Where customary rights are recognized an aboriginal take of these species would be allowed under criterion (iii), with appropriate consent under criterion (vii). By contrast, many marine mammal, bird and shark species have recently become ‘charismatic’ to the conservation movement, and legal bans on killing them reflect public revulsion at their use for human food. However, these views are volatile and local, so in the last resort, the choice of whether to exploit these types of animal will be locally or nationally determined. The only rational criterion is avoidance of excessive depletion and minimal risk of extirpation.
Exclude fishing on juvenile groups. Generally, heavy fishing on juveniles increases the risk of recruitment failure, so such fisheries would not normally be allowed. In some cases, traditional fisheries (criterion (iv)) include eggs, fry and juveniles of highly fecund species such as herring, anchovy, sardines, milkfish or hake, so such fisheries would be permissible where impacts can be proven to be minimal. Likewise, fisheries might be allowed on restricted numbers of juveniles where adults live and spawn in refuges from fishing, as in traditional Mediterranean fisheries (Caddy 2000).
Participatory vetting of fisheries. To maximize support, the local fishing community must vet and approve the list of fisheries, notwithstanding criteria (iii) and (iv). In addition, the management agency must be convinced with science-based evidence that gear is appropriate (criteria (i) and (ii)), that management and monitoring (criterion (ix)) are feasible for the chosen fisheries, and that the scientific basis of the forecasting (criterion (viii)) represents best practice.
Simulations show fisheries are sustainable. Fishery assessments must show that the biomass of the main ecosystem groups, biodiversity, and the fishery catches themselves are probably sustainable and will not fluctuate more than a predetermined and agreed amount over a 100 year period. A tougher criterion would be to insist that the forecasts are robust against climate fluctuations and uncertainty to a specified level of risk. The great importance of ecosystem-based analysis is evident here, because, on their own, single-species stock assessments cannot show risks to charismatic or non-target organisms, or sessile organisms that provide important structural cover. Criterion (viii) describes a critical part of the process: examining trade-offs of ecological with social and economic objectives using as a wide a range of indices as possible.
Adaptive management plan is in place. Because environmental changes (climate, pollution) and our ignorance of fundamental ecology often lead to the unexpected in natural ecosystems, it would be prudent for the restored ‘Lost Valley’ and its fisheries to be subject to regular monitoring of the indices from criterion (viii). This would allow adaptive shifts in fishing, much like the way that catch quotas and fishing locations are regulated today, but driven by an ecosystem approach.
Table 2.
Candidate list of criteria for sustainable and responsible fisheries to be opened in a restored ecosystem.
(For full discussion see § 5. Modified from Pitcher et al. 2004.)
| criteria for sustainable fisheries | notes |
|---|---|
| (i) minimal bycatch discards | technological modifications to gear |
| (ii) no damage to habitat by gear | technological modifications to gear |
| (iii) include aboriginal fisheries | customary rights recognized |
| (iv) include traditional target species | except where (i) and (ii) would bar |
| (v) minimize risk to charismatic species | except as under (iii) and (vii) |
| (vi) exclude fisheries on juveniles | except where minimal impact is proven |
| (vii) participatory vetting of fisheries | by management agency, local community and public |
| (viii) simulations show fishery sustainable | 100 year simulations are satisfactory |
| (ix) adaptive management plan in place | adaptive changes to the unexpected (e.g. climate change) |
A candidate responsible fishery designed with criteria (i)–(ix) could be evaluated by assessing its conformity with the FAO Code of Conduct for Responsible Fisheries (FAO 1995) using a rapid appraisal technique (Pitcher 1999). Although a portfolio of responsible fisheries may be designed for the ‘Lost Valley’ modelling process, in practice it is likely that continual adjustment will take place once a BTF policy was implemented.
6. Opening the ‘Lost Valley’
After an ‘ideal’ set of fisheries have been selected according to the criteria (i)–(vii) discussed earlier, simulations are used to forecast fishing and its effect over a long time period, typically 50 or 100 years (criterion (viii)). Relative fishing mortalities over the set of fisheries are adjusted until catches are sustainable and impacts on the ecosystem meet specified criteria: this process has been termed ‘opening the Lost Valley’ (Pitcher et al. 2004). The adjustment is performed automatically using an automated search routine.
A search routine in Ecosim seeks to maximize a specified objective function using a multi-dimensional Davidon–Fletcher–Powell search algorithm (Walters et al. 2002). The search iteratively varies the fishing mortality per gear type to maximize the objective function over the simulated time horizon, usually 50 or 100 years. Alternative fishery objectives may be selected, including economic value, numbers of jobs, the biomass of long-lived species, or a log portfolio utility function. When the search is started, for each fishery, the species landed, bycatch and discards are related to the model groups and initial small catches (e.g. 2.5% of starting biomass) are entered, along with any discarded bycatches, ex-vessel prices by species and gear, and relative operating costs by gear. (An interesting technical detail is that, at this point, the basic parameters of the underlying Ecopath model have to be readjusted slightly to achieve mass-balance: for replicability, this may be performed with an automated procedure (Kavanagh et al. 2004)). In practice, many searches have to be performed to reduce the chances of finding a local optimum.
The results of the search provide forecast fishery catches, biomass, economic values, numbers of jobs and biomass changes in all other groups in the fished ‘Lost Valley’ ecosystem. Results are examined and any scenarios that cause extirpation or severe depletion of species, are eliminated. In fact, the biomass of designated species may be protected from large changes in biomass as part of the policy search objective function (Cochrane 2002). Adjustments to the weightings in the objective function enable (after some iteration) policies that attempt to balance economic with ecological or social values. This search procedure is repeated for a wide range of policy objectives and for each candidate-restored ecosystem, producing several forecast scenarios that may be compared.
In addition, we may seek to challenge these results with climate changes that might realistically be expected for the locality in question, and taking account of the principal uncertainty in the simulation modelling. These can be achieved by driving the simulations with various types of climate-forcing function, and with semi-Bayesian Monte Carlo simulations (as shown in figure 2).
7. Choosing ecosystem restoration goals
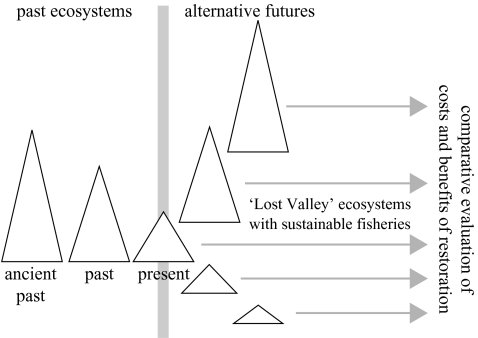
Once we have a snapshot of what a set of alternative restored ecosystems, complete with their sustainable fisheries, might look like, the remaining problem is to find an objective way to choose a rational policy goal from among them. This may be done by comparing the benefits that will accrue to society from each alternative future represented by a fished ‘Lost Valley’ ecosystem (figure 3). To show the full range of options that may be considered, included in this process is the present-day ecosystem (albeit with a new portfolio of fisheries designed to be sustainable). It might also be useful to include ecosystems even further depleted than that of the present day, especially if we wished to evaluate the advantages and risks for increased food production of fishing large amounts of lower trophic level organisms (krill, zooplankton).
