Abstract
In vitro inoculation of Vitis vinifera L. cv. Chardonnay explants with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN, increased grapevine growth and physiological activity at a low temperature. There was a relationship between endophytic bacterial colonization of the grapevine plantlets and their growth at both ambient (26°C) and low (4°C) temperatures and their sensitivities to chilling. The major benefits of bacterization were observed on root growth (11.8- and 10.7-fold increases at 26°C and 4°C, respectively) and plantlet biomass (6- and 2.2-fold increases at 26°C and 4°C, respectively). The inoculation with PsJN also significantly improved plantlet cold tolerance compared to that of the nonbacterized control. In nonchilled plantlets, bacterization enhanced CO2 fixation and O2 evolution 1.3 and 2.2 times, respectively. The nonbacterized controls were more sensitive to exposure to low temperatures than were the bacterized plantlets, as indicated by several measured parameters. Moreover, relative to the noninoculated controls, bacterized plantlets had significantly increased levels of starch, proline, and phenolics. These increases correlated with the enhancement of cold tolerance of the grapevine plantlets. In summary, B. phytofirmans strain PsJN inoculation stimulates grapevine growth and improves its ability to withstand cold stress.
Abiotic stress leads to a series of molecular, biochemical, physiological, and morphological changes that adversely affect plant growth and productivity. A low temperature is a major factor limiting the productivity and geographical distribution of many species, including important agricultural crops (3). Cold-hardy plants increase their freezing tolerance upon exposure to low, nonfreezing temperatures by a phenomenon known as cold acclimation (38).
The process of cold acclimation involves changes in gene expression profiles (32), membrane lipids, total protein content, and composition of soluble proteins (30). Increases in the activity of oxygen-scavenging enzymes (11), sugar, and proline content (2, 3, 41), anthocyanin accumulation, and altered growth morphology (30) have also been reported. Moreover, some plants produce specific proteins that protect cells under supercooling conditions, hence lowering the freezing point (6). These, among other changes, alter leaf ultrastructure (28) and modify plant growth patterns, affecting morphology (32).
Epiphytic bacterial species with ice-nucleating activity (Ice+ bacteria), such as Pseudomonas syringae, contribute to the frost injuries of many cold-sensitive plants by reducing the plants' ability to supercool, a process that prevents the formation of membrane-damaging ice crystals (21). Since the ice nucleation temperature increases with an increasing population size of Ice+ bacteria, preemptive competitive exclusion of Ice+ bacteria by naturally occurring but non-ice-nucleating active bacteria could be an effective and practical means of frost control in cold-sensitive plants (21). Recently, commercial formulations combining bacteria antagonistic to plant pathogenic microbes with ice nucleation-active bacteria have been utilized as an environmentally safe method to manage biotic and abiotic stress in plants (21). In addition, some of these bacteria, such as epiphytic or endophytic plant growth-promoting rhizobacteria (PGPR), enhance plant growth while improving their resistance to stress (10, 13, 15, 22, 26). PGPR can stimulate developmental changes in host plants (13, 17), disrupt phytopathogen organization (4, 5), induce systemic resistance to pathogens (7, 8, 13, 34), affect phytohormone production, and improve nutrient and water management (13, 25). In vitro-bacterized plantlets not only grew faster than nonbacterized controls but also were sturdier, with a better-developed root system (4) and significantly greater capacities for withstanding biotic (4, 5, 34) and abiotic (9) stresses.
In this study, we tested a known plant growth-promoting bacterium, Burkholderia phytofirmans strain PsJN, for its ability to enhance chilling resistance in grapes. This bacterium is capable of epiphytic and endophytic colonization of grapevine tissues and organs (4, 12) and has been shown to protect plants against heat stress (9). Our hypothesis was that endophytic colonization of grapevines by Burkholderia phytofirmans strain PsJN would cause physiological and biochemical changes in the plants that may enhance their cold tolerances upon exposure to low temperatures. To address this hypothesis, we examined the levels of electrolytes, proline, phenolics, and starch content and related them to gas exchange and chilling tolerance in bacterized and nonbacterized plantlets under cold stress. Morphologies and cytologies of grapevine organs before and after chilling were also examined.
Vineyards north of Reims (Champagne) are from 48° to 49°5′ latitude and occur at the northern limit of the area where grapes are grown for production in France. Nonfreezing but cool temperatures are common during the growing season in this area and can compromise grapevine productivity. Since grapes are clonally propagated, PGPR could be established in planta during in vitro propagation and potentially provide a carryover effect of enhancing chilling stress resistance in viticulture.
MATERIALS AND METHODS
Plant material and in vitro growth conditions.
Plantlets of Vitis vinifera L. cv. Chardonnay clone 7535 were micropropagated by nodal explants grown on 15 ml of semisolid medium (23) in 25-mm culture tubes under 200 μmol m−2 s−1 white fluorescent light for a 16-h photoperiod at 26°C (12). Uniform plantlets (n = 24) were selected for each treatment in each experiment.
