Abstract
To overcome the shortcomings of universal 16S rRNA gene primers 8F and 907R when studying the diversity of complex microbial communities, the 3′ termini of both primers were replaced with inosine. A comparison of the clone libraries derived using both primer sets showed seven bacterial phyla amplified by the altered primer set (8F-I/907R-I) whereas the original set amplified sequences belonging almost exclusively to Proteobacteria (95.8%). Sequences belonging to Firmicutes (42.6%) and Thermotogae (9.3%) were more abundant in a library obtained by using 8F-I/907R-I at a PCR annealing temperature of 54°C, while Proteobacteria sequences were more frequent (62.7%) in a library obtained at 50°C, somewhat resembling the result obtained using the original primer set. The increased diversity revealed by using primers 8F-I/907R-I confirms the usefulness of primers with inosine at the 3′ termini in studying the microbial diversity of environmental samples.
Universal primers are routinely employed in the study of microbial community structure and composition. The application of molecular biological methods to investigate the occurrence and distribution of bacteria in the environment has the advantage of providing direct information on community structure (5). Most studies of microbial diversity are based on the extraction of total bacterial DNA from samples, followed by PCR amplification of small-subunit rRNA genes. While this approach sounds relatively straightforward, it is in fact fraught with problems at almost every step, from the extraction of DNA to the selection of primers, right on through to the amplification of DNA (10, 18, 19, 26), producing an incomplete and often distorted view (8).
The choice of primers to be used in studies to assess the diversity of prokaryotes is not trivial (for reviews, see references 1 and 8). Primer complementarity to a large fraction of the gene sequences in a database, such as the ribosomal database project (RDP) database (16), does not necessarily mean that the primer is optimal. No database today represents the estimated total number of at least 10 million bacterial species, with possible high sequence divergence (4). Additionally, sequences in the database may be incomplete or corrupt. It may therefore be prudent to requestion the universality of these so-called “universal” primers (1, 8).
To enhance the universality of primers for the amplification of related sequences of 16S rRNA genes from different microorganisms, degenerate primers may be designed to have a number of nucleotide options at several positions in the internal primer sequence. This will allow annealing to and amplification of a variety of related sequences. When fourfold degeneracy is required for a given location, the natural base inosine may be used. Inosine is biologically found in the 5′ nucleotide of the tRNA anticodon, known as the Watson-Crick wobble (3). The primers containing inosine compensate for the high rate of degeneracy of the targeted codons and can substantially reduce overall primer degeneracy as well as false priming and nontarget gene amplification (11, 20). When designing a specific primer, inclusion of degenerate bases or inosine at the 3′ end of the primer is usually considered undesirable, as annealing of the last three bases on the 3′ end can be enough to initiate PCR at the wrong sites (30). On the other hand, mismatched nucleotides at positions at the 3′ terminus and 1 bp before the 3′ terminus have previously been shown to be detrimental to the amplification process (13, 23). This is primarily due to a need for a perfect 3′ base pair to allow enzymatic synthesis rather than to any thermodynamic effect on duplex formation (2).
A single base mutation at the 3′-end position homologous to that of a universal primer in a given organism will therefore have a much greater effect than mutations in neighboring bases. This may result in misrepresentation of that genotype in a PCR-based DNA library. Replacing the 3′-terminal position of a universal primer with the base inosine, thus lending the “one-eyed king” (8) an extra I, may compensate for such mutations, revealing higher microbial diversity. Here we demonstrate the utility of such primers in effective eubacterial 16S rRNA gene amplification and the subsequent study and analysis of the microbial diversity of saline industrial wastewater, compared to regular primers.
MATERIALS AND METHODS
Nucleic acid extraction.
The total genomic DNA from saline industrial wastewater samples was extracted from pellets obtained (30-ml samples) using a MoBio power soil DNA isolation kit (MoBio Laboratories, Inc., Solana Beach, CA) with one modification: DNA bound to the silica filter membrane was washed twice with C5 solution. The purified DNA was eluted in 60 μl of C6 solution (MoBio Laboratories, Inc.) and stored at −20°C. Genomic DNA from Escherichia coli was obtained by the same method, and the DNA concentrations were determined with an ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE).
