Abstract
This contribution, which reviews some broad trends in human history and in the history of fishing, argues that sustainability, however defined, rarely if ever occurred as a result of an explicit policy, but as result of our inability to access a major part of exploited stocks. With the development of industrial fishing, and the resulting invasion of the refuges previously provided by distance and depth, our interactions with fisheries resources have come to resemble the wars of extermination that newly arrived hunters conducted 40 000–50 000 years ago in Australia, and 12 000–13 000 years ago against large terrestrial mammals in North America. These broad trends are documented here through a map of change in fish sizes, which displays characteristic declines, first in the nearshore waters of industrialized countries of the Northern Hemisphere, then spread offshore and to the Southern Hemisphere. This geographical extension met its natural limit in the late 1980s, when the catches from newly accessed stocks ceased to compensate for the collapse in areas accessed earlier, hence leading to a gradual decline of global landing. These trends affect developing countries more than the developed world, which have been able to meet the shortfall by increasing imports from developing countries. These trends, however, together with the rapid growth of farming of carnivorous fishes, which consumes other fishes suited for human consumption, have led to serious food security issues. This promotes urgency to the implementation of the remedies traditionally proposed to alleviate overfishing (reduction of overcapacity, enforcement of conservative total allowable catches, etc.), and to the implementation of non-conventional approaches, notably the re-establishment of the refuges (also known as marine reserves), which made possible the apparent sustainability of pre-industrial fisheries.
Keywords: overfishing, overcapacity, trophic levels, food web
1. Introduction
Many lay people believe that widespread ‘pollution’ endangers ocean life, perhaps a lingering impact of books such as ‘The sea around us’ (Carson 1951), and the pronouncements of Jacques Cousteau. Fisheries, by contrast, have long been seen as benign, and their growth not related to the decline of their target species, which is usually attributed to ‘environmental change’ or some form of ‘pollution’.
Why is it that commercial fishing, which, after all, is devoted to killing fishes and removing them from their habitat so we can eat them, has so generally been perceived as having little, if any, impact on the populations that were being fished? We suspect that this has to do with notions from another age, when fishing was indeed a matter of wrestling one’s sustenance from a foreign, hostile sea, and from tiny boats, close to one’s village, using equipment barely capable of making a dent in the huge populations of fish known to inhabit the ocean’s unfathomable depths (Pauly & Maclean 2003). This perception is still present, and it is time to realize how ill conceived it is.
One of the effects of the perception of fisheries as local folklore, featuring self-reliant fishers as stewards of local resources, is that we fail to even see the giant enterprise now feeding the tightly integrated, global market that supplies the fish that we order in restaurants or purchase in supermarkets. The problem with this is that the giant enterprise in question is having so severe an impact on its own resources base that, if present trends continue, it will collapse in the next decades, and drag down with it, into oblivion, many of the fishes it exploits (Parrish 1995, 1998), together with their supporting ecosystems (Pauly et al. 2003). This is probably one reason why at least one among the major fish distributors in the world, Unilever, partnered a conservation NGO, the WWF, in creating the Marine Stewardship Council, designed to bring market pressure to bear on what is perceived as underperforming management regimes (see contributions in Phillips et al. 2003).
Unsustainable fisheries have been with us for a long time. The earliest fishing implements so far identified are sophisticated bone harpoons, recovered from 90 000-year-old old middens by archaeologists working a site in present-day Congo (formerly Zaire). The main species that was targeted is a now-extinct, 2 m long, freshwater catfish; most probably the fishers in question moved on to other species (Yellen et al. 1995). This pattern of fisheries exterminating the population upon which they originally relied, and then moving on to other species, has continued ever since (Cushing 1988; Ludwig et al. 1993; Jackson et al. 2001), with periods of ‘sustainability’ occurring as a result of certain species being exploited in only part of their range, owing to equipment or vessel limitations, or subsidies not yet secured (Pauly et al. 2002).
2. Wars of extermination
This dynamic obviously mimics the successive wars of extermination humans conducted on land, against large mammals and other animals. The best studied of these was conducted 12 000–13 000 years ago by ‘Clovis’ hunters in North America, so named after the site where their fluted arrow points were first found. Contrary to earlier conventional wisdom, the Clovis people were not the ‘first Americans’, these probably having been coastal people, who may have relied on fishing for their subsistence (Erlandson 2002). The Clovis people, by contrast, were apparently the first to tackle the large mammals of the interior. Archaeology and model studies confirm that their decimation of 30 species of large and slow-reproducing mammals (mastodon, giant ground sloth, giant armadillo, western camels, etc.) proceeded in the form of a giant wave sweeping across North America over a period of 800 to 1600 years (Alroy 2001). Given the difficulty preliterate societies can be expected to have in conveying quantitative information on animal abundance across generations (Pauly 1995), this timespan was sufficient for the Clovis hunters living past the crest of this wave to fail to realize what their ancestors had done and lost (Alroy 2001), a problem still occurring now, in the form of ‘shifting baselines’ (Pauly 1995).