Plant bacterization.
The bacterial inoculum was produced by transferring two loops of PsJN to 100 ml of King's B liquid medium in a 250-ml Erlenmeyer flask incubated at 20°C at 150 rpm for 48 h (4). Bacteria were collected by centrifugation (3,000 × g for 15 min) and washed twice with phosphate-buffered saline (PBS) (10 mM, pH 6.5). The pellet was resuspended in PBS and used as inoculum. The bacterial concentration was estimated by spectrophotometry (600 nm) and adjusted to 106 CFU/ml with PBS (5). The concentration was confirmed by plate counting. Nodal explants, approximately 1 cm long and taken from 6-week-old stock plantlets, were immersed in the inoculum for 1 min, blotted with sterile filter paper, and placed in culture tubes as described previously (5). Noninoculated control plantlets were dipped in PBS. The plants were incubated in the growth chamber as described above.
Cold treatment.
After 6 and 12 weeks, bacterized and nonbacterized grapevine plantlets were divided into two groups: one group was transferred to a cold growth chamber maintained at 4°C under a 16 h-photoperiod with light provided by white fluorescent lamps at an intensity of 200 μmol m−2 s−1, and the other (control) group was kept under the conditions described above. Each treatment consisted of 24 plantlets, and the experiment was repeated three times. Analyses were conducted after 2 weeks of treatment.
Electrolyte leakage.
Leaf samples comprising the fifth and sixth leaves from the basal end of the plantlets were taken from each plantlet (n = 24), rinsed with distilled water, and dried on filter paper. The leaves were incubated in 30 ml mannitol (0.4 M) in 50-ml plastic bottles at 24°C for 20 h on a rotary shaker (80 rpm) as described previously (18). The conductance of the incubation medium was measured using a conductivity meter (Orion, model 150; Thermo Electron Corporation, Breda, The Netherlands). Samples were autoclaved at 120°C for 3 min and cooled to room temperature, and the volumes were adjusted to 30 ml. The conductivity of the samples was measured again to determine the total electrolyte content of the tissue. The degree of electrolyte leakage was calculated as described earlier (1).
Free proline analysis.
Free proline content was determined as described by Ait Barka and Audran (3). Plantlets were frozen in liquid N2 and kept at −80°C until used. Leaves, stems, and roots were ground separately and homogenized in 3% (wt/vol) sulfosalicylic acid. The homogenate was filtered through filter paper (Whatman no. 1). After the addition of ninhydrin reagent (1% [wt/vol] ninhydrin in 60% acetic acid), the mixture was heated to 100°C for 20 min. The reaction was then stopped in ice. The mixture was extracted with 1 ml toluene, and the sample was vigorously shaken for 15 s. After 4 h in darkness at room temperature, sample absorbance of the toluene layer was read at 520 nm. Proline concentration was determined by using a calibration curve and expressed as μM proline g−1 fresh weight (FW).
Determination of total phenolics.
The content of total phenolics was determined by using a modified Folin-Ciocalteu colorimetric method (36). Fresh leaf tissue (600 mg) was ground in 5 ml ethanol (80%) using a tissue homogenizer. Samples were placed in 50-ml tightly covered plastic tubes and incubated at 4°C for 2 h in the dark and then filtered as above. The pellet was resuspended in 2.5 ml ethanol. Five replicates of 125 μl phenolic extract, 625 μl 1/10 diluted Folin-Ciocalteu reagent, and 250 μl 7.5% (wt/vol) Na2CO3 were vortexed for 10 s, and the mixture was incubated at 45°C in a water bath shaker for 15 min. Phenolics were measured at 750 nm using catechin as the standard. Total phenolics were expressed as ng g−1 FW.
Gas exchange measurements.
Carbon dioxide exchange rates were measured on 24 plantlets from each treatment using a Li-Cor 6200 portable photosynthetic system (Li-Cor, Lincoln, Neb.) and a 250-ml gas exchange chamber. During the measurement, the gas exchange chamber was illuminated with a white light source (1,000 μmol photons · m−2 · s−1) under a relative humidity of 65 to 75%, an air CO2 concentration of 420 to 460 μl liter−1, and a flow rate of 1,125 μmol · min−1. O2 evolution was measured with an oxygen electrode (Hansatech, Cambridge, United Kingdom). Three leaves were detached from each plant and placed in the electrode chamber. Saturating CO2 conditions were maintained using 2 M potassium carbonate-potassium bicarbonate buffer, pH 9.3. The electrode buffer contained saturated potassium chloride.
Starch extraction and analysis.