Figure 3.
Diagram illustrating the concepts of sustainable fishing of restored ‘Lost Valley’ ecosystems, and the comparative evaluation of their costs and benefits. Triangles represent ecosystem models of the past and possible futures, as in figure 1. Arrows represent sustainable fishing by responsibly designed fisheries in restored ecosystems.
One fundamental way to evaluate the benefits of alternative restored ecosystems is to compare the net present economic value of their fisheries, information that is readily estimated from the Ecosim simulations mentioned previously. In general, experience with this technique shows that evaluating economic objectives using conventional discounting will cause unacceptable depletion and loss of biodiversity, a problem referred to as the ‘conservationist dilemma’ that has been well known since the time of Clark (1973). To take account of this ‘intergenerational externality’ (Padilla 2002), various forms of low or zero discount rates algorithms have been argued to be more appropriate for natural resources (e.g. Chichilnisky 1996; Weitzman 2001; Fearnside 2002), although these attempts have generated some controversy (e.g. Goulder & Stavins 2002). In the BTF work, an intergenerational discounting equation is used that takes into account both conventional discounting and a societal view of the need to recognize the rights of the next generation (in a Pigouvian fashion; Sumaila et al. 2001). The ‘discount clock’ is partly reset each generation, and both market and societal intergenerational discount rates are used (Sumaila & Walters 2003, 2004). Using this algorithm, Ainsworth & Sumaila (2003) analyse how much of the collapse of Newfoundland cod may be attributed to a failure to take account of value to future generations. In BTF analysis, either intergenerational or conventional values may be calculated as options in the ecosystem simulations.
Purely economic considerations, however, are rarely considered sufficient for modern policy making. The simulation technique also provides an estimate of the number of jobs in the fishery, at least those directly involved: the processing and marketing sectors are not included but might, for a particular fishery, also be estimated from the total catch.
Because the simulation technique covers all biological components of the ecosystem, it is also possible to examine the effect of the candidate fisheries on several measures of integrity and diversity. For example, system resilience may be estimated by a whole-ecosystem index (Ulanowicz 1999; Heymans 2004). Comparisons may also be made using a biodiversity index modified for use with the functional groups in this type of modelling, rather than the samples of species for which they were designed (a modified Kempton index, Q-90; Ainsworth & Pitcher 2005), or an index based on life history and fishery parameters that expresses the risk of local extinction (Cheung & Pitcher 2004).
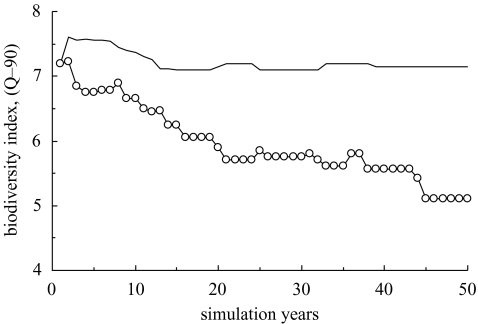
An example of biodiversity tracked over a 50 year simulation is shown in figure 4, which illustrates a northern British Columbia marine ecosystem restored to its estimated state in 1750, before contact of native peoples with European settlers. Two different objective functions have been maximized by searches. Note that the economic objective progressively reduces biodiversity while the other preserves the biodiversity of the ecosystem more or less in its restored state.
Figure 4.
Example of biodiversity (modified Kempton index, Q-90) tracked over 50 year optimal harvest scenarios in a northern British Columbia marine ecosystem restored to a candidate ‘Lost Valley’ and then fished (from year 0) with a designed responsible fishing fleet. This ‘Lost Valley’ example is the ecosystem in its estimated state in 1750 before contact of native peoples with Europeans. Objective functions have been maximized by searches. Open circles represent the results for an economic objective; the solid line represents the results for a mixed ecological/economic objective. Note that fishing under the purely economic objective progressively depletes biodiversity. (Modified from Ainsworth & Pitcher 2005b.)
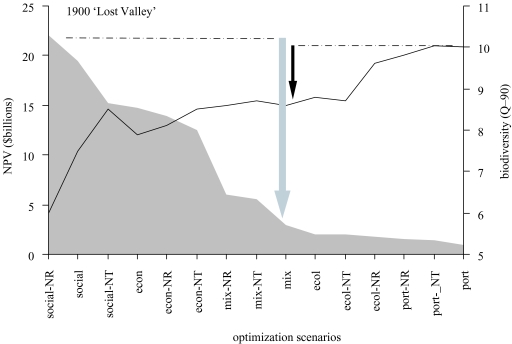
Comparisons among the candidate restoration scenarios may now be made. One useful technique is to sort them into order using each of the criteria in turn. Is this way, trade-offs among conflicting objectives, such as economic value and biodiversity, are made explicit. Policy choices for fisheries inevitably require such trade-offs, but they are rarely made transparent such that industrial, union and conservation stakeholders may attempt to achieve a consensus. An example from work on northern British Columbia is shown in figure 5, which illustrates the trade-offs between biodiversity and economic value for several scenarios under one of the candidate restoration goals (based on restoring the ecosystem as it was in 1900).
Figure 5.
The trade-offs between economic value (NPV, left y-axis, shaded area) and biodiversity (Q-90, right y-axis, solid line) for 15 fished ‘Lost Valley’ scenarios based on the northern British Columbia marine ecosystem restored to its state in 1900. Each scenario represents the result of a single optimal policy search that maximizes an objective function (see § 6). Scenarios are sorted in order by total value. Conventional NPV is in US$ billions over 100 years of fishing (discount rate: 4% per year); end-state biodiversity is represented by the modified Kempton Q-90 statistic. In the scenario labels, ‘social’ maximized jobs, ‘econ’ maximized NPV, ‘ecol’ maximized B/P ratios, ‘mixed’ represents balanced three-way objectives, and ‘port’ was a portfolio utility optimization. Scenarios that generate large revenues tend to sacrifice biodiversity and vice versa. Trade-offs for one of the ‘mixed’ objectives are indicated by arrows. (Modified from Ainsworth & Pitcher 2005b.)