Ironically, there are those who, despite the evidence provided by numerous Clovis points embedded in the bones of fossil mammals, still argue that it is ‘climate change’ that drove these 30 species to extinction. Here, environmental changes are supposed to have eliminated, in a few centuries, species that had endured millions of years of, yes, environmental change, including glaciations that covered the northern part of North America under 2 km of ice.
There is good evidence of a similar mammalian hecatomb 40 000–50 000 years ago in Australia, in this case associated with the very first arrival of Homo sapiens, who exterminated, over a short period, the larger representatives of the marsupial fauna that had evolved over millions of years on that continent (Roberts et al. 2001).
Our last example is the extermination of the large, ostrich-like moa in what is now New Zealand, by the ancestors of the present-day Maori, who arrived from Polynesia in the late thirteenth century, and who took only ca. 100 years to exterminate 11 species that had lived in the area for millions of years (Holdaway & Jacomb 2000).
In the marine realm, the serial depletions of large coastal animals, documented, for example, in Jackson et al. (2001), accelerated with the development, during the Industrial Revolution, of vessels of unprecedented fishing power, such as stream trawlers. Added to the substantial, pre-existing fishing effort of the rowed and sailed craft that tended to operate inshore, these industrial vessels, targeting stocks of larger fishes further offshore, quickly reduced populations that had previously been perceived as immune to the effects of fishing (Cushing 1988; Myers et al. 1997). Denial is, however, still rampant, sometimes taking absurd forms, as illustrated here by a representative of the French fishing industry recently asserting, for the demersal resources around France, that ‘the stocks are not declining, they are changing location’ (translation of the title of Bigot 2002).
3. The beginnings of change
The emergence of the United Nations’ Convention on Law of the Sea (UNCLOS), in the late 1970s, which enabled countries to claim EEZs reaching 200 miles into the open sea, including essentially all coastal shelves, put the responsibility for fisheries resource management squarely with maritime countries, thus ending many decades, even centuries in some cases, of fighting over traditional fishing grounds (Johnstone 1977). Unfortunately, the opportunity that this offered was lost by most countries. The international race for fish that had characterized earlier fisheries development continued unabated. Indeed, governments or supranational entities—the United Nations Development Programme, the EU— or international development banks (see Mannan 1997) subsidized the growth of national fisheries to replace the just-displaced DWF of foreign countries. As well, they enabled the DWF to come back through UNCLOS-sanctioned, and often bargain-priced, ‘fishing agreements’, as between the EU and individual West African countries (Kaczynski & Fluharty 2002).
Fisheries scientists contributed to this, notably by publishing estimates of potential yields now known to have been wildly over-optimistic (review in Pauly 1996).
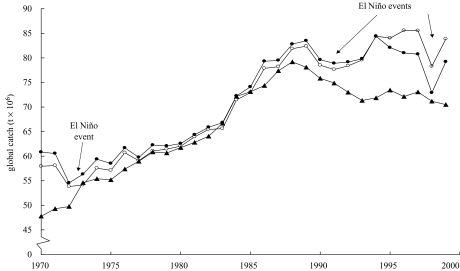
The post-UNCLOS technological and geographical expansion extended the trend of catch increase, if at a slower rate. Global catches began to decline in the late 1980s, a trend reversal due to broad-based collapse of the underlying ecosystems, long masked by systematic over-reporting by China (Watson & Pauly 2001; figure 1), and the targeting of deep water stocks (see figure 3). Several major studies, by Jackson et al. (2001), Christensen et al. (2003), and Myers & Worm (2003), showed that marine fisheries impact their resources base and their supporting ecosystems far more strongly than commonly assumed, thus providing further support for our explanation of observed catch trends.
Figure 1.
Global marine fisheries trends. Overall landings (open circles) are from the FAO, and suggest an increasing trend through the 1990s. Adjustment for over-reporting by China, as proposed by Watson & Pauly (2001), generates a decreasing trend through the 1990s, which becomes more visible, and is seen to have started in the late 1980s (filled triangles and thick black line) when the catch of only one species, the Peruvian anchoveta, which is strongly impacted by El Niño events, is not considered (filled circles and thin black line).
However, most government fisheries laboratories still work mainly along traditional lines, i.e. performing assessments for single-species fisheries, in view of estimating their TAC. At the same time, many of their staff attempt to fight off claims by conservationists asserting, with increasing public support, that these fisheries impact on numerous other (‘bycatch’) species, and, in fact, engage in serial destructions of their supporting ecosystems (Rosenberg 2003).
Formulating alternatives to these developments will require freeing of these laboratories, and the regulatory agencies they are part of, from their subservient relationship with the fishing industry, and the re-establishment of their role as guardians of what are, after all, publicly owned resources (Macinko & Bromley 2002; Okey 2003). Indeed, we believe that it is the perception of regulatory agencies as captive of the narrow interests of an extractive industry that is behind the widespread, if not well-articulated, public demands for some sort of EBFM, as expressed, for example, in the WSSD, held in Johannesburg in 2002.