Organs were sampled from each plantlet (n = 24) and homogenized individually at 4°C in a mortar containing 0.1 M phosphate buffer, pH 7.5. The homogenates were centrifuged at 12,000 × g for 15 min, and the pellets were used for starch analysis. The collected pellets were resuspended in dimethyl sulfoxide-8 M hydrochloric acid (4:1, vol/vol). Starch was dissolved over 30 min at 60°C with agitation (60 rpm). After centrifugation for 15 min at 12,000 × g, 100-μl supernatant samples were mixed with 100 μl iodine-HCl solution (0.06% KI and 0.003% I2 in 0.05 M HCl) and 1 ml distilled water. The absorbance was read at 600 nm after 15 min of incubation at room temperature.
Microscopic observations.
Roots, stems, and leaves were cut into 1-mm sections. The samples were immersed in cold fixative solution containing 8% glutaraldehyde, 2% paraformaldehyde in 0.2 M potassium buffer (pH 7.24), vacuum infiltered for 20 min, and immersed in fresh fixative solution for 20 h (18). Samples were subsequently washed with 0.2 M potassium buffer (pH 7.24), postfixed in 2% osmium tetroxide prepared in the same buffer for 4 h, washed with the buffer, and dehydrated in a graded ethanol series. The samples were then washed with acetone series and embedded in araldite (Fluka, France). Semithin sections (1 μm) were collected on glass slides and stained with toluidine blue, rinsed in distilled water, air dried, and mounted in Eukitt. The sections were examined under an optical microscope (model no. BH-2; Olympus, Tokyo, Japan).
Statistical analyses.
Unless stated otherwise, replicates of 24 plants were used per treatment. Collected data were analyzed statistically using analysis of variance. Means for each treatment were separated with at least significant difference (LSD; P < 0.05) multiple comparison test (Fisher's protected). All experiments were repeated three times.
RESULTS
Under the applied experimental conditions, the changes in all analyzed parameters were similar for both 6- and 12-week-old plantlets. The results of the experiments conducted on 12-week-old plantlets are presented.
Plant bacterization.
A comprehensive PsJN colonization study of grapevine plantlets was completed earlier using a PsJN::gfp2X derivative tagged with green fluorescent protein. We found that the bacterium initially colonized the root surface, followed by tissue penetration and colonization of the root interior, translocation via stem xylem vessels, and then endophytic colonization of leaf tissues (12). A study conducted under the same in vitro culture and bacterial inoculation conditions as the experiment reported here clearly demonstrated that B. phytofirmans strain PsJN establishes endophytic populations. The presence of PsJN in the root and shoot interior was reconfirmed in this study (data not shown).
Growth promotion.
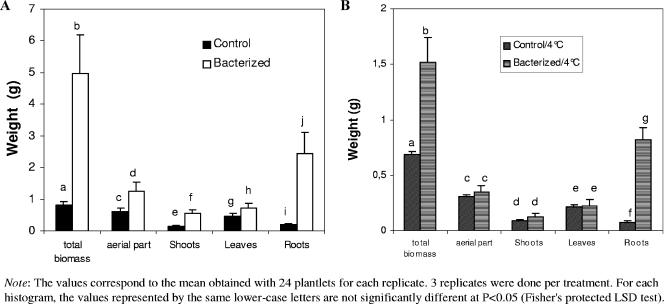
At 26°C, bacterized plantlets had sixfold-higher total biomasses compared to those of the nonbacterized controls (Fig. 1). Although there was a significant (P < 0.05) enhancement of the biomass of all organs, the stimulation of root growth was the greatest: approximately 12-fold. After 2 weeks of cold temperature treatment, the total biomass decreased for both treatments, and a significant difference between bacterized and control treatments was recorded for only root biomass (Fig. 1).
FIG. 1.
Effect of chilling injury on the weight of grapevine plantlets. (A) Nonchilled plantlets. (B) Chilled plantlets. Error bars indicate standard deviations.
Electrolyte leakage.
Electrolyte leakage results show that bacterization significantly (P < 0.05) improved the chilling tolerance of the grapevine plantlets (Table 1). Prior to plantlet exposure to the chilling treatment, ion leakage from leaves of both bacterized and nonbacterized control plantlets was similar, 13 to 14% of the total leaf electrolytes. After chilling, however, the electrolyte leakage was approximately twice as great in the controls as in bacterized plantlets, 39 versus 20%, respectively.
TABLE 1.