Primer design and PCR amplification.
Total DNA was amplified by PCR with a Mastercycler gradient thermocycler (Eppendorf, Westbury, N.Y.), using specific 16S rRNA primers for bacteria, namely, forward primer 8F (5′-GGATCCAGACTTTGATYMTGGCTCAG), as described by Felske et al. (7) but modified by being shortened from the 5′ end, and reverse primer 907R (5′-CCGTCAATTCMTTTGAGTTT), as described by Lane et al. (15). Another pair of primers, 8F-I and 907R-I, was obtained by replacement with inosine at the 3′ termini. The universal primers were tested to eliminate dimer or nonspecific annealing by using the Amplify 1.0 computer software package (Bill Engels, University of Wisconsin). The Probe Match program available at the Ribosomal Database Project II (RDP II) website (http://rdp.cme.msu.edu/index.jsp) (16) was used to assess the specificity and universality of the primers.
The primers used in the PCR amplifications were obtained from Sigma-Genosys. The reaction mixtures included 12.5 μl ReddyMix (PCR master mix containing 1.5 mM MgCl2 and a 0.2 mM concentration of each deoxynucleoside triphosphate; ABgene, Surrey, United Kingdom), 1 pmol each of the forward and reverse primers, 1 to 2 μl of the sample preparation, and water to bring the total volume to 25 μl. An initial denaturation hot start of 4 min at 95°C was followed by 30 cycles of the following incubation pattern: 94°C for 30 s, 50 to 54°C for 40 s, and 72°C for 70 s. A final extension at 72°C for 20 min concluded the reaction.
When primers with inosine at the 3′ termini were used, a significant improvement in yield was observed when the MgCl2 concentration was increased to 5.5 mM with a supplement of 0.1 μg/μl analytical-grade bovine serum albumin (data not shown).
Clone library construction and sequencing.
The PCR products were purified by electrophoresis on an 0.8% agarose gel (Sigma), stained with ethidium bromide, and visualized with a UV transilluminator. The approximately 0.9-kbp heterologous 16S rRNA DNA products were excised from the gel, and the DNA products were purified from the gel slice by using a Wizard PCR prep kit (Promega, Madison, Wis.). The gel-purified PCR products were cloned into the pCRII-TOPO-TA cloning vector as specified by Invitrogen (Carlsbad, CA) and transformed into calcium chloride-competent E. coli DH5α cells according to the manufacturer's instructions and standard techniques (22).
Plasmid DNA was isolated from individual clones by using a Wizard Plus SV minipreps DNA purification system (Promega, Madison, Wis.). Aliquots from a subset of the samples of purified plasmid DNA were digested with the restriction enzyme EcoRI (MBI Fermentas) for more than 4 h at 37°C, and the digested product was separated by electrophoresis on a 1% agarose gel (agarose low electroendosmosis; Hispanagar, Spain). After being stained with ethidium bromide, the bands were visualized using a UV transilluminator to select clones containing the appropriately sized insert.
The clones with the correct plasmid insert were then used for sequencing. Sequencing (with M13-F and M13-R primers annealed to the plasmid) was performed using an ABI PRISM dye terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS and an ABI model 373A DNA sequencer (Perkin-Elmer).
Sequence analyses.
MEGA (Molecular Evolutionary Genetics Analysis, version 3.1) (12) was used to dereplicate the libraries of 16S rRNA gene sequences for subsequent analyses by comparing all the sequences in a data set to each other, grouping sequences with ≥97% and ≥90% identity together, and outputting a representative sequence from each group.