Thus, we suggest that, rather than railing about the imprecision of EBFM, we should treat it as a guiding principle, as is done in Canada with the concept of ‘good government’, which underlies federal public policy; or the right to the ‘pursuit of happiness’, which underlies much jurisdiction in the USA; or ‘liberté, egalité, fraternité’, which inspire much of the public and political discourse in France. Indeed, it is our impression that broad concepts of this sort, despite their vagueness, provide one of the few avenues for framing debates about complex, value-laden issues. Moreover, we venture that a consensus could quickly emerge around the notion that EBFM should maintain, or where necessary re-establish, the structure and function of the ecosystems within which fisheries are embedded (NRC 1999; see also Garcia & de Leiva Moreno 2003). This could involve, among other things, regulating fisheries such that the mix of species caught maintains the relative abundance of the same species in the ecosystems, just as the overall gas mileage of the cars in a country is, or can be regulated, by putting a cap on the aggregate mileage of the cars sold by each manufacturer.
4. Fishing down marine food webs
Two measures that can be easily estimated from fisheries landings have shown themselves to be highly indicative of the status of the underlying ecosystems, and thus could be used for such monitoring: the mean TL of fish landed and the mean ML of the species in the landings.
Eating and not being eaten is, besides reproduction, the main concern of organisms in ecosystems, and the latter can largely be described, therefore, as a meshing of food chains into complex food webs, within which an organism occupies a given position determined by its size, the anatomy of its mouth parts and its feeding preferences. One dimension of this position is the TL, expressing how many steps away an organism is located away from the base of marine food webs, i.e. phytoplanktonic and benthic algae, assigned a definitional TL of 1, the same as for detritus, mainly derived from ungrazed, dead algae and the excreta of herbivores (Odum & Heald 1975).
Phytoplankton is grazed mostly by copepods and other small crustaceans, with a TL of 2, in stark contrast to terrestrial food chains, where the herbivores are often very large. The zooplankton, in turn is consumed mainly by small pelagic fishes (herring, sardine, anchovies), with a TL of approximately 3, the imprecision stemming from the fact that they often consume a variable mix of phytoplankton, herbivorous and carnivorous zooplankton, and detritus. Small pelagics are caught in enormous quantities (38 million tonnes in 2000, i.e. 44% of global marine landings), are either consumed by people (e.g. as canned ‘oil sardine’) or ‘reduced’ to fishmeal and oil, a key component of the chicken and pig feeds, and of farmed salmon (Naylor et al. 2000). The typical table fish, however (cod, snapper, tuna, halibut, etc.), that restaurants serve whole, or as a steak or fillet, are predators on the small pelagics and other smaller fishes and invertebrates, and tend to have a TL of ca. 4, with 4.5 an upper limit reached by large sharks (Cortés 1999), bluefin tuna and other large predators such as some marine mammals (Pauly et al. 1998c). TLs are variable in space and time, the latter variability referring both to seasons and to the age (size) of fishes. We shall ignore the issue of TL variability here, addressed in some detail in Pauly et al. (2001).
Important, also, is that in the sea, the high TL organisms tend to be larger (typically three to four times in terms of body length) than their prey (Ursin 1973), and need more time to reach maturity and reproduce (Denney et al. 2002), which renders them very susceptible to overfishing (e.g. Sadovy & Cheung 2003).
In summary, we can conclude that, given the current technical ability to catch whatever marine species are abundant within an ecosystem, and the fact that large fishes are usually more valuable than smaller fishes, increased landings of fishes with lower TL imply a reduction of the abundance of the higher TL species. Or put differently, non-sustainable fishing should manifest itself, at the ecosystem level, in a gradual shift of mean TL towards lower values, even if the individual species for which TACs exist appear to be fished sustainably (Valtysson & Pauly 2003).
This process, now known as ‘fishing down marine food webs’ (FD) was originally presented in 1998 based on the global database of landings created and maintained by the FAO, itself relying on data supplied by its member countries, some of them with only rudimentary fisheries monitoring systems (Pauly et al. 1998a). In particular, these data tend to be over-aggregated in terms of species landed (i.e. many species are combined as ‘mixed fishes’), and areas covered (e.g. fishes caught by a distant-fishing nation in the EEZs of different countries are combined without indication as to their origins). Following a critique by Caddy et al. (1998) suggesting that these defects of the FAO database may invalidate the conclusion in Pauly et al. (1998a), several replications of their findings based on disaggregated datasets were published (table 1), establishing the validity of the FD concept and its ubiquity.
Table 1.