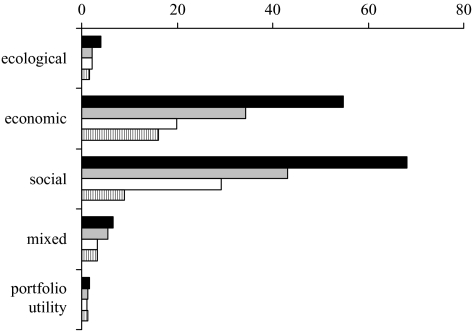
Figure 6 shows forecast economic benefits generated by four different ‘Lost Valley’ restoration goals: northern British Columbia ecosystems based on 1750, 1900, 1950 and 2000. Results for four different objective functions are shown. Note that, in this example, maximizing jobs produces slightly more cash than maximizing economic value, but the impact on biodiversity is greater.
Figure 6.
Forecast economic benefits (intergenerational NPV) generated by four ‘Lost Valley’ restoration goals over 50 years of fishing in northern British Columbia. Black bars show values based on a ‘Lost Valley’ that restores the 1750 ecosystem; grey bars show 1900; white bars show 1950; striped bars show 2000. Results for four different objective functions are shown. (From Ainsworth & Pitcher 2005b.)
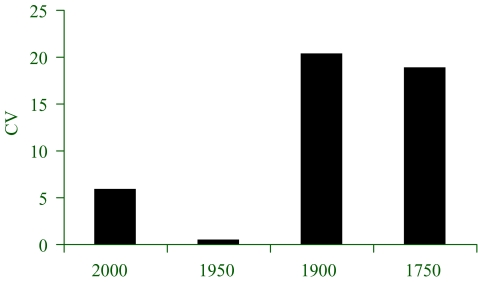
These results may be challenged by uncertainty in the principal model parameters using Monte Carlo sampling. Figure 7 shows the coefficients of variation for four restoration goals for northern British Columbia, averaged across 54 functional groups at the end state of 50 year ecosystem simulations. The value for the 1950 restoration goal is anomalously low because of deficiencies in the model and the data upon which it is based. It would be unwise therefore to include this goal in any realistic discussion of policy until this ecosystem model is improved.
Figure 7.
Variability in biomass (CV, coefficient of variation) for four restoration goals for northern British Columbia, averaged across 54 functional groups at the end state of 50 year ecosystem simulations. The model was subjected to Monte Carlo sampling from even distributions of all biomass parameters, based on quality of the data used in each a case, and filtered by the need to achieve mass-balance. (From Ainsworth & Pitcher 2005b.)
8. Implementing back-to-the-future policy: how do we get there?
The intention of the process is to provide a clear, cognitive map of a future ecosystem that, as far as possible, resembles one from the past, and a policy goal to which all may agree and aspire. For a full evaluation, the costs of restoration should be considered alongside the benefits, because a true policy goal cannot be known until a full cost–benefit analysis is performed. However, there are two problems with this ostensibly logical approach.
First, there is a fundamental problem in that estimating the costs of restoration may depend on precisely what techniques are adopted, and the actual instruments may themselves generate conflict. For example, MPAs set up adjacent to a traditional fishing community will often trigger protests from inshore and local fishers if they cannot be convinced of the long-term advantages. Moreover, reducing quotas for some sectors as fisheries are modified to become more sustainable is bound to generate conflict.
Second, there are considerable psychological advantages in emphasizing the possibility of restored biomass, which may be considerable in long-lived target species such as halibut or cod. Hence, it may be easier to achieve agreement by putting forward an attractive long-term goal than by diverting attention to the means by which one might get there. Agreement on one element of a policy generally eases subsequent steps, although final agreement may always be difficult. However, once management aims to make progress towards a specific goal, the use of adaptive management (e.g. Walters 1986), at least in its passive form, is the wisest course, to try to avoid the difficulties that a changing environment and an imperfectly understood ecology can throw at any management plan.
When BTF is implemented, the ‘Lost Valley’ ecosystems are depleted from their original state by the sustainable fisheries designed to exploit them. So, rather than allow expensive restoration to go all the way to the ‘Lost Valley’ state only to be subsequently depleted, it would clearly be expedient and cheaper to try to restore the system to one of these fished ecosystem states directly. Consequently, we may regard the ‘fished Lost Valley’ as comprising a suite of organisms at their ORB (Ainsworth & Pitcher 2005b). A full quantitative basis for this ORB concept is currently under development, but includes a bioeconomic evaluation of the costs of restoration.
Surprisingly, implementation of BTF may engender some conflict between managers and the conservation community as the system moves towards a ‘fished Lost Valley’ objective. Higher biodiversity may entail reductions in some species while others increase. For example, over the past 100 years in the North Sea, seabird populations have increased at least twofold as a result of human activities, including fishing (Mackinson 2001; Furness 2002). Thus, attempts to restore older systems might involve active or passive management to reduce some species. In this manner, it is interesting to contemplate some of the trade-offs that may have to be faced in a BTF restoration process. For example, restoration of habitat and wild populations in terrestrial systems usually has to be strictly managed. The common taboo on killing mammals and birds in marine ecosystems does not extend to terrestrial systems, and we see many examples of elephants, kangaroos, elk, wolves and crocodiles whose populations are controlled by active culling at the same time as they are protected from hunting. So it is important to realize that, once marine mammal, bird, fish or carnivorous turtle populations have recovered to sustainable levels within the meaning of the target ‘Lost Valley’ ecosystem, they may have to be controlled within the management boundaries. Similarly, Walters & Martell (2004) show that active culling may lead to higher fishery values when predator and prey are linked in a depensatory relationship. Monitoring programmes should be set up to ensure that all changes, including charismatic fauna, are within the expected bounds of the transitional path towards new management objectives.
9. Implementing Back-To-the-Future policy: participation
Implementing a policy goal that has been chosen using any science-based process, including BTF, is, of course a difficult matter. When fishing communities and other essential stakeholders actively participate in the policy agenda, compliance and consent may be high (Harris 1998; Hart & Pitcher 1998). Sometimes even voluntary agreements with a strong local base may operate surprisingly well (e.g. English Channel area agreements by gear sectors; Blyth et al. 2002). Haggan (2000) identifies four elements as critical to successful participation: recognition of the scope of the problem and our collective responsibility whether fishers, scientists, managers or policy makers; respect for different systems of knowledge; agreement to share knowledge in the interest of conservation and restoration; and commitment to share in the benefits of restored systems. Unfortunately, much recent fisheries management operates at very large scales. Degnbol (2003) considers that this scale is often too large to reflect local needs of fishing communities. Pauly and Ommer have called for ‘putting fisheries back in local places’ (Pauly 1999). Because ecosystem simulations generally work on a per-square-kilometre basis, and wide-ranging important species that are seasonal visitors can be emulated with a range of techniques that deal with migration, these methods may be adapted to quite small local areas (e.g. Hong Kong, less than 2000 km2; Pitcher et al. 1998). This means that a BTF analysis can hope to reflect local needs and issues reasonably well. An example of pilot work in a local community in British Columbia is presented in Pitcher et al. (2002c).