The all-rRNA gene sequences of each group were first compared with those in the GenBank database using the basic local alignment search tool BLAST (http://www.ncbi.nlm.nih.gov/BLAST/BLAST.cgi). Classifier version 1.0 (to assign 16S rRNA sequences to a taxonomical hierarchy) and Library Compare (to compare two sequence libraries using the RDP classifier), available at the Ribosomal Database Project II website (16), were used to find diversity in different ranks of related sequences. A 97 to 100% match of the unknown clone with the GenBank data set was considered an accurate identification to the species level, 93 to 96% similarity was accepted as genus-level identification, and an 86 to 92% match was considered an accurate identification of a related organism (25). The sequences from appropriate libraries were aligned using ClustalW (EMBL-EBI Center for Research and Services in Bioinformatics; http://www.ebi.ac.uk/clustalw/index.html), and positions not sequenced for all isolates or with alignment uncertainties were removed. Phylogenetic trees were constructed by the neighbor-joining method (21) with the MEGA package (12). Bootstrap resampling analysis (6) of 100 replicates was performed to estimate the confidence levels of the tree topologies.
Statistical analysis.
The statistical significance of the difference between the primers' abilities to enhance different phyla and subclasses was determined by Kruskal-Wallis nonparametric analysis of variance, followed by post hoc multiple comparisons. Analyses were performed using STATISTICA (a data analysis software system) version 7.0 (StatSoft, Inc.). Calculation of the regression lines was performed using SigmaPlot 2000 V.6 (SPSS Science, Chicago, IL).
Nucleotide sequence accession numbers.
The sequences from this study have been deposited in the NCBI GenBank database under accession numbers DQ458316 to DQ458468.
RESULTS AND DISCUSSION
PCR has changed the field of microbial ecology, facilitating the detection of unculturable microorganisms from virtually any environmental source, and has been used extensively in the assessment of environmental microbial diversity. This technique relies on the assumption that the universal primers used for amplification are complementary to conserved regions of the gene sequences present in the environment, while heterogeneity is to be found in the fragment enclosed by these primers (1, 8). However, the recent discovery of new taxa with 16S rRNA gene sequences not complementary to standard universal primers suggests that current 16S rRNA gene libraries are not representative of true prokaryotic biodiversity (1). Poor complementarity of universal primers, especially at the 3′ end, has a detrimental effect on the amplification process (13, 23). A single mismatch to a given taxon in environmental samples will, in this case, lead to underrepresentation of these genotypes within 16S rRNA gene libraries (1). To overcome this difficulty, we replaced the 3′ termini of the 16S rRNA gene universal primers 8F and 907R with inosine.
The 8F and 907R primers were designed from sequences of culturable bacteria, mostly proteobacteria (15, 29). Using these primers and similar ones is likely to produce a highly biased database. The 8F primer was assessed by using Probe Match at the RDP II website (16) and was found to complement relatively few sequences, half of them belonging to the phylum Proteobacteria; this is disconcerting as this primer is commonly used. This may be attributed to the fact that sequences of the region complementary to 8F are often unknown or ambiguous (1, 8). The 907R primer was complementary to 97,600 of 197,833 bacterial sequences existing in the RDP II database at the time the test was performed, with 38,625 being complementary to sequences belonging to the phylum Proteobacteria. Furthermore, in a comparison with 500 bacterial sequences, the base homologous to the guanine at the 3′ terminus of 8F was found to be variable, while the base homologous to the thymine at the 3′ terminus of 907R is considered conserved (but not totally conserved) (1). Therefore, with the number of known sequences continuously increasing, the specificity and utility of primers that were previously developed using a much smaller data set should frequently be reassessed.
In the current study, the microbial diversity of a high-salinity industrial wastewater evaporation pond was chosen to assess the efficacy of replacing nucleotides with inosine at the 3′ termini of universal primers 8F and 907R. The pond (pond 204) is part of an array of evaporation ponds located in the Ramat Hovav industrial area in the Negev Desert, Israel. The salt concentration in the pond at the time of sampling was around 12%. The ponds receive a mixture of high-strength industrial wastewaters from various industries in the area, making a unique habitat for various microorganisms (14). The 8F/907R universal primer pair was compared with the 8F-I/907R-I primer pair, where nucleotides at the 3′ termini were replaced with inosine. The annealing temperatures of the two primer pairs were tested using genomic DNA from E. coli with a gradient PCR technique. The conventional universal primers 8F/907R displayed a maximum yield at about 49°C, with a maximal functional temperature of 58°C. The 8F-I/907R-I primers, with inosine at the 3′ termini, displayed a maximum yield at about 47°C, with a maximal functional temperature of 56°C.