Some contributions demonstrating the occurrence of FD using locally disaggregated datasets, following the original presentation of this phenomenon by Pauly et al. (1998a), based on the global FAO catch dataset.
| country/area | years | decline | source and remarks |
|---|---|---|---|
| Iceland | 1900–1999 | 1918–1999 | Valtysson & Pauly (2003), based on database in Valtysson (2001) |
| Celtic Sea | 1945–1998 | 1946–2000 | Pinnegar et al. (2002), with TLs based on stable isotopes of nitrogen |
| Gulf of Thailand | 1963–1982;1963–1997 | 1965–1982;1965–1997 | Christensen (1998); Pauly & Chuenpagdee (2003) |
| eastern Canada | 1950–1997 | 1957–1997 | Pauly et al. (2001), based on data submitted to the FAO by Canada’s Department of Fisheries & Oceans |
| western Canada | 1873–1996 | 1910–1996 | Pauly et al. (2001), based on comprehensive dataset assembled by Wallace (1999) |
| Greek waters | 1964–1997 | from mid-1980s | Stergiou & Koulouris (2000) |
| Cuban EEZ | 1960–1995 | 1960–1995 | Pauly et al. (1998b); Baisre (2000) |
| Chinese EEZ | 1950–1998 | 1970–1998 | Pang & Pauly (2001) |
| west Central Atlantic | 1950–2000;1950–2000 | 1950–2000;1950–2000 | Pauly & Palomares (2005), based on FAO data (Area 41), disaggregated into USA (north) and other countries (south) |
| world, tuna and billfishes | 1950–2000 | 1950–2000 | Pauly & Palomares (2004), based on FAO data (ISSCAAP group 36 only) |
| world, all fishes | 1950–2000 | 1950–2000 | Pauly & Watson (2005) |
In the process, a rule-based mapping technique was developed (Watson et al. 2004), which allowed assignment for the years 1950–2000 of the FAO fisheries catches to the more than 180 000 half latitude/longtitude degree cells comprising the world ocean, together with the key attributes of these landings (i.e. their species composition, and hence their mean TL and their ML).
This allowed mapping of the FD phenomenon, and simultaneously, elimination of the bias that was caused by fisheries statistics from small islands and some other states, which combine landings of inshore reef fishes with those of adjacent large oceanic, high-TL pelagics such as tunas (Pauly & Palomares 2005).
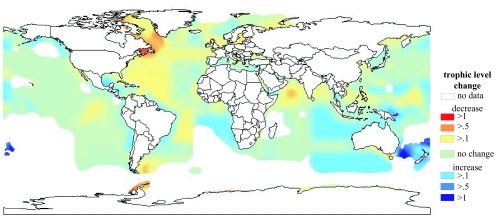
The resulting maps (in Pauly & Watson 2005) (figure 2) show how widespread the FD phenomenon is. Indeed, it can be said to occur everywhere it matters, as the shelf areas where TL have strongly declined contribute a large fraction of the world fisheries catches. Indeed, the rate of TL decline has mostly increased since the 1950s, with the strongest rate of decline in the 1980s. Global fisheries were operating, on average, at a TL of 3.37 in the early 1950s; now their mean TL is ca. 3.29, but this was as low as 3.25 in 1983. Remember: so far, humans do not eat zooplankton (although exceptions exist: there is a market for jellyfish in East Asia, to which some western countries have now begun to export this product).
Figure 2.
Differences between the mean ML of fish and invertebrate species in fisheries landing in the 1950s, and that in the 1990s, mapped into 180 000 cells of 1/2 latitude/longitude degrees according to the procedure in Watson et al. (2004). Note areas of strong decline (greater than 1 m) around the countries bordering the North Atlantic and other industrialized countries. The distribution of the size reductions shown here largely matches those of the TL, as may be expected given the high correlation between TL and body size (Pauly & Watson 2005).
This analysis is confirmed by figure 2, a map of the mean ML reached by the species explicitly mentioned in global landing statistics. As can be seen, declines of up to 1 m over the 50 year period considered here occurred, mainly in the North Atlantic, but also in other areas where highly industrialized fisheries have removed most of the fishes capable of reaching large sizes. Figure 2 shows that this process, viewed globally, is proceeding in a rather smooth fashion, notwithstanding the mesh size and other single-species regulations meant to prevent the size of certain target species from falling below some critical levels (note that this does not consider the well-documented reduction in the average size within species).
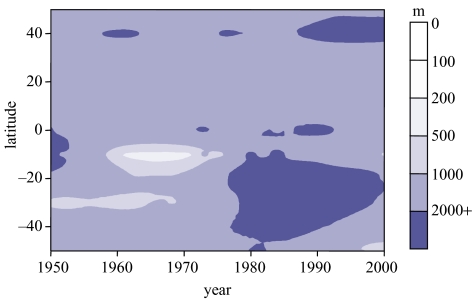
In 1950, when the FAO began to assemble the global fisheries dataset analysed here, coastal fisheries had already impacted on inshore populations of fishes and invertebrates of both industrialized and non-industrialized countries (Cushing 1988; Butcher 1996). However, the serial depletion induced by the first industrial fisheries in areas such as the North Sea or New England, expanded, after World War II, to deeper waters, especially in the Southern Hemisphere. Figure 3 which shows, by latitude, the mean depth of marine fisheries catches from 1950 to the present, illustrates these trends, and thus explains how the overfishing of local fish populations has been largely masked by landings from new fishing grounds.