In BTF, the aim is to encourage a greater chance of success because a sense of ownership of the process is fostered and developed from the earliest stages of the work. The BTF process includes community participation in building models of the past, in the choice of sustainable fisheries and in the evaluation of the costs and benefits of alternative restoration goals. Moreover, the mental maps shaped by awareness of past abundance and diversity developed in the BTF process may serve to assist consent and compliance with a restoration agenda (Pitcher & Haggan 2003). Participatory elements that are integral to three phases of the BTF process are summarized in table 3.
Table 3.
Summary of integral participatory elements from local fishing communities in the BTF process.
(TEK, traditional ecological knowledge; LEK, local ecological knowledge. All stages are intended to work in concert with science-based decision making.)
| model development phase |
|
| policy development phase |
|
| operational phase |
|
10. Discussion: unresolved Issues
At this stage, BTF is work in progress, and so it is not surprising that several methodological and procedural challenges remain unresolved.
The principal modelling difficulties relate to the scope, magnitude and time-scale of changes experienced by the ecosystems. In theory, eliminating fishing pressure in a model of the present day would lead to models resembling the past, but in practice this may prove impossible because of changes in species composition, ecosystem structure and environment. In the BTF process, past models are not built by ‘winding back the present’, but by using specific historical information on the presence and absence of species, trends in past biomasses from stock assessment or surveys, where they exist. In addition, we may use information from archives; historical documents (Heymans & Pitcher 2004); archaeological investigations (e.g. Heymans 2003; Orchard & Mackie 2004); interviews that collate traditional or local environmental knowledge (e.g. Haggan et al. 1998; Salas et al. 1998; Simeone 2004), even language (Danko 1998) and ancient artwork (Williams 1998). The many sources of uncertainty in this process need to be addressed and the way in which historical information is turned into inputs for the ecosystem modelling needs considerable improvement. Better semi-quantitative assessments of relative biomass, diet and sizes need to be devised (e.g. Ainsworth & Pitcher 2005a). In general, historical information needs a more rigorous and replicable transduction into the quantitative data needed for ecosystem modelling to become a part of the science of historical ecology.
BTF has an advantage in not relying exclusively on complex stock assessment (Walters 1998), although such work can help in the tuning of the ecosystem models (Mace 2001). Nevertheless, the quantitative ecosystem modelling used for BTF so far relies almost exclusively on Ecopath and Ecosim techniques. Yet, many of the assumptions in this modelling system, although plausible, remain unvalidated. Of especial concern are the Ecosim ‘vulnerability’ parameters, to which specific results often appear very sensitive. Moreover, these parameters not only shape predator–prey interactions (which they do in an entirely credible fashion for evolutionary ecologists), but also predetermine the scope for further biomass growth in relation to current levels. For any series of ‘time-shot’ BTF ecosystem models, this problem creates a conflict between the need to compare the outcomes of various fisheries options while other parameters remain fixed, and setting parameters correctly for biomasses that were closer to unexploited levels in the past. These modelling problems have yet to be resolved.
As pointed out by Heymans & Pitcher (2004), our past ecosystem models may resemble the actual past as a painting by Picasso resembles reality. An important question is whether our comparative restoration policy scenarios can be made robust against such distortions. A deeper insight of the dynamics of ecosystems under change will be required before we can answer this question.
Moreover, the logistics and cost of mounting a quantitative, robust and credible BTF analysis are considerable. An interdisciplinary team needs to be assembled to gather, validate and analyse the historical, archaeological and ecological information needed for BTF. Moreover, like other synoptic work, the scope of BTF work appears to be beyond the capacity of one graduate student thesis, and therefore hard to fund.
11. Two common criticisms are often made of the Back-to-the-future process, and therefore are discussed here
The ‘ecosystems do not rewind’ argument. First, it is held that, even if the past would form a desirable goal, it is not possible to get there starting with today’s state, because the environment and the ecosystem have changed too much. The ecosystem, in short, will fail to ‘rewind’, thereby fatally compromising the BTF policy goal. In fact, the experience with fisheries management is that most exploited species will ‘rewind’ if they are not too depleted (e.g. Hilborn 1996; Hall 1999, p. 202; Hilborn et al. 2003). It is worth reflecting that the assumptions of conventional single-species stock assessment would never be valid if this were not true. Nevertheless, there are some instances where a ‘rewind’ does not happen as expected. This may be because of habitat destruction or pollution, loss of keystone species or predator–prey cascades in the trophic web (e.g. Newfoundland after the cod collapse; Fu et al. 2001), and these factors will vary among the different life histories of species in the ecosystem. Small pelagic fishes are likely to be governed by ocean cycles (e.g. Chavez et al. 2003) as well as fishing (e.g. De Oliviera et al. 1998). Alternate stable states may exist for some ecosystems (e.g. Baltic cod/clupied switch; Rudstam et al. 1994), and invasions may alter ecosystem structure irreversibly (e.g. Black Sea; Zaitsev 1992). In the light of scientific evidence like this, some irreversible changes or switches might be anticipated, and in this case, restoration possibilities will have to take what is feasible into account. Thus the answer to this criticism is, if a BTF policy is challenged by irreversible changes, let it be so. We can probably have some insight into what may be different and can try to model it. The rest of the BTF procedures, in devising sustainable fisheries and evaluating the most beneficial reconstruction that may be achieved, remain valid.
The ‘past was different’ argument. Second, a variant of the first criticism is that, because the past was very different, models of the past, even if accurate, would not represent viable restoration goals. This is because the past was different in terms of species composition and climate, primary production was lower because of recent eutrophication, and pollution was lower from smaller populations of humans. All these things are true, but differences between the past and the present can be dealt with in a similar way as for the first criticism. Ecological processes in the past must realistically have obeyed the same sets of rules and laws as we see today, even if climate and other factors had different values. Past models often deliberately span several years to minimize some of the shorter-term changes. Although it would be satisfying to have models of the past that could be derived directly by ‘winding back’ models of the present using past time-series of fisheries and climate, beyond a certain date, this would be increasingly uncertain. As shown above, this is not necessary for BTF and a series of snapshots in time will suffice.
A third common criticism, often brought by social scientists, is based on a misunderstanding. BTF does not aim to bring back the human social conditions of the past.