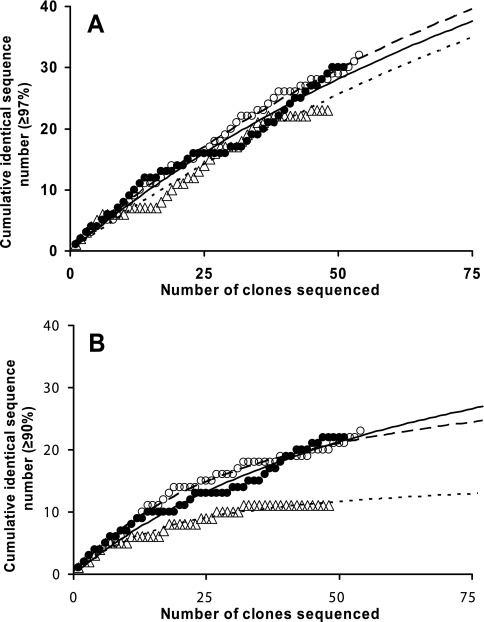
The total genomic DNA from the wastewater was amplified using the 8F/907R primers at a 54°C annealing temperature and the 8F-I/907R-I primers at 54°C and 50°C annealing temperatures to construct three libraries of 16S rRNA genes, with 48, 54, and 51 clones sequenced, respectively. From a plot of the cumulative number of different sequences against the number of clones, we could estimate the differences in microbial diversity yielded by the two primer sets at various annealing temperatures. We depicted approximations of the cumulative number of sequences versus the number of clones and estimated the total possible number of different sequences based on an infinite number of clones being obtained (Fig. 1), as proposed by Sekiguchi et al. (24). When the first 48 sequences of each library were grouped on the basis of ≥97% similarity, use of the 8F-I/907R-I primers at annealing temperatures of 54°C and 50°C and the 8F/907R primers at 54°C yielded 28, 29, and 23 separate 16S rRNA gene groups, respectively (Fig. 1A). Grouping on the basis of ≥90% identity returned a somewhat different image, with 20, 22, and 11 groups, respectively (Fig. 1B). From this estimation, it is suggested that the microbial diversity depicted by the use of 8F-I/907R-I with inosine at the 3′ termini may be almost twice as high as that depicted by the primers without inosine at that position. The comparatively low number of sequence groups obtained by the 8F/907R primers implies a greater universality of the 8F-I/907R-I primers. When all 153 sequences (from the three clone libraries) were similarly reviewed on the basis of 97 and 90% identities, 56 and 27 different sequence groups, respectively, were obtained. These values are lower than the theoretical number of groups for 153 clones according to the regression line fitted to the cumulative number of sequences from primers with inosine at the 3′ termini at either temperature (data not shown).
FIG. 1.
Estimation of microbial diversity in a wastewater environment as determined by using the 8F/907R primer set at an annealing temperature of 54°C (▵, dotted line) and the 8F-I/907R-I primer set at 54°C (○, dashed line) and 50°C (•, solid line).The cumulative number of identical 16S rRNA gene sequences is plotted against the total number of clones with either ≥97% (A) or ≥90% (B) identity. The regression lines were calculated using the modified hyperbolic equation y = x/(ax − b), where y is the cumulative number of different sequences, x is the total number of clones, and a and b are the coefficients proposed by Sekiguchi et al. (24). R2 values are higher than 0.967 for all regression lines.