Figure 3.
Mean depth of global fisheries landings, by latitude, from 1950 to 2000, based on catch data originally mapped into 180 000 cells of 1/2 latitude/longitude degree according to the procedure in Watson et al. (2004). Note the trend toward greater depths, particularly in the Southern Hemisphere.
5. Impacts on food security
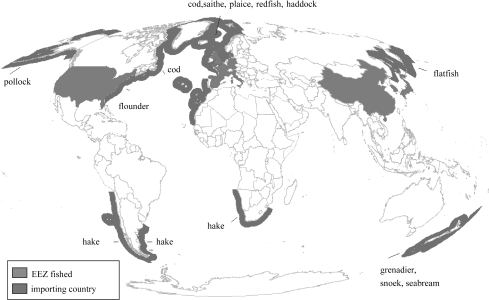
The masking effect of geographical expansion would not have worked, however, were it not for a tightly integrated global market resulting from the relaxation of investment regulations, the opening of international banking and advances in telecommunications, capable of compensating through imports from the Southern Hemisphere, for the shortfall in meeting the increasing demand for fish in the Northern Hemisphere owing to increased recognition of the benefits of eating seafood and increasing affluence. In many developing countries, the need to generate hard currency to repay their debts is most easily accomplished either by selling fishing access rights to countries willing to pay relatively high prices or by exporting high-value fishes. This has resulted in many coastal areas being overfished by DWFs, leaving few fishes for small-scale and artisanal fishers to generate income and subsistence, as seen in many African countries (Atta-Mills et al. 2004). Indeed, nine of the top 40 fish-exporting countries globally are considered to have a low-income food deficit. This is illustrated for demersal table fish in figure 4, but also applies to other high-end seafood (e.g. shrimps to Japan, the USA or the EU) and to small pelagics used for fishmeal and fed to farmed animals, both terrestrial and aquatic (e.g. salmon).
Figure 4.
Major fishing grounds for demersal (table) fishes, represented here through the EEZ (blue) of key exporting countries, and the countries (red) where the bulk of these fishes are consumed. As might be seen, most of these fishes originate and are traded within the Northern Hemisphere. However, there is an increased tendency for the shortfall to originate from the Southern Hemisphere (see figure 3). Similar maps (with the USA, the EU, Japan and China as major importers and the Southern Hemisphere as supplier) emerge when pelagic fishes, or invertebrates (shrimps, squids, etc.), are considered.
The masking effect (to consumers in developed countries) of serial depletion coupled with a global market for seafood is further enhanced by fish farming, which many believe will ‘relieve the pressure on overfished stocks’. In fact, it can do so only if the fishes and other organisms that are farmed do not require fishmeal or fish oils for their production (as is the case for clams and mussels, for the herbivorous tilapia farmed in much of tropical Asia, or for catfish in the USA). When they do, as in the case of salmon or other carnivorous fishes, farming adds to the pressure, as it transforms small pelagics and other fishes perfectly fit for human consumption into animal feeds whose nutritive value is largely lost to humans when they must pass through the gut of a carnivore (Naylor et al. 2000).
6. What needs to be done
Given the FD and related trends discussed here, it appears rather urgent to now implement the reforms long proposed by most fisheries scientists and economists: to radically reduce fishing capacity (Mace 1997), notably, by abolishing the government subsidies that keep otherwise unprofitable fleets afloat (Munro & Sumaila 2002), and to strictly enforce various equipment restrictions (Chuenpagdee et al. 2003).
Such measures may not allow us to increase future landings, i.e. to continue to meet an ever-increasing human demand (Pauly et al. 2003). Rather, these measures may allow us to sustain what we have, and which we are in the process of losing (Dulvy et al. 2003; Pauly et al. 2003), thus intensifying the food security issues that reduced per capita fish supply in developing countries has begun to create (see figure 5 in Garcia & de Leiva Moreno 2003).
However, we believe that these traditional measures, even if they succeed in stabilizing bulk fish supply, will not be sufficient to prevent the loss of large and hence vulnerable fish species. Given the ‘shifting baseline syndrome’ mentioned above, re-establishing functional ecosystems and sustainable fisheries will require us to identify firmly anchored baselines representing earlier states of the population and ecosystems in question, and to rebuild our stocks accordingly. This makes the reconstruction and description (or simulation) of earlier states of ecosystems important, and new areas of research (Pitcher 2001), which will have to be multidisciplinary if they are to be successful.