BTF provides a consistent long-term policy goal that may never be reached, but which short-term variation in ecology, climate and human influences need not deflect. A broad participation by scientists, researchers, stakeholders, government, managers, NGOs and the public is critical for the success of any restoration policy that might be set up under the BTF banner. Such broad participation might perhaps be assisted by an explicitly ecosystem-based policy that could be seen to employ data inputs from all sectors of society (Pitcher 2000). However, we have barely scratched the surface of the deep issues raised by the need for this level of participation in the BTF policy searches and analyses. Nor have we enough experience of asking fishing communities to choose what kind of future they might wish to aim for. We are not yet sure how to convey the uncertainty in our work, which to many may seem arcane. Perhaps a cadre of ‘barefoot ecologists’, the equivalent of rural development generalists for fisheries as envisaged by Jeremy Prince, might be able to help (Prince 2003).
Acknowledgments
The development of the Back-to-the-future concept has been supported at various stages by small grants from the Peter Wall Institute for Advanced Studies and the Fisheries Centre, both at the University of British Columbia; the World Wildlife Fund, Canada; and the Department of Fisheries and Oceans, Canada. The author is indebted to Coasts under Stress (CUS) for a major grant supporting BTF that made possible most of the quantitative work, interviews and workshops outlined in this paper. CUS is an interdisciplinary initiative supported by the National Science and Environmental Research Council and the Social Science and Humanities Research Council of the Government of Canada. The author is also grateful for the enthusiasm and commitment from BTF team members Dr Sheila (J.J.) Heymans, Dr Marcelo Vasconcellos, Dr Rashid Sumaila, Dr Melanie Power, Cameron Ainsworth, Eny Buchary, William Cheung, Aftab Erfan, Robyn Forrest, Nigel Haggan, Hector Lozano and Telmo Morato. Dr Villy Christensen and Professor Carl Walters provided invaluable and long-suffering support for the modelling. Comments from two anonymous referees improved the manuscript.
Glossary
- BTF
back-to-the-future
- MPA
marine protected area
- NGO
non-governmental organizations
- NPV
net present value
- ORB
optimum restorable biomass
References
- Ainsworth, C. H. & Pitcher, T. J. 2005a Using local ecological knowledge as a data supplement for ecosystem models. (In the press.)
- Ainsworth, C. H. & Pitcher, T. J. 2005b Evaluating goals for restoration in the marine ecosystem of British Columbia. (In the press.)
- Ainsworth C.H., Pitcher T.J. Modifying Kempton’s biodiversity index for use with dynamic ecosystem simulation models. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004. pp. 91–93. Fisheries Centre Research Reports. [Google Scholar]
- Ainsworth C.H., Sumaila U.R. Intergenerational discounting and the conservation of fisheries resources. A case study in Canadian Atlantic cod. Pages 26–33 in Sumaila, R.(ed) In: Sumaila R., editor. Three essays on the economics of fishing. 3. Vol. 11. 2003. pp. 26–33. Fisheries Centre Research Reports. [Google Scholar]
- Ainsworth C.H., Heymans J.J., Pitcher T.J., Vasconcellos M. Ecosystem models of Northern British Columbia for the time periods 2000, 1950, 1900 and 1750. 4. Vol. 10. 2002. Fisheries Centre Research Reports. [Google Scholar]
- Andersen P.K. Competition, predation, and the evolution and extinction of Steller’s sea cow, Hydrodamalis gigas. Mar. Mamm. Sci. 1995;11:391–394. [Google Scholar]
- Arcese P., Sinclair A.R.E. The role of protected areas as ecological baselines. J. Wildl. Mngmt. 1997;61:587–602. [Google Scholar]
- Balmford A. Economic reasons for conserving wild nature. Science. 2002;297:950–953. doi: 10.1126/science.1073947. (and 18 others) [DOI] [PubMed] [Google Scholar]
- Bakun A. Patterns in the ocean: ocean processes and marine population dynamics. University of California Sea Grant; San Diego, CA: 1996. [Google Scholar]
- Baum J.K., Myers R.A., Kehler D.G., Worm B., Harley S.J., Doherty P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2002;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- Blyth R.E., Kaiser M.J., Edwards-Jones G., Hart P.J.B. Voluntary management in an inshore fishery has conservation benefits. Environ. Conserv. 2002;29:493–508. [Google Scholar]
- Brothers N.P., Cooper J., Løkkeborg S. The incidental catch of seabirds by longline fisheries: worldwide review and technical guidelines for mitigation. 1999. FAO Fisheries Circular 937. [Google Scholar]
- Brown C.R., Brown B.E., Carpenter C. Some of the traditional food gathering of the Heiltsuk Nation. BC Ministry of Education, Skills and Training; Victoria, Canada: 1997. [Google Scholar]
- Caddy J.F. Marine catchment basin effects versus impacts of fisheries on semi-enclosed seas. ICES J. Mar. Sci. 2000;57:628–640. [Google Scholar]
- Callenbach E. Bring back the buffalo! A sustainable future for America’s great plains. Island Press; Washington, DC: 1995. [Google Scholar]
- Chavez F.P., Ryan J., Lluch-Cota S.E., Ñiquen M.C. From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- Cheung W.L., Pitcher T.J. An index expressing risk of local extinction for usewith dynamic ecosystem simulation models. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. 2004. pp. 94–102. Fisheries Centre Research Reports. [Google Scholar]
- Chichilnisky G. An axiomatic approach to sustainable development. Social Choice Welfare. 1996;13:219–248. [Google Scholar]
- Christensen V. Fishery induced changes in a marine ecosystem: insight from models of the Gulf of Thailand. J. Fish Biol. A. 1998;53:128–142. [Google Scholar]
- Christensen V. Ecosystems of the past: how can we know since we weren’t there? In: Guénette S., Christensen V., Pauly D., editors. Fisheries impacts on North Atlantic ecosystems: models and analyses. 4. Vol. 9. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2002. pp. 26–34. Fisheries Centre Research Reports. [Google Scholar]
- Christensen V., Pauly D. Changes in models of aquatic ecosystems approaching carrying capacity. Ecol. Applic. 1997;8:104–109. [Google Scholar]
- Christensen V., Guenette S., Heymans J.J., Walters C.J., Watson R., Zeller D., Pauly D. Hundred-year decline of North Atlantic predatory fishes. Fish and Fisheries. 2003;4:1–24. [Google Scholar]
- Clark C.W. The economics of overexploitation. Science. 1973;181:630–634. doi: 10.1126/science.181.4100.630. [DOI] [PubMed] [Google Scholar]