Effect of chilling injury on electrolyte leakage from leaves, leaf-free proline, total phenolics, and starch content of grapevine plantletsa
| Treatment | Electrolyte leakageb (%) | Free proline (μmoles g−1 FW)
|
Total phenols (nanogram g−1 FW) | Starch content (mg/g of FW)
|
||||
|---|---|---|---|---|---|---|---|---|
| Shoots | Leaves | Roots | Shoots | Leaves | Roots | |||
| Control at 26°C | 13.00 a | 0.95 a | 2.85 a | ND a | 13.11 a | 0.005 a | 0.010 a | 0.010 a |
| Control at 4°C | 39.00 b | 1.89 b | 5.75 b | 0.09 a | 14.51 b | 0.005 a | 0.010 a | 0.002 a |
| Bacterized at 26°C | 14.00 a | 1.24 c | 3.5 c | 0.15 b | 19.85 c | 0.060 b | 0.050 b | 0.004 a |
| Bacterized at 4°C | 19.95 c | 2.19 d | 6.45 d | 0.18 b | 21.55 d | 0.050 c | 0.080 c | 0.002 a |
Values correspond to the mean obtained with 24 plantlets for each replicate. Three replicates were performed per treatment. In each row, the values represented by the same lowercase letters were not significantly different at a P value of <0.05 (Fisher's protected LSD test). ND, not detected.
Expressed as percentage of total electrolyte content.
Free proline and total phenolics.
Both cold and bacterization treatments significantly (P < 0.005) increased free proline content in leaves and stems in all tissues of the control plantlets (Table 1). There was almost no proline found (it was either not detected or detected in trace amounts) in the roots of the control plantlets not subjected to chilling and 0.15 μmol · g−1 FW in the bacterized ones. Similarly, bacterization as well as cold stress significantly (P < 0.05) enhanced the content of total phenolics in leaves (Table 1). Independently of temperature, bacterization enhanced the content of phenolics by approximately 50% and, independently of bacterization, low-temperature treatment enhanced their titers by 10%.
Photosynthetic capacity.
Independently of the temperature treatment, bacterization significantly enhanced photosynthetic activity of the grapevine leaves, compared to that of the control (Table 2). When subjected to chilling, the photosynthetic activities of both bacterized and nonbacterized plantlets decreased, although the cold treatment affected the nonbacterized plantlets significantly (P < 0.05) more (Table 2). O2 evolution was also negatively affected by the low temperature treatment. In both temperatures, O2 evolution and CO2 fixation were significantly (P < 0.05) greater in the bacterized plantlets.
TABLE 2.
Effect of chilling and bacterization on photosynthetic activity (CO2 fixation and O2 production) and photosynthesis of grapevine plantletsa
| Treatment | CO2 fixation (μmol m−2 s−1) | O2 production (nmol min−1 cm−2) | Photosynthesis level (μmoles CO2 cm−2 s−1) |
|---|---|---|---|
| Control at 26°C | 3.15 a | 0.30 a | 0.47 a |
| Control at 4°C | 1.51 b | 0.15 b | 0.15 b |
| Bacterized at 26°C | 3.98 c | 0.65 c | 0.80 c |
| Bacterized at 4°C | 2.68 d | 0.33 d | 0.40 a |
Values correspond to the mean obtained with 24 plantlets for each replicate. Three replicates were performed per treatment. In each row, the values represented by the same lowercase letters were not significantly different at a P value of <0.05 (Fisher's protected LSD test). Photosynthetic activity is determined by CO2 fixation and O2 production.
Starch content and distribution.
Compared to the nonbacterized controls, the enhanced photosynthetic activity of the bacterized plantlets corresponded with significantly (P < 0.05) higher starch content, particularly in leaves and shoots (Table 1). Chilling treatment had no significant (P < 0.05) effect on starch content in all organs of the control plantlets (Table 1). Under similar conditions, the bacterized plantlets continued accumulating starch in leaves and shoots (P < 0.05). However, no significant differences (P < 0.05) were found in roots between all treatments.
Microscopic observations.
