Abstract
This study assessed changes in depressive symptoms over time in 57 patients with first-episode psychosis, and investigated the relationships of these symptoms during the acute psychotic episode and the post-psychotic period with treatment outcome. Assessment instruments included the Calgary Depression Scale (CDS) and the Positive and Negative Syndrome Scale (PANSS). For the evaluation of treatment outcome, recently proposed operational remission criteria were used. PANSS factor analysis identified a depression/anxiety factor (PANSSD/ A) at baseline, which separated into "pure" depression (PANSS-D) and anxiety (PANSS-A) factors at 24 months. There were strong correlations between the CDS and the PANSS-D/A, PANSS-D and PANSS-A scores at baseline, but at 24 months significance was lost between CDS and PANSS-A. Compared to non-remitters, patients who achieved remission had significantly higher baseline CDS scores, but depressive symptoms resolved with antipsychotic treatment. Non-remitting patients had relatively low baseline CDS scores, but their depressive symptoms persisted throughout the study. These findings suggest that depressive symptoms in the acute psychotic episode differ from those in the post-psychotic period in terms of their phenomenology, temporal relationship to psychosis, and treatment response.
Keywords: Schizophrenia, depression, remission, outcome, factor analysis
While schizophrenia and depression have historically been regarded as separate disorders, it is now well recognized that depressive symptoms are common in schizophrenia (1). Prevalence estimates differ considerably, probably as a result of differences in samples studied (e.g., acute versus chronic) and assessment instruments used. Reported prevalence rates vary between 7% and 70% (2,3). Depressive symptoms pose a challenge for the clinician, as they may mimic negative symptoms (4) and antipsychotic-induced akinesia (5) or akathisia (6), and are therefore frequently difficult to recognize. Furthermore, depressive symptoms in schizophrenia may have several different origins, requiring specific treatment interventions. For example, they may represent a psychological response to the illness and its accompanying adverse life events (7). Alternatively, they may be due to substance abuse (4), or reflect a comorbid major depressive disorder or anxiety disorder, or be induced by antipsychotic treatment (8). Finally, it has been suggested that depressive symptoms may represent a core feature of the schizophrenic illness itself (9,10).
It has been noted that most depressive symptoms occur concurrently with the acute psychotic symptoms, and resolve once antipsychotic treatment is implemented and the psychosis remits (10). However, there are patients with schizophrenia who experience persistent depressive symptoms that are not responsive to antipsychotic treatment alone. A depressive syndrome was found in 12.9% of patients with chronic schizophrenia, and these symptoms persisted beyond 3 months in 60% of the subjects (3). Another study found that 30 to 40% of schizophrenia patients evidenced full depressive syndromes during the longitudinal course of their illness (11). These persistent (or emergent) depressive symptoms may be particularly important in the post-psychotic period, as they have been associated with an increased risk of relapse (7), suicidality (12), and impaired social and vocational functioning (11,13,14). Although the relationship of depressive symptoms to treatment outcome is still a subject of some debate, it would appear that their presence in the acute phase of the illness is associated with a favourable outcome (15-17). On the other hand, when present in the chronic course of schizophrenia, depressive symptoms appear to be negative prognostic indicators (13,18).
Studies of depressive symptoms in schizophrenia have used a variety of measures, including the depression factor of the Positive and Negative Syndrome Scale (PANSS) (16), the depression factor of the Brief Psychiatric Rating Scale (BPRS) (19), and the Montgomery-Asberg Depression Rating Scale (4). However, the Calgary Depression Scale (CDS) was the first scale specifically designed and validated for the evaluation of depressive symptoms in patients with schizophrenia (20). The CDS is based on items selected from the Hamilton Depression Rating Scale and the Present State Examination (20). Exploratory factor analysis indicated that the CDS is monodimensional (21). The items of the scale have been shown not to overlap with negative symptoms and extrapyramidal symptoms (22-24), but in one study a correlation was found with positive symptoms (25). A high degree of correlation has been reported between CDS and PANSS-D scores (26). The PANSS-D factor includes a number of anxiety items, and may be more correctly referred to as the PANSS depression/ anxiety factor.
The primary purpose of this study was to assess changes in depressive symptoms over time in patients with firstepisode psychosis, and particularly, to investigate the relationships of these symptoms in the acute psychotic episode and in the post-psychotic period with treatment outcome. One problem in previous studies was the lack of standard outcome measures. To overcome this, we used recently published operationally defined criteria for remission (27).