- Cochrane K. The use of ecosystem models to investigate ecosystem-based management strategies for capture fisheries: introduction. In: Pitcher T.J., Cochrane K., editors. The use of ecosystem models to investigate multispecies management strategies for capture fisheries. 2. Vol. 10. 2002. pp. 5–10. Fisheries Centre Research Reports. [Google Scholar]
- Danko J.P. Building a reliable database from native oral tradition using fish related terms from the Saanich language. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 29–33. Fisheries Centre Research Reports. [Google Scholar]
- Degnbol P. Science and the user perspective: the gap co-management must address. In: Wilson D.C., Nielsen J.R., Degnbol P., editors. The fisheries co-management experience. Accomplishments, challenges and prospects. Kluwer; Dordrecht, The Netherlands: 2003. pp. 31–50. [Google Scholar]
- De Oliviera J.A.A., Butterworth D.S., Bole B.A., Cochrane K.L., Brown J.P. The application of a management procedure to regulate the directed and bycatch fishery of South African sardine, Sardinops sagax. S. Afr. J. Mar. Sci. 1998;19:449–469. [Google Scholar]
- Diamond J. Guns, germs, and steel: the fates of human societies. Random House; London: 1997. [Google Scholar]
- Dobson A.P., Bradshaw A.D., Baker A.J.M. Hopes for the future: restoration ecology and conservation biology. Science. 1997;277:515–522. [Google Scholar]
- Dulvy N.K., Sadovy Y., Reynolds J.D. Extinction vulnerability in marine populations. Fish and Fisheries. 2003;4:25–64. [Google Scholar]
- FAO . Code of conduct for responsible fisheries. FAO; Rome: 1995. [Google Scholar]
- Fearnside P.M. Time preference in global warming calculations: a proposal for a unified index. Ecol. Econ. 2002;41:21–31. [Google Scholar]
- Fu C., Mohn R., Fanning L.P. Why the Atlantic cod (Gadus morhua) stock off eastern Nova Scotia has not recovered. Can. J. Fish. Aquat. Sci. 2001;58:1613–1623. [Google Scholar]
- Furness R.W. Management implications of interactions between fisheries and sandeel-dependent seabirds and seals in the North Sea. ICES J. Mar. Sci. 2002;59:261–269. [Google Scholar]
- Gibbons W., Odum E.P. Keeping all the pieces: perspectives on natural history and the environment. Smithsonian Institution Press; Washington, DC: 1993. [Google Scholar]
- Goulder L.H., Stavins R.N. Discounting: an eye on the future. Nature. 2002;419:673–674. doi: 10.1038/419673a. [DOI] [PubMed] [Google Scholar]
- Haggan N. Back to the future and creative justice: recalling and restoring forgotten abundance in Canada’s marine ecosystems. In: Coward H., Ommer R., Pitcher T., editors. Just fish: ethics in the Canadian coastal fisheries. ISER Books; St Johns, Newfoundland: 2000. pp. 83–99. [Google Scholar]
- Haggan N., Beattie A. Back to the future: reconstructing the Hecate Strait ecosystem. 1999;7(3) Fisheries Centre Research Reports. [Google Scholar]
- Haggan N., Archibald J., Salas S. Knowledge gains power when shared. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 8–13. Fisheries Centre Research Reports. [Google Scholar]
- Hall M. An ecological view of the tuna-dolphin problem. Rev. Fish Biol. Fish. 1988;8:1–34. [Google Scholar]
- Hall S.J. The effects of fishing on marine ecosystems and fisheries. Blackwell Scientific; Oxford: 1999. [Google Scholar]
- Harris C. Social regime formation and community participation in fisheries management. In: Pitcher T.J., Hart P.J.B., Pauly D., editors. Reinventing fisheries management. Kluwer; Dordrecht, The Netherlands: 1998. pp. 261–276. [Google Scholar]
- Hart P.J.B., Pitcher T.J. Conflict, cooperation and consent: the utility of an evolutionary perspective on individual human behaviour in fisheries management. In: Pitcher T.J., Hart P.J.B., Pauly D., editors. Reinventing fisheries management. Kluwer; Dordrecht, The Netherlands: 1998. pp. 215–225. [Google Scholar]
- Hays G.C., Broderick A.C., Godley B.J., Luschi P., Nichols W.J. Satellite telemetry suggests high levels of fishing-induced mortality in marine turtles. Mar. Ecol. Prog. Ser. 2003;262:305–309. [Google Scholar]
- Heymans J.J. Revised models for Newfoundland for the time periods 1985–87 and 1995–97. In: Heymans J.J., editor. Ecosystem models of Newfoundland and southeastern Labrador: additional information and analyses for ‘back to the future’. 5. Vol. 11. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2003a. pp. 40–61. Fisheries Centre Research Reports. [Google Scholar]
- Heymans J.J. First nations impact on the eastern Newfoundland and southern Labrador ecosystem during pre-contact times. In: J.J. Heymans., editor. Ecosystem models of Newfoundland and southeastern Labrador: additional information and analyses for ‘back to the future’. 5. Vol. 11. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2003b. pp. 4–11. Fisheries Centre Research Reports. [Google Scholar]
- Heymans J.J. Evaluating the ecological effects on exploited ecosystems using information theory. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004. pp. 87–90. Fisheries Centre Research Reports. [Google Scholar]
- Heymans J., Pitcher T.J. Synoptic methods for constructing models of the past. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004. pp. 1–17. Fisheries Centre Research Reports. [Google Scholar]
- Hilborn R. The frequency and severity of fish stock collapses and declines. In: Hancock D.A., Smith D.C., Grant A., Beumer J.P., editors. Developing and sustaining world fisheries resources: the state of science and management. Proc. 2nd World Fisheries Congr. CSIRO; Canberra, Australia: 1996. pp. 36–38. [Google Scholar]
- Hilborn R., Branch T.A., Ernst B., Magnusson A., Minte-Vera C.V., Scheuerell M.D., Valero J.L. State of the world’s fisheries. A. Rev. Environ. Resources. 2003;28:359–399. [Google Scholar]
- Jackson J.B.C. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. (and 18 others) [DOI] [PubMed] [Google Scholar]
- Johnson T.C., Barry S.L., Chan Y., Wilkinson P. Decadal record of climate variability spanning the last 700 years in the southern tropics of East Africa. Geology. 2001;29:83–86. [Google Scholar]
- Kavanagh P., Newlands N., Christensen V., Pauly D. Automated parameter optimization for Ecopath ecosystem models. Ecol. Model. 2004;172:141–149. [Google Scholar]
- Kennelly S.J., Broadhurst M.K. Bycatch begone: changes in the philosophy of fishing technology. Fish and Fisheries. 2002;3:340–355. [Google Scholar]
- Lenihan H.S., Peterson C.H. How habitat degradation through fishery disturbance enhances impacts of hypoxia on oyster reefs. Ecol. Applic. 1998;8:128–140. [Google Scholar]
- Lichatowich J. Salmon without rivers: a history of the Pacific salmon crisis. Island Press; New York: 2001. [Google Scholar]
- Ludwig D., Hilborn R., Walters C. Uncertainty, resource exploitation, and conservation: lessons from history. Science. 1993;260:17–18. doi: 10.1126/science.260.5104.17. [DOI] [PubMed] [Google Scholar]