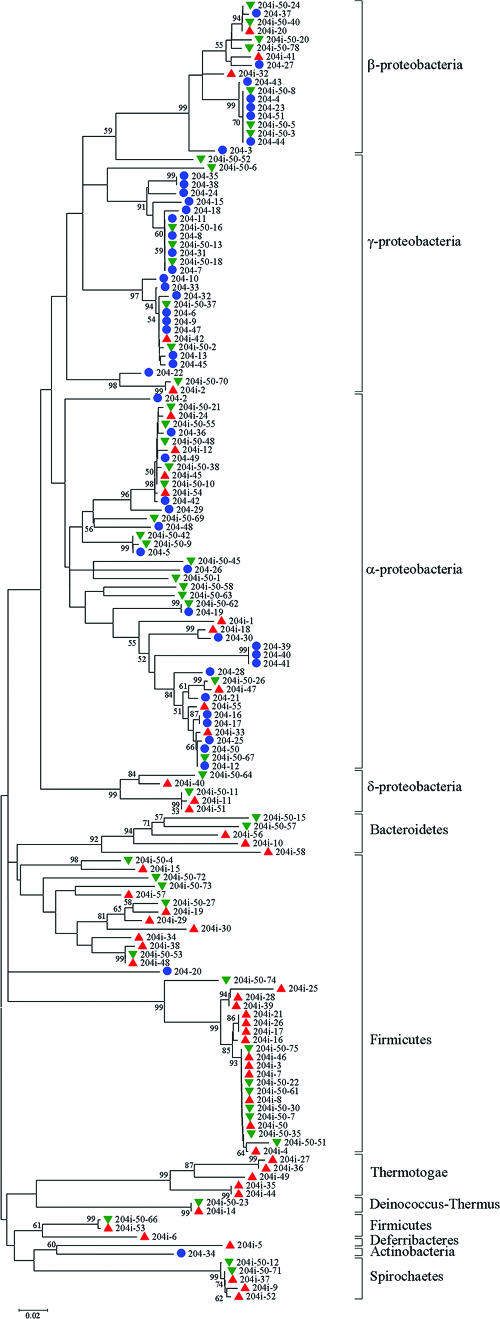
Classification of the sequences from the libraries at the level of bacterial phyla revealed that sequences amplified using conventional 8F/907R primers belonged almost exclusively to Proteobacteria (95.8%), whereas primers with inosine at the 3′ termini expanded the diversity to seven bacterial phyla (Table 1). At a 50°C permissive annealing temperature, close to 63% of the sequences amplified by use of the 8F-I/907R-I primers belonged to Proteobacteria, with 27.5% belonging to Firmicutes. At a restrictive annealing temperature, only 33.3% of sequences amplified using primers with inosine at the 3′ termini belonged to Proteobacteria, with close to 43% of the sequences originating from Firmicutes and more than 9% from Thermotogae (Table 1 and Fig. 2). Moreover, Proteobacteria sequences obtained by use of the 8F-I/907R-I primers included five sequences belonging to the δ-Proteobacteria genus Desulfovibrio (204i-11, 204i-40, 204i-51, 204i-50-11, and 204i-50-64) (Fig. 2), a subclass completely undetected by the 8F/907R primer pair in the course of this study. However, the 8F-I/907R-I primers appeared to be biased against γ-Proteobacteria at a 54°C annealing temperature, with only one sequence (204i-42) (Fig. 2) obtained representing that particular subclass. The results of the Kruskal-Wallis nonparametric analysis of variance test showed significant differences between the microbial community structures on the phylum level (P < 0.001). Multiple post hoc comparisons of the mean ranks for all groups showed significant differences (P < 0.02) between all the primer pairs. This excluded the possibility that these differences are due to an insufficient number of sequences obtained for each primer pair. The increased diversity obtained with primers 8F-I/907R-I indeed confirms the usefulness of the primers with inosine substituted for the nucleotides at the 3′ termini for enhancing our knowledge regarding microbial diversity in environmental samples. The value of inosine at the 3′ positions of primers has been previously demonstrated by Batzer et al. (2) for the detection of evolutionary mutations in primate species that could not be amplified using regular primers.
TABLE 1.