METHODS
This was a post-hoc analysis of a sample comprising 57 participants in a prospective study of first episode of psychosis admissions to the Stikland-Tygerberg academic hospital complex in Cape Town, South Africa. Full details of the study participants and methodology have been described elsewhere (28). Briefly, inclusion criteria comprised inpatients or outpatients aged 16 to 55 years meeting DSM-IV diagnostic criteria for schizophreniform disorder, schizophrenia or schizoaffective disorder, who had been exposed to less than four weeks of antipsychotic treatment. Exclusion criteria were a DSM-IV axis I diagnosis other than schizophreniform disorder, schizophrenia or schizoaffective disorder, alcohol or drug dependence, depot antipsychotic treatment, a significant general medical condition and mental retardation. The study protocol and patient information and consent procedures were approved by the Institutional Review Board of the University of Stellenbosch. Subjects and/or their guardians provided written informed consent.
Subjects were treated with low doses of haloperidol in an open label design according to a fixed protocol. Briefly, doses were restricted to 1 mg/day for the first four weeks. For non-responders (<20% reduction in PANSS total score), the dose was increased to 2 mg/day for three weeks, followed by weekly increments of 1 mg/day until response was achieved, or intolerable side effects developed, or a maximum dose of 10 mg/day was reached. Any non-responders at this stage were switched to thioridazine, up to a maximum of 600 mg/day. Non-responders after three weeks of thioridazine at maximum dose were switched to treatment with clozapine. Downward titration of haloperidol was permitted at any point if side effects emerged. Lorazepam was permitted for sedation, and orphenadrine and benzhexol were prescribed for the treatment of extrapyramidal symptoms.
Although the participants were assessed with a variety of instruments, those relevant to this study were the Structured Clinical Interview for DSM-IV (SCID) (29), the PANSS (30) and the CDS (20). The subjects were evaluated weekly with the PANSS and the CDS for the first 9 weeks (or until they were stabilized), at 12 weeks, and then at three-monthly intervals over a two-year period, with additional unscheduled assessments when required.
In an attempt to improve the assessment of treatment outcome, we used recently proposed operational criteria for remission (27). These criteria define remission according to a threshold of severity of items representing the three major symptom domains identified in factor analyses (negative, psychosis and disorganized factors) and the five criteria specified in DSM-IV for a diagnosis of schizophrenia. The symptom severity threshold for the PANSS comprises a score of 3 (mild) or less on each of the following eight items: delusions, conceptual disorganization, hallucinatory behaviour, blunted affect, social withdrawal, lack of spontaneity, mannerisms/posturing and unusual thought content. These criteria must have been met for at least six months.
Factor analysis was performed on the PANSS individual items at baseline and at 24 months, by the method of maximum likelihood. A principal component analysis was performed as a guide for choosing the number of factors. The factor solution was rotated using the equamax procedure. When selecting the number of factors we considered the following: those with eigenvalues greater than unity, inspection of the scree plot, and percent variance explained by the solution. The Pearson product moment correlation coefficient (r) was used to investigate relations between CDS and PANSS factors. For assessing changes in CDS scores over time for remitters versus non-remitters, a general linear model repeated measures analysis of variance was conducted. Student's t test was used to compare the remitting and non-remitting subjects in terms of CDS and PANSS factor scores. Analyses were performed on the intent to treat (ITT) population (all subjects who received treatment and provided efficacy data for at least one visit), with the last value carried forward (LVCF). A significance level of 0.05 was used throughout.
RESULTS
The participants' mean age was 28.2 ± 8.6 years, and 29 (50.8%) were female. Three patients failed to complete at least one post-baseline assessment, so that the ITT sample for the analysis comprised 54 participants. Twenty-three (40%) achieved the full remission criteria. Twenty-eight (49%) were followed for the entire 24 months. Reasons for discontinuation were: lost to follow-up (N=23), withdrawn from trial (N=5), and deceased (N=1).
On the basis of the scree plot inspection, we chose a five factor solution for the PANSS individual items at baseline, accounting for 60% of the variance. The third principal component was a depression/anxiety factor, comprising the items anxiety, guilt feelings, tension and depression, which accounted for 11.5% of the variance. The factor analysis for the PANSS individual items at 24 months yielded a different picture. Again limiting the number of factors to five, the depression/anxiety factor separated into "pure" depression (guilt feelings and depression) and anxiety (anxiety and tension) factors, accounting for 7.8% and 8.6% of the variance, respectively. The separation into depression and anxiety factors is consistent with a previous PANSS factor analysis in a large first-episode psychosis sample (31). Although the item somatic concern did not load with any of the components, we decided to include it in our further analyses in the interest of consistency with the majority of previously published studies (31). Thus, we used three PANSS factors: a depression/ anxiety factor (PANSS-D/A), a depression factor (PANSS-D) and an anxiety factor (PANSS-A).