As another important change, we will have to re-establish, as also demanded by the WSSD, the refugia earlier fish populations enjoyed, and which made it possible for some of our fisheries to last for centuries, although they were unregulated. Some of these refugia, now called ‘marine reserves’ or no-take zones, should be inshore, to protect coastal species; some will have to be large and offshore to protect oceanic fishes (Russ & Zeller 2003; Balmforth et al. 2004). The alternative is that we lose many of the species upon which our fisheries have so far depended.
No-take marine reserves will have to be perceived not as scattered and small concessions to conservationist pressure, but as a legitimate and obvious management tool, required for preventing the entire distribution area of various exploited species from being accessible to fishing. Indeed, avoiding the extinction of species previously protected by their inaccessibility to fishing gear should become a major goal for future management regimes. This would not only enable fisheries, for the first time in their history, to become truly sustainable, but also to address the issue of uncertainty, as eloquently stated in a posthumous edition of some of Rachel Carson’s re-discovered writings:
… To convert some of the remaining wild areas into State and National parks, however, is only part of the answer. Even public parks are not what nature created over the eons of time, working with wind and wave and sand. Somewhere we should know what was nature’s way; we should know that the earth would have been had not man interfered. And so, besides public parks for recreation, we should set aside some wilderness area of seashore where the relations of sea and wind and shore—of living things and their physical world—remain as they have been over the long vistas of time in which man did not exist. For there remains, in this space-age universe, the possibility that man’s way is not always best. (Carson 1998, p. 124)
Acknowledgments
The authors thank Adrian Kitchingman for his work on the maps presented here as figures 2–3, and their colleagues in the Sea Around Us Project for discussions. Support from the Pew Charitable Trusts is gratefully acknowledged; D. P. also acknowledges support from Canada’s Natural Scientific and Engineering Research Council.
Glossary
- DWF
distant water fleet
- EBFM
ecosystem-based fisheries management
- EEZ
exclusive economic zone
- EU
European Union
- FAO
Food and Agricultural Organization
- FD
fishing down
- ML
maximum length
- NGO
non-governmental organization
- TAC
total allowable catch
- TL
trophic level
- WSSD
World Summit on Sustainable Development
- WWF
World Wide Fund for Nature
References
- Alroy J. A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction. Science. 2001;292:1893–1896. doi: 10.1126/science.1059342. [DOI] [PubMed] [Google Scholar]
- Atta-Mills J., Alder J., Sumaila U.R. The decline of a regional fishing nation: the case of Ghana in West Africa. Natural Resources Forum. 2004;28:13–21. [Google Scholar]
- Baisre, J. 2000 Chronicles of Cuban marine fisheries (1935–1995). Trend analysis and fisheries potential. FAO Fisheries Technical Paper no. 394. Rome: FAO.
- Balmforth A., Gravestock P., Hockley N., McClean C., Roberts C.M. The worldwide cost of marine protected areas. Proc. Natl Acad. Sci. USA. 2004;101:9694–9697. doi: 10.1073/pnas.0403239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot, J. 2002 Les stocks ne diminuent pas, ils se déplacent. L’Usine Nouvelle 12 (Dec).
- Butcher J. The marine fisheries of the Western Archipelago: toward an economic history, 1850 to the 1960s. In: Pauly D., Martosubroto P., editors. Baseline studies in biodiversity: the fish resources of western Indonesia. ICLARM; Manila, Philippines: 1996. pp. 24–39. ICLARM Studies and Reviews 23. [Google Scholar]
- Caddy J., Csirke J., Garcia S., Grainger R. How pervasive is ‘fishing down marine food webs’. Science. 1998;282:183. [Google Scholar]
- Carson R. The sea around us. Oxford University Press; New York: 1951. [Google Scholar]
- Carson R. Lost woods: the discovered writings of Rachel Carson. Beacon Press; Boston, MA: 1998. [Google Scholar]
- Christensen V. Fishery-induced changes in the marine ecosystem: insights from models of the Gulf of Thailand. J. Fish Biol. A. 1998;53:128–142. [Google Scholar]
- Christensen V., Guénette S., Heymans J.J., Walters C.J., Watson R., Zeller D., Pauly D. Hundred year decline of North Atlantic predatory fishes. Fish Fish. 2003;4:1–124. [Google Scholar]
- Chuenpagdee R., Morgan L.E., Maxwell S.M., Norse E.A., Pauly D. Shifting gears: assessing collateral impacts of fishing methods in the U.S. waters. Frontiers Ecol. Environ. 2003;10:517–524. [Google Scholar]
- Cortés E. Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 1999;56:707–717. [Google Scholar]
- Cushing D.H. The provident sea. Cambridge University Press; 1988. [Google Scholar]