- McClanahan T.R. The near future of coral reefs. Environ. Conserv. 2002;29:460–483. [Google Scholar]
- Mace P.M. A new role for MSY in single-species and ecosystem approaches to fisheries stock assessment and management. Fish and Fisheries. 2001;2:2–32. [Google Scholar]
- Mackinson S. Representing trophic interactions in the North Sea in the 1880s, using the Ecopath mass-balance approach. In: Guénette S., Christensen V., Pauly D., editors. Fisheries impacts on North Atlantic ecosystems: models and analyses. 4. Vol. 9. 2001. pp. 35–98. Fisheries Centre Research Reports. [Google Scholar]
- McLoughlin L.C. Questioning assumptions and using historical data in developing an information base for estuarine management. Coast to Coast. 2002;202:281–285. [Google Scholar]
- Martell S., Wallace S. Estimating historical lingcod biomass in the Strait of Georgia. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 45–48. Fisheries Centre Research Reports. [Google Scholar]
- Mercer M.C. Records of the Atlantic walrus, Odobenus rosmarus rosmarus, from Newfoundland. J. Fish. Res. Board Can. 1967;24:2631–2635. [Google Scholar]
- Merilees W. The humpback whales of Georgia Strait. Waters: J. Vancouver Aquarium. 1985;8:1–24. [Google Scholar]
- Montevecchi W.A., Kirk D.A. Great auk (Pinguinus impennis) In: Poole A., Gill F., editors. The birds of North America. vol. 260. The American Ornithologists’ Union and The Academy of Natural Sciences; Philadelphia, PA: 1996. pp. 1–20. [Google Scholar]
- Morales A., Rosello E., Canas J.M. Cueva de Nerja (prov. Malaga): a close look at a twelve thousand year ichthyofaunal sequence from southern Spain. Ann. Sci. Zool. Mus. R. L’Afrique Centrale, Tervuren, Belg. 1994;274:253–262. [Google Scholar]
- Morrison M.L. Wildlife restoration: techniques for habitat analysis and animal monitoring. Island Press; New York: 2002. [Google Scholar]
- Mowat F. Sea of slaughter. Seal Books, McClelland-Bantam Inc; Toronto: 1984. [Google Scholar]
- Myers R.A., Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- Orchard T., Mackie Q. Environmental archaeology: principles and case studies. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004. Fisheries Centre Research Reports. [Google Scholar]
- Padilla E. Intergenerational equity and sustainability. Ecol. Econ. 2002;41:69–83. [Google Scholar]
- Parsons T.R. The impact of industrial fisheries on the trophic structure of marine ecosystems. In: Polis G.A., Winemiller K.D., editors. Food webs: integration of patterns and dynamics. Chapman & Hall; New York: 1996. pp. 352–357. [Google Scholar]
- Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- Pauly D. Fisheries management: putting our future in places. In: Newell D., Ommer R., editors. Fishing people fishing places: issues in Canadian small-scale fisheries. University of Toronto Press; 1999. pp. 355–362. [Google Scholar]
- Pauly D., Christensen V., Dalsgaaard J., Froese R., Torres F., Jr Fishing down marine food webs. Science. 1998a;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Pauly D., Pitcher T.J., Preikshot D. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998b. Fisheries Centre Research Reports. [Google Scholar]
- Pauly D., Christensen V., Pitcher T.J., Sumaila U.R., Walters C.J., Watson R., Zeller D. Diagnosing and overcoming fisheries’ lack of sustainability. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Pitcher T.J. ‘Back to the future’: a novel methodology and policy goal in fisheries. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998a. pp. 4–7. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J. Pleistocene pastures: Steller’s sea cow and sea otters in the Strait of Georgia. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998b. pp. 49–52. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J. Rapfish, a rapid appraisal technique for fisheries, and its application to the code of conduct for responsible fisheries. 1999. FAO Fisheries Circular No. 947. [Google Scholar]
- Pitcher T.J. Fisheries management that aims to rebuild resources can help resolve disputes, reinvigorate fisheries science and encourage public support. Fish and Fisheries. 2000;1:99–103. [Google Scholar]
- Pitcher T.J. Fisheries managed to rebuild ecosystems: reconstructing the past to salvage the future. Ecol. Applic. 2001;11:601–617. [Google Scholar]
- Pitcher T.J. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004a. Introduction to the methodological challenges in ‘back-to-the-future’ research; pp. 4–10. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J. The problem of extinctions. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004b. pp. 21–28. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J. What was the structure of past ecosystems that had many top predators? In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004c. pp. 18–20. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Forrest R. Challenging ecosystem simulation models with climate change: the ‘Perfect Storm’. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. Fisheries Centre at the University of British Columbia; Vancouver, Canada: 2004. pp. 29–38. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Haggan N. Cognitive maps: cartography and concepts for ecosystem-based fisheries policy. In: Haggan N., Brignall C., Wood L., editors. Putting fishers’ knowledge to work. 1. Vol. 11. 2003. pp. 456–463. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Courtney A., Watson R., Pauly D. Assessment of Hong Kong’s Inshore fishery resources. 1. Vol. 6. 1998. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Haggan N., Preikshot D., Pauly D. Ecosystem considerations in fisheries management. Alaska Sea Grant; Anchorage: 1999. ‘Back to the future’: a method employing ecosystem modelling to maximise the sustainable benefits from fisheries; pp. 447–465. [Google Scholar]
- Pitcher T.J., Vasconcellos M., Heymans S.J.J., Brignall C., Haggan N. Information supporting past and present ecosystem models of northern British Columbia and the Newfoundland Shelf. 1. Vol. 10. 2002a. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Heymans J.J., Vasconcellos M., editors. Ecosystem models of Newfoundland for the time periods 1995, 1985, 1900 and 1450. 5. Vol. 10. 2002b. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Power M., Wood L., editors. Restoring the past to salvage the future: report on a community participation workshop in Prince Rupert, BC. 7. Vol. 10. 2002c. Fisheries Centre Research Reports. [Google Scholar]