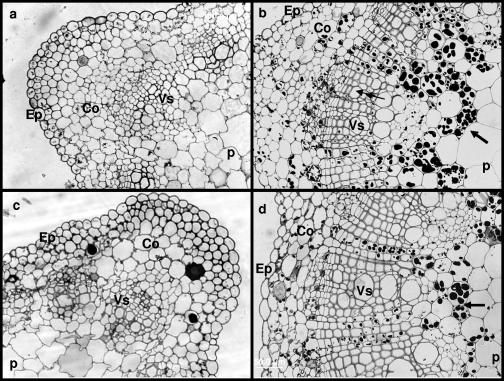
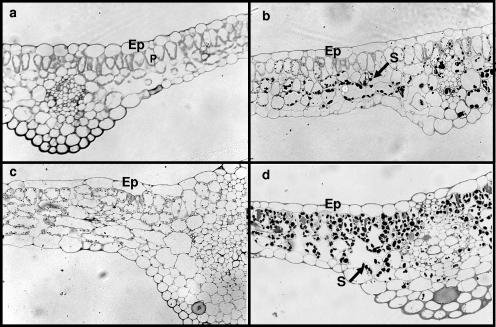
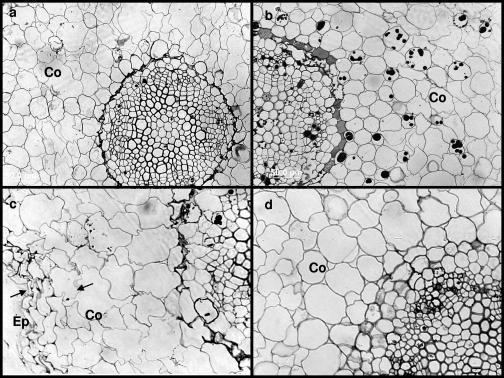
The vascular system is organized into “bundles,” with water-conducting xylem vessels located internally (on the pith side) and the food/organic material-transporting phloem tissue on the exterior (see Fig. 3). No obvious differences were observed between the temperature treatments in the structure of the cell wall or the tissue organization in shoots and leaves of the nonbacterized plantlets (Fig. 2 and 3). The nonbacterized control plantlets chilled at 4°C exhibited less growth but had larger vascular systems than did the controls grown at 26°C (Fig. 4a and c). The chilling treatment also caused cell wall alterations and some damage in the epidermis and the cortex of control chilled roots (Fig. 4c). The bacterized plantlets had secondary vascular structures, with relatively large-diameter xylem cells, indicating enhanced vascular cambial activity (Fig. 3b and d). The thick cell walls of the xylem provide the principal structural support for aerial plant parts. Microscopic examination of sections taken from different organs confirmed the results of the starch analysis reported in Table 1. More starch was observed in leaf and stem sections of bacterized plantlets than in controls, with no clear difference detected in roots (Fig. 4). Increased starch accumulation in leaves of bacterized plantlets upon chilling could also be seen on cross sections of leaf parenchyma (Fig. 2) and stele (Fig. 3).
FIG. 3.
Light micrographs of grapevine shoots. Twelve-week-old plantlets were subjected to 4°C treatment during two weeks, whereas the control remained at 26°C. (a) Control plantlets. (b) Bacterized plantlets. (c) Control plantlets chilled at 4°C. (d) Bacterized plantlets chilled at 4°C. For both bacterized plantlets grown at 26°C and bacterized chilled plantlets, a cross-section showed an accumulation of starch grains in shoot stele as indicated by the arrows. Bacterized plantlet shoots indicate the presence of secondary structure as evidenced by a net development of xylem (black arrowheads). No obvious correlation between cold treatment and marked host wall alterations or heavy tissue damage was observed. Ep, epidermis; Co, cortex; P, pith; Vs, vascular stele. Magnification, ×40.
FIG. 2.
Light micrographs of grapevine leaves. Twelve-week-old plantlets were subjected to 4°C treatment during 2 weeks, whereas the control remained at 26°C. (a) Control plantlets. (b) Bacterized plantlets. (c) Control plantlets chilled at 4°C. (d) Bacterized plantlets chilled at 4°C. No obvious correlation between cold treatment and marked host wall alterations or heavy tissue damages was observed. For both bacterized plantlets grown at 26°C and bacterized chilled plantlets, the cross-section showed an accumulation of starch grains in leaf parenchyma as indicated by the arrows. Ep, epidermis; P, parenchyma; S, starch. Magnification for panels a, b, and d, ×20; magnification for panel c, ×40.
FIG. 4.
Light micrographs of grapevine roots. Twelve-week-old plantlets were subjected to 4°C treatment during 2 weeks, whereas the control remained at 26°C. (a) Control plantlets. (b) Bacterized plantlets. (c) Control plantlets chilled at 4°C. (d) Bacterized plantlets chilled at 4°C. Roots of chilled plantlets showed obvious correlations between cold treatment and marked host wall alterations and tissue damage in the epidermis (Ep) and the cortex (Co), as indicated by the arrows. Magnification, ×40.
DISCUSSION
Burkholderia phytofirmans strain PsJN is capable of colonization of internal tissue while cocultured in vitro with grape (12), tomato (34), and other plants (26). Based on the colonization pathway we observed with a PsJN::gfp2X derivative tagged with green fluorescent protein (12) and on the effectiveness of our surface sterilization protocol, which was proven with grape (5, 12), tomato (34), and potato (17) plants, the bacterium can be considered a true endophyte. The grapevine plantlets cocultured with PsJN grew faster and had significantly (P < 0.05) more secondary roots (4, 5, 12). The bacterium also enhances the resistance of tissue culture plantlets to fungal disease (4, 5, 34) and heat stress (9). The strain expresses a high level of 1-aminocyclopropane-1-carboxylate deaminase (33), the enzyme that hydrolyzes the ethylene precursor 1-aminocyclopropane-1-carboxylate to ammonia and α-ketobutyrate. By lowering the production of this hormone in planta, the bacterium can decrease inhibitory effects of ethylene on root elongation and its stimulation of senescence under stress (19).