- Denney N.H., Jennings S., Reynolds J.D. Life-history correlates of maximum population growth rates in marine fishes. Proc. R. Soc. B. 2002;269:2229–2237. doi: 10.1098/rspb.2002.2138. doi:10.1098/rspb.2002.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulvy N.K., Sadovy Y., Reynold J.D. Extinction vulnerability in marine populations. Fish and Fisheries. 2003;4:25–64. [Google Scholar]
- Erlandson J. Anatomically modern humans, maritime voyaging, and the Pleistocene colonization of the Americas. Mem. Calif. Acad. Sci. 2002;27:59–92. [Google Scholar]
- Garcia S., de Leiva Moreno I. Global overview of mature fisheries. In: Sinclair M., Valdimarsson G., editors. Responsible fisheries in the marine ecosystem. FAO; Rome: 2003. pp. 1–24. [Google Scholar]
- Holdaway R.N., Jacomb C. Rapid extinction of the moas (Aves: Dinornithiformes): model, test, and implications. Science. 2000;287:2250–2254. doi: 10.1126/science.287.5461.2250. [DOI] [PubMed] [Google Scholar]
- Jackson J.B.C. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. (and 16 others) [DOI] [PubMed] [Google Scholar]
- Johnstone K. The aquatic explorers: a history of the Fisheries Research Board of Canada. University of Toronto Press; 1977. [Google Scholar]
- Kaczynski V.M., Fluharty D.L. European policies in West Africa: who benefits from fisheries agreements? Mar. Pol. 2002;26:75–93. [Google Scholar]
- Ludwig D., Hilborn R., Walters C. Uncertainty, resource exploitation and conservation: lessons from history. Science. 1993;260:17–36. doi: 10.1126/science.260.5104.17. [DOI] [PubMed] [Google Scholar]
- Mace P.M. Developing and sustaining world fisheries resources: the state of fisheries and management. In: Hancock D.H., Smith D.C., Grant A., Beumer J.P., editors. Proc 2nd World Fisheries Congr. CSIRO Publishing; Collingwood, Australia: 1997. pp. 1–20. [Google Scholar]
- Macinko S., Bromley D.W. Who owns America’s fisheries? Island Press; Washington, DC: 2002. [Google Scholar]
- Mannan M.A. Foreword to: status and management of tropical coastal fisheries in Asia. In: Silvestre G., Pauly D., editors. ICLARM Conf. Proc. 53, Manila, Philippines. ICLARM; Manila, Philippines: 1997. [Google Scholar]
- Munro G.R., Sumaila U.R. Subsidies and their potential impact on the management of the ecosystems of the North Atlantic. In: Pitcher T., Sumaila U.R., Pauly D., editors. Fisheries impacts on North Atlantic ecosystems: evaluations and policy explorations. 2002. pp. 10–27. University of British Columbia Fisheries Centre Research Report 9(5). See http://www.fisheries.ubc.ca. [Google Scholar]
- Myers R.A., Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- Myers R.A., Hutchings J.H., Barrowman N.J. Why do fish stock collapse? The example of cod in Atlantic Canada. Ecol. Applic. 1997;7:91–106. [Google Scholar]
- Naylor R.L., Goldberg J., Primavera J.H., Kautsky N., Beveridge M.C.M., Clay J., Folke C., Lubchenco J., Mooney H., Troell M. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- NRC. Sustainable marine fisheries. National Academy Press; Washington, DC: 1999. US National Research Council. [Google Scholar]
- Odum W.E., Heald E.J. The detritus-based food web of an estuarine mangrove community. In: Cronin L.E., editor. Estuarine research. vol. 1. Academic Press; New York: 1975. pp. 265–286. [Google Scholar]
- Okey T. Membership of the eight Regional Fishery Management Councils in the United States: are special interests over-represented? Mar. Pol. 2003;27:193–206. [Google Scholar]
- Pang L., Pauly D. The marine fisheries of China: development and reported catches. Fisheries Centre; Vancouver, Canada: 2001. Part 1 Chinese marine capture fisheries from 1950 to the late 1990s: the hopes, the plans and the data; pp. 1–27.pp. 1–27. Fisheries Centre Research Report 9(2). See www.fisheries.ubc.ca. [Google Scholar]
- Parrish R.H. Lanternfish heaven: the future of world fisheries? Naga. 1995;18:7–9. [Google Scholar]
- Parrish R.H. Life history strategies for marine fishes in the late Holocene. In: Durand M.H., Cury P., Mendelssohn R., Bakun A., Roy C., Pauly D., editors. Global versus global change in upwelling areas. ORSTOM; Séries Colloques et Séminaires. Paris: 1998. pp. 524–535. [Google Scholar]
- Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- Pauly D. One hundred million tonnes of fish, and fisheries research. Fish. Res. 1996;25:25–38. [Google Scholar]
- Pauly D., Chuenpagdee R. Development of fisheries in the Gulf of Thailand large marine ecosystem: analysis of an unplanned experiment. In: Hempel G., Sherman K., editors. Large marine ecosystems of the world: change and sustainability. Elsevier Science; Amsterdam: 2003. pp. 337–354. [Google Scholar]
- Pauly D., Maclean J. In a perfect ocean: fisheries and ecosystem in the North Atlantic Ocean. Island Press; Washington, DC: 2003. [Google Scholar]
- Pauly, D. & Palomares, M. L. 2005 Fishing down marine food web: it is far more pervasive than we thought. Bull. Mar. Sci (In the press.)