As expected, there were very strong correlations between the CDS and the PANSS-D/A, PANSS-D and PANSS-A at baseline. However, at 24 months, while the correlation between CDS and PANSS-D remained highly significant, significance was lost between CDS and PANSS-A (Table 1).
Table 1.
Correlations between the CDS and the PANSS-D/A, PANSS-D and PANSS-A factors during the acute psychosis phase (baseline) and the post-psychotic period (24 months)
| Calgary Depression Scale (CDS) | ||||
|---|---|---|---|---|
| Baseline | 24 months | |||
| r | p | r | p | |
| PANSS-D/A | .76 | <0.0001 | .53 | <0.0001 |
| PANSS-A | .51 | <0.0001 | .03 | 0.8 |
| PANSS-D | .83 | <0.0001 | .90 | <0.0001 |
PANSS-D/A - Positive and Negative Syndrome Scale depression/anxiety factor; PANSS-A - PANSS anxiety factor; PANSS-D - PANSS depression factor
Relationships between depressive symptoms and the other symptom domains of psychosis were sought by examining correlations between CDS, PANSS-D/A, PANSS-D and PANSS-A factors, and the other PANSS factors at baseline. Results are shown in Table 2. The only significant correlation was between the PANSS-D factor and the PANSS negative factor at baseline.
Table 2.
Correlations of CDS, PANSS-D/A, PANSS-D and PANSS-A factors with other PANSS factors at baseline
| PANSS | PANSS | PANSS | PANSS | |||||
|---|---|---|---|---|---|---|---|---|
| negative | disorganized | positive | excited | |||||
| r | p | r | p | r | p | r | p | |
| PANSS-D/A | .09 | 0.5 | -.09 | 0.5 | .04 | 0.7 | -.04 | 0.8 |
| PANSS-A | .01 | 0.9 | -.13 | 0.3 | .03 | 0.8 | .01 | 0.9 |
| PANSS-D | .40 | 0.002 | .07 | 0.6 | -.03 | 0.8 | -.18 | 0.2 |
| CDS | .21 | 0.1 | .02 | 0.8 | .016 | 0.9 | -.06 | 0.6 |
CDS - Calgary Depression Scale; PANSS - Positive and Negative Syndrome Scale; PANSS-D/A - PANSS depression/anxiety factor; PANSS-A - PANSS anxiety factor; PANSS-D - PANSS depression factor
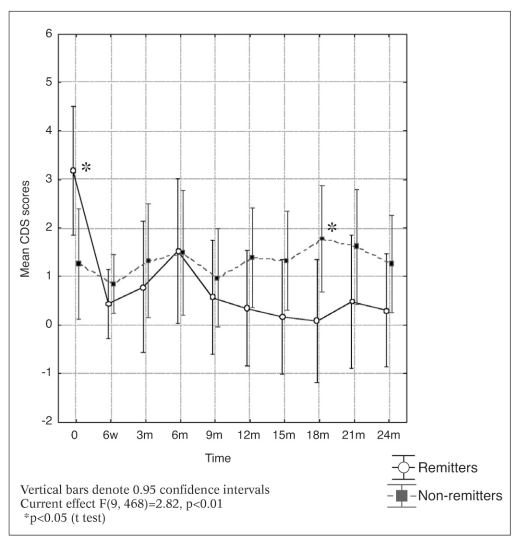
Changes in CDS scores over time for the remitters and non-remitters are depicted in Figure 1. Patients who achieved remission had significantly higher baseline CDS scores, but depressive symptoms resolved with antipsychotic treatment. Non-remitting patients had relatively low baseline CDS scores, but depressive symptoms persisted throughout the study. In the maintenance phase of treatment, non-remitting patients generally had higher levels of depressive symptoms, the difference reaching statistical significance at 18 months, but not at the other time points.
Figure 1.
Changes in CDS scores over time for remitters and nonremitters
Differences between remitters and non-remitters in the CDS and PANSS factor scores during the acute psychotic (baseline) and maintenance (24 month) phases are given in Table 3. In the acute phase, depressive and anxiety symptoms tended to be higher in the remitters, reaching significance for the CDS scores. In the maintenance phase, there was a trend in the opposite direction: higher scores were observed in the non-remitters.