Diversity at the phylum level of three libraries of 16S rRNA genes obtained by use of universal primer sets with and without inosine at the 3′ termini
| Phylum | Diversity (%) obtained using:
|
||
|---|---|---|---|
| 8F-I/907R-I at 54°C | 8F-I/907R-I at 50°C | 8F/907R at 54°C | |
| Proteobacteria | 33.3 | 62.7 | 95.8 |
| Firmicutes | 42.6 | 27.5 | 2.1 |
| Thermotogae | 9.3 | ||
| Bacteroidetes | 5.5 | 3.9 | |
| Spirochaetes | 5.5 | 3.9 | |
| Deinococcus-Thermus | 1.9 | 2 | |
| Deferribacteres | 1.9 | ||
| Actinobacteria | 2.1 | ||
FIG. 2.
Phylogenetic tree based on 16S rRNA gene sequences that were retrieved from industrial wastewater by use of primer set 8F/907R at an annealing temperature of 54°C (blue circles) and primer set 8F-I/907R-I at 54°C (red triangles) and at 50°C (green triangles). The tree was constructed by the neighbor-joining method (21) with the MEGA package (12), using partial sequences of 16S rRNA genes. The bar represents two substitutions per 100 nucleotide positions. Bootstrap probabilities (6) are indicated at branch nodes.
The dissimilarity of the results obtained with the 8F-I/907R-I primers at the 54°C and 50°C annealing temperatures (Table 1, Fig. 2) may be explained by the different thermodynamic stabilities of inosine in relation to the four nucleotides (17, 28) and by the variability of the nucleotides in different bacterial phyla. A definite advantage in the use of inosine is to enhance primer universality while retaining specificity. Inosine is clearly the best base to use, especially at positions with A/C or G/T ambiguities and at positions with three- and fourfold base ambiguities, since it is the least selective. The order of stability of inosine (I) with the bases is I · C > I · A > I · T ≈ I · G > I · I (17). Watkins and SantaLucia (28) demonstrated the importance of the inosine nearest-neighbor parameters which have a large influence on the hybridization stability of I · X pairs. The stability trend for the base pair in the positions 5′ and 3′ of the I · X pair is G · C > C · G > A · T > T · A. This information may be useful in designing a probe/primer of optimal stability (17, 28).
The problems associated with universal primer design have been discussed previously (1, 8). So-called “conserved regions” in the 16S rRNA genes used for the bacterial universal primers were aligned by Watanabe et al. (27) and found to include many mismatches in the cores and in the 3′ ends of the primers. The introduction of inosine residues into the cores of the bacterial universal primers homologous to these regions enabled the amplification and detection by PCR/denaturing gradient gel electrophoresis of phylotypes that were not detected using the original primers with the same groundwater samples (27). Inosine-containing primers are therefore considered useful in detecting more-diverse populations in the environment.
The advantage of inosine at the 3′ termini of the primers (8F-I/907R-I) is in its ability to match all four nucleotides; instead of 16 possible variations at the 3′ terminus, one of a pair of degenerate primers is used. However, the limitation of inosine is in its different thermodynamic stabilities in relation to each of the four nucleotides. This can be partially overcome by using low annealing temperatures (as shown by the results at the 54°C and 50°C annealing temperatures [Table 1 and Fig. 2] and possibly also by altering the magnesium and primer concentrations. The capabilities of different DNA polymerases used in the PCR to synthesize DNA with an inosine-containing template should also be taken into consideration (9). Another disadvantage of inosine is its recognition by Taq polymerase as guanine, which prevents the study of nucleotide diversity by subsequent sequencing analysis.
New primers that are both universal and specific are thus required. Ideally, they must be specific to the domain in question while being complementary to sequences in all taxa within that domain. Detection of a base analog which is more discriminatory against mismatches than the normal bases (the ideal base analog would pair with the same stability to all bases) could be used to increase the selectivity of the probe/primer at positions of unambiguous sequence (17).