- Pauly, D. & Watson, R. 2005 Background and interpretation of the ‘Marine Trophic Index’ as a measure of biodiversity. Phil. Trans. R. Soc. B360 (In the press.) (doi:10.1098/rstb.2004.1597). [DOI] [PMC free article] [PubMed]
- Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F.C., Jr Fishing down marine food webs. Science. 1998a;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Pauly, D., Froese, R. & Christensen, V. 1998b How pervasive is ‘fishing down marine food webs’: response to Caddy et al Science282, 183. Full text on p. 1383a on www.sciencemag.org/cgi/content/full/282/5393/1383 [DOI] [PubMed]
- Pauly D., Trites A.W., Capuli E., Christensen V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998c;55:467–481. [Google Scholar]
- Pauly D., Palomares M.L., Froese R., Sa-a P., Vakily M., Preikshot D., Wallace S. Fishing down Canadian aquatic food webs. Can. J. Fish. Aquat. Sci. 2001;58:51–62. [Google Scholar]
- Pauly D., Christensen V., Guénette S., Pitcher T., Sumaila U.R., Walters C., Watson R., Zeller D. Toward sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Pauly D., Alder J., Bennett E., Christensen V., Tyedmers P., Watson R. World The future for fisheries. Science. 2003;302:1359–1361. doi: 10.1126/science.1088667. [DOI] [PubMed] [Google Scholar]
- Phillips B., Ward T., Chaffee C., editors. Eco-labelling in fisheries. What is it all about? Blackwell Science; Oxford: 2003. [Google Scholar]
- Pinnegar J.K., Jennings S., O’Brien C.M., Polunin N.V.C. Long-term changes in the trophic level of the Celtic Sea fish community and fish market price distribution. J. Appl. Ecol. 2002;39:377–390. [Google Scholar]
- Pitcher T. Fisheries managed to rebuild ecosystems? Reconstructing the past to salvage the future. Ecol. Applic. 2001;11:601–617. [Google Scholar]
- Roberts R.G. New ages for the last Australian megafauna: continental-wide extinction about 46 000 years ago. Science. 2001;292:1888–1892. doi: 10.1126/science.1060264. (and 10 others) [DOI] [PubMed] [Google Scholar]
- Rosenberg A. Managing to the margins: the overexploitation of marine fisheries. Frontiers Ecol. Environ. 2003;1:102–106. [Google Scholar]
- Russ G.R., Zeller D. From mare liberum to mare reser varum. Mar. Pol. 2003;27:75–78. [Google Scholar]
- Sadovy Y., Cheung W.L. Near extinction of a highly fecund fish: trouble among the croakers. Fish Fish. 2003;4:86–99. [Google Scholar]
- Stergiou K.I., Koulouris M. Fishing down the marine food webs in the Hellenic seas. In: Durand F., editor. Proc. CIESM Workshop, Kerkyra, Greece, 26–30 July 2000. 2000. pp. 73–78. CIESM Workshop Series No 12. Monaco: International Commission for the Scientific Exploration of the Mediterranean Sea. [Google Scholar]
- Ursin E. On the prey preference of cod and dab. Medd. Danm. Havunders. N.S. 1973;7:85–98. [Google Scholar]
- The sea around Icelanders: catch history and discards in Icelandic waters. In: Valtysson H., editor; Zeller D., Watson R., Pauly D., editors. Fisheries impacts on North Atlantic ecosystems: catch, effort and national/regional data sets. Fisheries Centre; Vancouver, Canada: 2001. pp. 52–87. Fisheries Centre Research Reports 9(3). See www.fisheries.ubc.ca. [Google Scholar]
- Valtysson H., Pauly D. Fishing down the food web: an Icelandic case study. In: Guðmundsson E., Valtysson H., editors. Competitiveness within the global fisheries. University of Akureyri; Akureyri: 2003. pp. 12–24. [Google Scholar]
- Wallace, S. 1999 Fisheries impacts on marine ecosystems and biological diversity: the role of marine protected areas in British Columbia. PhD thesis, University of British Columbia, Vancouver.
- Watson R., Pauly D. Systematic distortion in world fisheries catch trends. Nature. 2001;424:534–536. doi: 10.1038/35107050. [DOI] [PubMed] [Google Scholar]
- Watson R., Kitchingman A., Gelchu A., Pauly D. Mapping global fisheries: sharpening our focus. Fish Fish. 2004;5:168–177. [Google Scholar]
- Yellen J.E., Brooks A.S., Cornelissen E., Mehlman M.J., Stewart K. A middle stone-age worked bone industry from Katanda, Upper Semliki Valley, Zaire. Science. 1995;268:553–556. doi: 10.1126/science.7725100. [DOI] [PubMed] [Google Scholar]