Table 3.
Mean CDS, PANSS-D/A, PANSS-D and PANSS-A scores at baseline and at 24 months in remitters and non-remitters
| Baseline | 24 months | |||||
|---|---|---|---|---|---|---|
| Remitters | Non-remitters | p | Remitters | Non-remitters | p | |
| CDS | 3.2±4.1 | 1.3±2.2 | 0.03 | 0.3±0.6 | 1.3±3.6 | 0.22 |
| PANSS-D/A | 11.9±5.5 | 9.6±4.3 | 0.09 | 6.6±2.3 | 7.5±3.0 | 0.24 |
| PANSS-A | 7.7±3.3 | 6.4±3.3 | 0.15 | 4.2±1.7 | 4.6±2.4 | 0.43 |
| PANSS-D | 5.8±3.0 | 4.8±2.1 | 0.15 | 3.5±1.0 | 4.0±2.4 | 0.3 |
CDS - Calgary Depression Scale; PANSS - Positive and Negative Syndrome Scale; PANSS-D/A - PANSS depression/anxiety factor; PANSS-A - PANSS anxiety factor; PANSS-D - PANSS depression factor
DISCUSSION
This study confirms previous findings that depressive symptoms are more prominent during the acute psychotic phase than in the post-psychotic phase of schizophrenia (10,15,32,33). The majority of these symptoms resolve shortly after initiation of antipsychotic therapy. The levels of depression in the post-psychotic phase are generally low, although such symptoms persist or emerge in some patients.
Our study provides further evidence that depressive symptoms in the acute psychotic phase are positive prognostic indicators (15-17,32). Although less clear cut, depressive symptoms in the post-psychotic phase of the illness appear to be associated with poorer outcome. In fact, 4 of 31 (13%) non-remitters had CDS scores >3 at endpoint, compared to none of 23 remitters. This finding is consistent with some previous studies where persistent depressive symptoms were associated with a poorer outcome (13,14). The lack of clear statistical significance in our study may be due to our relatively small sample size.
It would seem that depressive symptoms experienced during an acute psychotic episode are fundamentally different from those experienced in the post-psychotic period. The former appear to be temporally related to the psychosis itself and improve as psychotic symptoms resolve, in response to antipsychotic treatment. In contrast, persistent depressive symptoms are not responsive to antipsychotic therapy alone and they may require additional treatment interventions. In this regard, second-generation antipsychotics appear to be more effective than conventional antipsychotics (34). Although not effective in treating depressive symptoms in actively psychotic patients (35), antidepressant supplementation may be effective (36,37), as well as a mood stabilizer (38). It could be further argued that some persistent negative symptoms may in fact be masked depressive symptoms. The significant association between the PANSS-D factor and the PANSS negative factor found in our study could be explained on this basis. Further, a recent finding that negative symptoms respond favourably to antidepressant supplementation would support this hypothesis (39).
The superiority of the CDS over the PANSS in detecting depressive syndromes is apparent in our study. However, it may be that the two scales are measuring different things. The PANSS-D/A factor may reflect a mixed depression and anxiety syndrome occurring more commonly in the acute phase of psychosis, while the PANSS-D is more closely associated with the CDS and detects a purer depressive syndrome. Thus, it may be that acute and chronic depressive symptoms differ not only temporally and in terms of response to treatment, but also phenomenologically.
Limitations of our study include the relatively small sample, the inherent limitations of factor analysis (40), and the fact that our study was not designed to examine the origins of the depressive symptoms. To further elucidate the nature of depressive symptoms in schizophrenia, future studies should focus on the relative contributions of factors such as environmental stress, substance abuse and family history of mood disorders. In the meantime, clinicians would do well to be aware of the importance of depressive symptoms in schizophrenia, and treat such symptoms vigorously should they persist.
Acknowledgement
This work was supported in part by the Medical Research Council of South Africa.