- Pitcher T.J., Heymans J.J., Ainsworth C., Buchary E.A., Sumaila U.R., Christensen V. Opening the lost valley: implementing a ‘back to the future’ restora tion policy for marine ecosystems and their fisheries. In: Knudsen E.E., MacDonald D.D., Muirhead J.K., editors. Sustainable management of North American fisheries. Vol. 43. American Fisheries Society; MD: 2004. pp. 165–193.pp. 165–193. American Fisheries Society Symposium, Bethesda. [Google Scholar]
- Prince J.D. The barefoot ecologist goes fishing. Fish and Fisheries. 2003;4:359–371. [Google Scholar]
- Reidman M.L., Estes J.A. The sea otter (Enhydra lutris): behavior, ecology and natural history. Biol. Rep. US Fish Wildl. Serv. 1990;90:1–126. [Google Scholar]
- Richardson A. The control of productive resources on the northwest coast of North America. In: Williams N.M., Hunn E.S., editors. Resource managers: North American and Australian hunter–gatherers. Australian Institute of Aboriginal Studies; Canberra: 1992. pp. 93–11. [Google Scholar]
- Roman J., Palumbi S.R. Whales before whaling in the North Atlantic. Science. 2003;301:508–510. doi: 10.1126/science.1084524. [DOI] [PubMed] [Google Scholar]
- Rosello-Izquierdo E., Morales-Muniz A. A new look at the fish remains from Cueva de Nerja. Fish Remains Working Group; Paihia, New Zealand: 2001. October 2001. [Google Scholar]
- Rudstam L., Aneer G., Hildren M. Top-down control in pelagic Baltic ecosystem. Dana. 1994;10:105–129. [Google Scholar]
- Sadovy Y., Cheung W.L. Near extinction of a highly fecund fish: the one that nearly got away. Fish and Fisheries. 2003;4:86–99. [Google Scholar]
- Salas S., Archibald J., Haggan N. Aboriginal knowledge and ecosystem reconstruction. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 22–28. Fisheries Centre Research Reports. [Google Scholar]
- Schindler D.E., Essington T.E., Kitchell J.F., Boggs C., Hilborn R. Sharks and tunas: fisheries impacts on predators with contrasting life histories. Ecol. Applic. 2002;12:735–748. [Google Scholar]
- Simenstad C.A., Estes J.A., Kenyan K.W. Aleuts, sea otters and alternate stable state communities. Science. 1978;200:403–411. doi: 10.1126/science.200.4340.403. [DOI] [PubMed] [Google Scholar]
- Simeone W. How traditional knowledge can contribute to environmental research and resource management. In: Pitcher T.J., editor. Back to the future: advances in methodology for modelling and evaluating past ecosystems as future policy goals. 1. Vol. 12. 2004. pp. 74–77. Fisheries Centre Research Reports. [Google Scholar]
- Sinclair A.R.E., Hik D.S., Schmitz O.J., Scudder G.G.E., Turpin D.H., Larter N.C. Biodiversity and the need for habitat renewal. Ecol. Applic. 1995;5:579–587. [Google Scholar]
- Stanford R., Pitcher T.J. Ecosystem simulations of the English Channel: climate and trade-offs. 3. Vol. 12. 2004. Fisheries Centre Research Reports. [Google Scholar]
- Steneck R.S., Graham M.H., Bourque B.J., Corbett D., Erlandson J.M., Estes J.A., Tegner M.J. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 2002;29:436–459. [Google Scholar]
- Sumaila U.R., Walters C.J. Intergenerational discounting. In: Sumaila R., editor. Three essays on the economics of fishing. 3. Vol. 11. 2003. pp. 19–25. Fisheries Centre Research Reports. [Google Scholar]
- Sumaila U.R., Walters C.J. Intergenerational discounting: a new intuitive approach. Ecol. Econ. 2004 10.1016/j.ecolecon.2003.11.012 Published online 29 December 2004. [Google Scholar]
- Sumaila U.R., Pitcher T.J., Haggan N., Jones R. Evaluating the benefits from restored ecosystems: a back to the future approach. In: Johnston R.S., Shriver A.L., editors. Proc. 10th Int. Conf. of the Int. Inst. of Fish. Econ. & Trade, Corvallis, Oregon, USA 2000. 2001. [Google Scholar]
- Ulanowicz R.E. Life after Newton: an ecological metaphysic. BioSystems. 1999;50:127–142. doi: 10.1016/s0303-2647(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Wallace W.S. Changes in human exploitation of marine resources in British Columbia pre-contact to present day. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 58–64. Fisheries Centre Research Reports. [Google Scholar]
- Walters C.J. Adaptive management of renewable resources. Macmillan; New York: 1986. [Google Scholar]
- Walters C.J. Designing fisheries management systems that do not depend on accurate stock assessment. In: Pitcher T.J., Hart P.J.B., Pauly D., editors. Reinventing fisheries management. Kluwer; Dordrecht, The Netherlands: 1998. pp. 279–288. [Google Scholar]
- Walters C.J., Martell S.J.D. Fisheries ecology and management. Princeton University Press; 2004. [Google Scholar]
- Walters C.J., Christensen V., Pauly D. Structuring dynamic models of exploited ecosystems from trophic mass-balance assessment. Rev. Fish Biol. Fish. 1997;7:139–172. [Google Scholar]
- Walters C., Pauly D., Christensen V., Kitchell J.F. Representing density dependent consequences of life history strategies in aquatic ecosystems: EcoSim II. Ecosystems. 2000;3:70–83. [Google Scholar]
- Walters C.J., Christensen V., Pauly D. Searching for Optimum Fishing Strategies for Fishery Development, Recovery and Sustainability. In: Pitcher T.J., Cichrane K., editors. The use of ecosystem models to investigate multispecies management strategies for capture fisheries. 2. Vol. 10. 2002. pp. 11–15. Fisheries Centre Research Reports. [Google Scholar]
- Ward P. The call of distant mammoths: why the ice age mammals disappeared. Copernicus Press; New York: 1997. [Google Scholar]
- Watling L., Norse E.A. Disturbance of the sea bed by mobile fishing gear: a comparison to forest clearcutting. Conserv. Biol. 1998;12:1180–1197. [Google Scholar]
- Weitzman M.L. Gamma discounting. Am. Econ. Rev. 2001;91:260–271. [Google Scholar]
- Whipple S.J., Link J.S., Garrison L.P., Fogarty M.J. Models of predation and fishing mortality in aquatic ecosystems. Fish and Fisheries. 2000;1:22–40. [Google Scholar]
- Williams J. Reading rocks: on the possibilities of the use of northwest coast petroglyph and pictograph complexes as source material for ecological modelling of prehistoric sites. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. pp. 34–37. Fisheries Centre Research Reports. [Google Scholar]
- Williams N.M., Baines G., editors. Traditional ecological knowledge: wisdom for sustainable development. Centre for Resource and Environmental, Studies Australian National University; Canberra: 1993. [Google Scholar]
- Winship A. Pinnipeds and cetaceans in the Strait of Georgia. In: Pauly D., Pitcher T.J., Preikshot D., editors. Back to the future: reconstructing the Strait of Georgia ecosystem. 5. Vol. 6. 1998. p. 53.p. 57. Fisheries Centre Research Reports. [Google Scholar]
- Zaitsev Y.P. Recent changes in the trophic structure of the Black Sea. Fish. Oceanogr. 1992;1:180–189. [Google Scholar]