Despite the ability of plants to adapt partially to low-temperature stress in temperate climates (30), plant growth and overall productivity generally decline under chilling conditions (20). The extent of a plant's ability to withstand such stress is determined by metabolic alterations (32). In this study, we demonstrated for the first time that the plant growth-promoting bacteria colonizing grape plantlets can significantly influence the plantlets' resistance to chilling. Plantlet bacterization with Burkholderia phytofirmans strain PsJN had a pronounced effect on grapevine growth, development, and responses to low temperatures, i.e., diminished rates of biomass reduction and electrolyte leakage during chilling and stimulated postchilling recovery.
In our previous study with frost-treated organs of grapevines, electrolyte leakage was a good indicator of plant sensitivity to cold (1). The data thus indicate that the strain PsJN may have the potential to lower the sensitivity of this crop to chilling injury.
Bacterization significantly (P < 0.05) elevated the level of proline and phenolics and enhanced the rate of photosynthesis and starch deposition. This, combined with an enlarged root system and improved sucrose uptake from the medium, may have contributed to the stimulation of growth, development, and adaptation to stress (29). Decrease of photosynthesis induced by exposure to low temperatures is a well-known response of chilling-sensitive plants (6, 27).
Our results show that the differences in cold tolerance between the control and bacterized plantlets were reflected by differences in their abilities to significantly accumulate (P < 0.05) carbohydrates under cold stress conditions. This is in agreement with our earlier report of the accumulation of starch in grapevine buds subjected to low temperatures (2). This enhancement was explained in part by an inhibition of amylase activity under cold conditions. In cabbage seedlings, starch accumulated during cold acclimation and decreased during deacclimation (31). A comparison between the changes in chilling susceptibility of grapevine plantlets and their starch content indicates that starch may also play a role in protecting plant tissues against chilling.
Proline is a dominant organic molecule that accumulates in many organisms upon exposure to environmental stress (14) and plays multiple roles in plant adaptation to stress (24, 37), including chilling (37, 40). We found a significant correlation between freezing tolerance and an increase of proline concentration in shoot and bud tissue of grapevines after exposure to low temperatures (3). In this study, B. phytofirmans strain PsJN significantly (P < 0.05) increased proline accumulation in grapevine plantlets upon chilling. The enhancement of proline accumulation in leaf tissues was also reported with an avirulent strain of Pseudomonas syringae pv. tomato but not with the isogenic virulent bacteria (16).
The observed accumulation of phenolic compounds induced by PsJN confirms our earlier results (12). This phenomenon is linked to the host defense response, which also includes the strengthening of cell walls in the exodermis and several cortical cell layers. The activation of secondary responses associated with the onset of induced resistance, including the oxidation and polymerization of preexisting phenols and the synthesis of new phenolic compounds via the activation of the phenylpropanoid pathway, has been demonstrated with another endophytic bacterium, Serratia plymuthica, in cucumbers (8) and with two PGPR strains (Pseudomonas fluorescens strain Pf4 and P. aeruginosa strain Pag) in chickpeas (35).
We reported earlier that B. phytofirmans strain PsJN protected grapevines against Botrytis cinerea (4, 5). The mechanism of this protection was not localized but systemic. This type of response usually confers an enhancement of plant resistance to both biotic and abiotic stress (26). This phenomenon is known as rhizobacteria-mediated induced systemic resistance and was described as the mode of action of disease suppression by nonpathogenic rhizosphere bacteria (39). Host defense response pathways are preinduced by the colonizing beneficial bacteria, allowing a much faster response to pathogen infection, i.e., formation of structural barriers, such as thickened cell wall papillae due to the deposition of callose and the accumulation of phenolic compounds at the site of pathogen attack (8, 12). Our results indicate that a plant's reaction to cold stress could be similar to the one previously reported during pathogen attacks. Thus, the present results linked the biotic stress to the abiotic stress in the way by which plants react to the stress.
Conclusions.
The concept of PGPR is now well established (13), and some consideration of the relationship of PGPR to biocontrol is worthwhile. Positive interactions between bacterial endophytes and their host plants can result in a range of beneficial effects, which are similar if not complementary to those reported for exorhizobacteria. These include increased plant growth and development, resistance to disease, and improvements in the host plant's ability to withstand environmental stress (e.g., chilling).
B. phytofirmans strain PsJN has been well characterized as a PGPR that triggers induced systemic resistance against fungal pathogens (12, 34). Our findings indicate that this mechanism also enhances grapevine resistance to cold stress.
The beneficial effect of endophyte bacteria may be through their induction of the synthesis of proteins, which reduces the development of symptoms, and also through the prevention of some sets of reactions, which produce the symptoms of chilling injury. Because the nucleation temperature of plants increases with increasing population sizes of Ice+ bacteria, preemptive competitive exclusion of Ice+ bacteria with naturally occurring non-ice nucleation-active bacteria could be an effective and practical means of frost control. The management of frost injury by reducing Ice+ bacterial populations might become an important new method of frost control.