References
- 1.Siris SG. Depression in schizophrenia: perspective in the era of "atypical" antipsychotic agents. Am J Psychiatry. 2000;157:1379–1389. doi: 10.1176/appi.ajp.157.9.1379. [DOI] [PubMed] [Google Scholar]
- 2.Siris SG. Addington D. Azorin JM, et al. Depression in schizophrenia: recognition and management in the USA. Schizophr Res. 2001;47:185–97. doi: 10.1016/s0920-9964(00)00135-3. [DOI] [PubMed] [Google Scholar]
- 3.Barnes TR. Curson DA. Liddle PF, et al. The nature and prevalence of depression in chronic schizophrenic in-patients. Br J Psychiatry. 1989;154:486–91. doi: 10.1192/bjp.154.4.486. [DOI] [PubMed] [Google Scholar]
- 4.Tollefson GD. Sanger TM. Lu Y, et al. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. Arch Gen Psychiatry. 1998;55:250–258. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]
- 5.Van Putten T. May RP. "Akinetic depression" in schizophrenia. Arch Gen Psychiatry. 1978;35:1101–1107. doi: 10.1001/archpsyc.1978.01770330075006. [DOI] [PubMed] [Google Scholar]
- 6.van Putten T. May PRA. The many faces of akathisia. Compr Psychiatry. 1975;16:43–47. doi: 10.1016/0010-440x(75)90019-x. [DOI] [PubMed] [Google Scholar]
- 7.Birchwood M. Mason R. MacMillan F, et al. Depression, demoralization and control over psychotic illness: a comparison of depressed and non-depressed patients with a chronic psychosis. Psychol Med. 1993;23:387–395. doi: 10.1017/s0033291700028488. [DOI] [PubMed] [Google Scholar]
- 8.Harrow M. Yonan CA. Sands JR, et al. Depression in schizophrenia: are neuroleptics, akinesia, or anhedonia involved? Schizophr Bull. 1994;20:327–338. doi: 10.1093/schbul/20.2.327. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DA. Depressions in schizophrenia: some observations on prevalence, etiology, and treatment. Acta Psychiatr Scand. 1981;64(Suppl. 291):137–144. doi: 10.1111/j.1600-0447.1981.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 10.Koreen AR. Siris SG. Chakos M, et al. Depression in first-episode schizophrenia. Am J Psychiatry. 1993;150:1643–1648. doi: 10.1176/ajp.150.11.1643. [DOI] [PubMed] [Google Scholar]
- 11.Sands JR. Harrow M. Depression during the longitudinal course of schizophrenia. Schizophr Bull. 1999;25:157–171. doi: 10.1093/oxfordjournals.schbul.a033362. [DOI] [PubMed] [Google Scholar]
- 12.Roy A. Thompson R. Kennedy S. Depression in chronic schizophrenia. Br J Psychiatry. 1983;142:465–470. doi: 10.1192/bjp.142.5.465. [DOI] [PubMed] [Google Scholar]
- 13.McGlashan TH., Jr Carpenter WT., Jr Postpsychotic depression in schizophrenia. Arch Gen Psychiatry. 1976;33:231–239. doi: 10.1001/archpsyc.1976.01770020065011. [DOI] [PubMed] [Google Scholar]
- 14.Mandel MR. Severe JB. Schooler NR, et al. Development and prediction of postpsychotic depression in neuroleptic-treated schizophrenics. Arch Gen Psychiatry. 1982;39:197–203. doi: 10.1001/archpsyc.1982.04290020051010. [DOI] [PubMed] [Google Scholar]
- 15.Kay SR. Lindenmeyer J. Outcome predictors in acute schizophrenia: prospective significance of background and clinical dimensions. J Nerv Ment Dis. 1987;175:152–160. doi: 10.1097/00005053-198703000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Emsley RA. Oosthuizen PP. Joubert AF, et al. Depressive and anxiety symptoms in patients with schizophrenia and schizophreniform disorder. J Clin Psychiatry. 1999;60:747–751. doi: 10.4088/jcp.v60n1105. [DOI] [PubMed] [Google Scholar]
- 17.Oosthuizen P. Emsley RA. Roberts MC, et al. Depressive symptoms at baseline predict fewer negative symptoms at follow-up in patients with first-episode schizophrenia. Schizophr Res. 2002;58:247–252. doi: 10.1016/s0920-9964(01)00375-9. [DOI] [PubMed] [Google Scholar]
- 18.Falloon I. Watt DC. Shepherd M. The social outcome of patients in a trial of long-term continuation therapy in schizophrenia: pimozide vs. fluphenazine. Psychol Med. 1978;8:265–274. doi: 10.1017/s0033291700014318. [DOI] [PubMed] [Google Scholar]
- 19.Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multinational, multicentre, double-blind, parallelgroup study versus haloperidol. Br J Psychiatry. 1995;166:712–726. doi: 10.1192/bjp.166.6.712. [DOI] [PubMed] [Google Scholar]