Therefore, understanding molecular mechanisms behind the chill-induced effects on photosynthesis by examining the myriad changes in gene expression will be our next step toward a better understanding of the role of PGPR in low-temperature acclimation.
Acknowledgments
We thank Richard Veilleux, Department of Horticulture, Virginia Polytechnic Institute and State University, Blacksburg, VA, for his editorial advice.
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Ait Barka, E., and J. C. Audran. 1996. Utilisation de la conductivité spécifique comme critère d'estimation de la viabilité au niveau de l'appareil aérien des vignes champenoises soumises aux températures négatives. Can. J. Bot. 74:413-418. [Google Scholar]
- 2.Ait Barka, E., and J. C. Audran. 1996. Réponse des vignes champenoises aux températures négatives: Effet d'un refroidissement contrôlé sur les réserves glucidiques du complexe gemmaire avant et au cours du débourrement. Can. J. Bot. 74:492-505. [Google Scholar]
- 3.Ait Barka, E., and J. C. Audran. 1997. Response of champenoise grapevine to low temperatures: Changes of shoot and bud proline concentrations in response to low temperatures and correlations with freezing tolerance. J. Hortic. Sci. Biotechnol. 72:577-582. [Google Scholar]
- 4.Ait Barka, E., A. Belarbi, C. Hachet, J. Nowak, and J. C. Audran. 2000. Enhancement of in vitro growth and resistance to gray mold of Vitis vinifera co-cultured with plant growth-promoting rhizobacteria FEMS Microbiol. Lett. 186:91-95. [DOI] [PubMed] [Google Scholar]
- 5.Ait Barka, E., S. Gognies, J. Nowak, J. C. Audran, and A. Belarbi. 2002. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol. Control 24:135-142. [Google Scholar]
- 6.Antikainen, M., and M. Griffith. 1997. Antifreeze protein accumulation in freezing-tolerant cereals. Physiol. Plant. 99:423-432. [Google Scholar]
- 7.Benhamou, N. 1996. Elicitor-induced plant defence pathways. Trends Plant Sci. 1:233-240. [Google Scholar]
- 8.Benhamou, N., S. Gagné, D. L. Quéré, and L. Dehbi. 2000. Bacterial-mediated induced resistance in cucumber: beneficial effect of the endophytic bacterium Serratia plymuthica on the protection against infection by Pythium ultimum. Phytopathology 90:45-56. [DOI] [PubMed] [Google Scholar]
- 9.Bensalim, S., J. Nowak, and S. Asiedu. 1998. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am. Potato J. 75:145-152. [Google Scholar]
- 10.Bloemberg, G. V., and B. J. J. Lugtenberg. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4:343-350. [DOI] [PubMed] [Google Scholar]
- 11.Brüggemann, W., V. Beyel, M. Brodka, H. Poth, M. Weil, and J. Stockhaus. 1999. Antioxidants and antioxidative enzymes in wild-type and transgenic Lycopersicon genotypes of different chilling tolerance. Plant Sci. 140:145-154. [Google Scholar]
- 12.Compant, S., B. Reiter, A. Sessitsch, J. Nowak, C. Clément, and E. Ait Barka. 2005. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 71:1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compant, S., B. Duffy, J. Nowak, C. Clément, and E. Ait Barka. 2005. Use of plant growth-promoting bacteria for bioncontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71:4951-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delauney, A. J., and D. P. S. Verma. 1993. Proline biosynthesis and osmoregulation in plants. Plant J. 4:215-223. [Google Scholar]
- 15.Dobbelaere, S., J. Vanderleyden, and Y. Okon. 2003. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 22:107-149. [Google Scholar]
- 16.Fabro, G., I. Kovács, V. Pavet, L. Szabados, and M. E. Alvarez. 2004. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact. 17:343-350. [DOI] [PubMed] [Google Scholar]
- 17.Frommel, M. I., J. Nowak, and G. Lazarovits. 1991. Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum ssp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiol. 96:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gognies, S., A. Belarbi, and E. Ait Barka. 2001. Saccharomyces cerevisiae, a potential pathogen towards grapevine, Vitis vinifera. FEMS Microbiol. Ecol. 1271:143-150. [Google Scholar]
- 19.Grichko, V. P. S., and B. R. Glick. 2001. Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Phys. Biochem. 39:11-17. [Google Scholar]