- 20.Addington D. Addington J. Maticka-Tindale E. Assessing depression in schizophrenia: the Calgary depression scale. Br J Psychiatry. 1993;163:S39–S44. [PubMed] [Google Scholar]
- 21.Addington D. Addington J. Maticka-Tindale E, et al. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- 22.Addington D. Addington J. Maticka-Tindale E. Specificity of the Calgary Depression Scale for schizophrenics. Schizophr Res. 1994;11:239–244. doi: 10.1016/0920-9964(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Addington D. Addington J. Atkinson M. A psychometric comparison of the Calgary Depression Scale for Schizophrenia and the Hamilton Depression Rating Scale. Schizophr Res. 1996;19:205–212. doi: 10.1016/0920-9964(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 24.Collins AA. Remington G. Coulter K, et al. Depression in schizophrenia: a comparison of three measures. Schizophr Res. 1996;20:205–209. doi: 10.1016/0920-9964(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 25.Lancon C. Auquier P. Reine G, et al. Evaluation of depression in schizophrenia: psychometric properties of a French version of the Calgary Depression Scale. Psychiatry Res. 1999;89:123–132. doi: 10.1016/s0165-1781(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 26.El Yazaji M. Battas O. Agoub M, et al. Validity of the depressive dimension extracted from principal component analysis of the PANSS in drug-free patients with schizophrenia. Schizophr Res. 2002;56:121–127. doi: 10.1016/s0920-9964(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC., Jr Carpenter WT., Jr Kane JM, Jr, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 28.Oosthuizen P. Emsley RA. Turner J, et al. Determining the optimal dose of haloperidol in first-episode psychosis. J Psychopharmacol. 2001;15:251–255. doi: 10.1177/026988110101500403. [DOI] [PubMed] [Google Scholar]
- 29.First MB. Spitzer RL. Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1994. [Google Scholar]
- 30.Kay SR. Fizbein A. Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 1987;13:261–267. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Emsley R. Rabinowitz J. Torreman M. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61:47–57. doi: 10.1016/s0920-9964(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 32.Siris SG. Diagnosis of secondary depression in schizophrenia: implications for DSM-IV. Schizophr Bull. 1991;17:75–98. doi: 10.1093/schbul/17.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Addington J. Leriger E. Addington D. Symptom outcome 1 year after admission to an early psychosis program. Can J Psychiatry. 2003;48:204–207. doi: 10.1177/070674370304800309. [DOI] [PubMed] [Google Scholar]
- 34.Emsley RA. Buckley P. Jones AM, et al. Differential effect of quetiapine on depressive symptoms in patients with partially responsive schizophrenia. J Psychopharmacol. 2003;17:210–215. doi: 10.1177/0269881103017002010. [DOI] [PubMed] [Google Scholar]
- 35.Kramer MS. Vogel WH. DiJohnson C, et al. Antidepressants in 'depressed' schizophrenic inpatients. A controlled trial. Arch Gen Psychiatry. 1989;46:922–928. doi: 10.1001/archpsyc.1989.01810100064012. [DOI] [PubMed] [Google Scholar]
- 36.Siris SG. Adan F. Cohen M, et al. Targeted treatment of depressionlike symptoms in schizophrenia. Psychopharmacol Bull. 1987;23:85–89. [PubMed] [Google Scholar]
- 37.Mazeh D. Shahal B. Saraf R, et al. Venlafaxine for the treatment of depressive episode during the course of schizophrenia. J Clin Psychopharmacol. 2004;24:653–655. doi: 10.1097/01.jcp.0000144894.37611.0a. [DOI] [PubMed] [Google Scholar]
- 38.Hogarty GE. McEvoy JP. Ulrich RF, et al. Pharmacotherapy of impaired affect in recovering schizophrenic patients. Arch Gen Psychiatry. 1995;52:29. doi: 10.1001/archpsyc.1995.03950130029004. [DOI] [PubMed] [Google Scholar]
- 39.Jockers-Scherubl MC. Bauer A. Godemann F, et al. Negative symptoms of schizophrenia are improved by the addition of paroxetine to neuroleptics: a double-blind placebo-controlled study. Int Clin Psychopharmacol. 2005;20:27–31. doi: 10.1097/00004850-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Peralta V. Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49:269–285. doi: 10.1016/s0920-9964(00)00071-2. [DOI] [PubMed] [Google Scholar]