- 20.Haldiman, P. 1998. Low growth temperature induced changes to pigment composition and photosynthesis in Zea mays genotypes differing in chilling sensitivity. Plant Cell Environ. 21:200-208. [Google Scholar]
- 21.Lindow, S. E., and J. H. J. Leveau. 2002. Phyllosphere microbiology. Curr. Opin. Biotechnol. 13:238-243. [DOI] [PubMed] [Google Scholar]
- 22.Lodewyckx, C., J. Vangronsveld, F. Porteous, E. R. B. Moore, S. Taghavi, M. Mezgeay, and D. van der Lelie. 2002. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 21:583-606. [Google Scholar]
- 23.Martin, C., R. Vernoy, M. Carr, G. Vesselle, A. Collas, and C. Bougerey. 1987. The vine and techniques of in vitro cultivation. Bull. Org. Int. Vigne. 675:676:447-458. [Google Scholar]
- 24.Nanjo, T., T. M. Kobayashi, Y. Yoshida, Y. Kakubari, K. Yamaguchi-Shinozaki, and K. Shinozaki. 1999. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461:205-210. [DOI] [PubMed] [Google Scholar]
- 25.Nowak, J., and G. Lazarovits. 1997. Rhizobacteria for improvement of plant growth and establishment. Hortic. Sci. 32:188-192. [Google Scholar]
- 26.Nowak, J., and V. Shulaev. 2003. Priming for transplant stress resistance in in vitro propagation. In Vitro Cell. Dev. Biol. Plant 39:107-124. [Google Scholar]
- 27.Oquist, G., and N. P. Huner. 2003. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 54:329-355. [DOI] [PubMed] [Google Scholar]
- 28.Ristic, Z., and E. N. Ashworth. 1993. Changes in leaf ultrastructure and carbohydrates in Arabidopsis thaliana L. (Heynh) cv. Columbia during rapid cold acclimation. Protoplasma 172:111-123. [Google Scholar]
- 29.Saladin, G., C. Clément, and C. Magné. 2003. Stress effects of flumioxazin herbicide on grapevine (Vitis vinifera L.) grown in vitro. Plant Cell Rep. 21:1221-1227. [DOI] [PubMed] [Google Scholar]
- 30.Saltveit, M. E. 2000. Discovery of chilling injury, p. 423-448. In S. D. Kung and S. F. Yang (ed.), Discoveries in plant biology, vol. 3. World Scientific Publishing, Singapore. [Google Scholar]
- 31.Sasaki, H., K. Ichimura, and M. Oda. 1996. Changes in sugar content during cold acclimation and deacclimation of cabbage seedlings. Ann. Bot. 78:365-369. [Google Scholar]
- 32.Seki, M., A. Kamei, K. Yamaguchi-Shinozaki, and K. Shinozaki,. 2003. Molecular responses to drought, salinity and frost: common and different paths for plant protection. Curr. Opin. Biotechnol. 14:194-199. [DOI] [PubMed] [Google Scholar]
- 33.Sessitsch, A., T. Coenye, A. V. Sturz, P. Vandamme, E. Ait Barka, J. F. Salles, J. D. van Elsas, D. Faure, B. Reiter, B. R. Glick, G. Wang-Pruski, and J. Nowak. 2005. Burkholderia phytofirmans sp. nov., a novel plant associated bacterium with plant beneficial properties. Int. J. Syst. Evol. Microbiol. 55:1187-1192. [DOI] [PubMed] [Google Scholar]
- 34.Sharma, V. K., and J. Nowak. 1998. Enhancement of Verticillium wilt resistance in tomato transplants by in vitro co-culture of seedlings with a plant growth promoting rhizobacterium (Pseudomonas sp. strain PsJN). Can. J. Microbiol. 44:528-536. [Google Scholar]
- 35.Singh, U. P., K. Birinchi, K. Sarma, and D. P. Singh. 2003. Effect of plant growth-promoting Rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicylic acid contents in chickpea (Cicer arietinum). Curr. Microbiol. 46:131-140. [DOI] [PubMed] [Google Scholar]
- 36.Singleton, V. L., and J. A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158. [Google Scholar]
- 37.Sung, D. Y., F. Kaplan, K. J. Lee, and C. L. Guy. 2003. Acquired tolerance to temperature extremes. Trends Plant Sci. 8:179-187. [DOI] [PubMed] [Google Scholar]
- 38.Thomashow, M. F. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:571-599. [DOI] [PubMed] [Google Scholar]
- 39.van Loon, L. C., P. A. H. M. Bakker, and C. M. J. Pieterse. 1998. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36:453-483. [DOI] [PubMed] [Google Scholar]
- 40.Verma, D. P. S. 1999. Osmotic stress tolerance in plants: role of proline and sulfur metabolism, p. 153-168. In K. Shinozaki and K. Yamaguchi-Shinozaki (ed.), Molecular responses to cold, drought, heat and salt stress in higher plants. R.G. Landes Company, Austin, Tex.
- 41.Wanner, L. A., and O. Junttila. 1999. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 120:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]