In terms of microbial community structure, the difference between the results obtained with the two primer pairs (8F-I/907R-I and 8F/907R) with and without the substitution of inosine for the nucleotides at the 3′ termini was clear (Table 1 and Fig. 1 and 2). Using a pair of universal primers with inosine at the 3′ termini can expand the observed diversity of a microbial community under study but is not guaranteed to amplify all species existing in the environment. When approaching a given environmental sample, it may be prudent to use primers with and without inosine at the 3′ termini, thus increasing the richness of 16S rRNA gene PCR amplicons, to better reflect the true diversity of the microbial community.
Acknowledgments
This work was supported by research funds from the Ramat-Hovav Council and BMBF-MOST Cooperation in Water Technologies grant WT-501.
We thank Esti Kramarsky-Winter for useful comments on the manuscript.
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Baker, G. C., J. J. Smith, and D. A. Cowan. 2003. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55:541-555. [DOI] [PubMed] [Google Scholar]
- 2.Batzer, M. A., J. E. Carlton, and P. L. Deininger. 1991. Enhanced evolutionary PCR using oligonucleotides with inosine at the 3′-terminus. Nucleic Acids Res. 19:5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crick, F. H. C. 1966. Codon-anticodon pairing: the wobble hypothesis. J. Mol. Biol. 19:548-555. [DOI] [PubMed] [Google Scholar]
- 4.Curtis, T. P., W. T. Sloan, and J. W. Scannell. 2002. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 99:10494-10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly, K., R. J. Sharp, and A. J. McCarthy. 2000. Development of oligonucleotide probes and PCR primers for detecting phylogenetic subgroups of sulfate-reducing bacteria. Microbiology 146:1693-1705. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein, J. 1985. Confidence limits of phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 7.Felske, A., H. Rheims, A. Wolterink, E. Stackebrandt, and A. D. Akkermans. 1997. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143:2983-2989. [DOI] [PubMed] [Google Scholar]
- 8.Forney, L. J., Zhou, X., and C. J. Brown. 2004. Molecular microbial ecology: land of the one-eyed king. Curr. Opin. Microbiol. 7:210-220. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara, H., K. Fujiwara, and K. Hashimoto. 1995. PCR with deoxyinosine-containing primers using DNA polymerases with proofreading activity. PCR Methods Appl. 4:239-240. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, M. C., T. Tolker-Neilson, M. Givskov, and S. Molin. 1998. Biased 16S rDNA PCR amplication caused by interference from DNA flanking template region. FEMS Microbiol. Ecol. 26:141-149. [Google Scholar]
- 11.Kilpatrick, D. R., B. Nottay, C.-F. Yang, S.-J. Yang, M. N. Mulders, B. P. Holloway, M. A. Pallansch, and O. M. Kew. 1996. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J. Clin. Microbiol. 34:2990-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, S., K. Tomura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 13.Kwok, S., D. E. Kellogg, N. McKinney, D. Spasic, L. Goda, C. Levenson, and J. J. Sninsky. 1990. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 18:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahav, R., P. Fareleira, A. Nejidat, and A. Abeliovich. 2002. The identification and characterization of osmotolerant yeast isolates from chemical wastewater evaporation ponds. Microb. Ecol. 43:388-396. [DOI] [PubMed] [Google Scholar]
- 15.Lane, D. J., B. Pace, G. J. Olsen, D. A. Stahlt, M. L. Sogint, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maidak, B. L., G. J. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey, and C. R. Woese. 1997. The RDP (Ribosomal Database Project). Nucleic Acids Res. 25:109-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, F. H., M. M.Castro, F. Aboul-ela, and I. Tinoco, Jr. 1985. Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 13:8927-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Laurent, F., L. Philippot, S. Hallet, R. Chaussod, J. C. Germon, G. Soulas, and G. Catroux. 2001. DNA extraction from soils: old bias for new microbial diversity analysis methods. Appl. Environ. Microbiol. 67:2354-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polz, M. F., and C. M. Cavanaugh. 1998. Bias in template-to-product ratios in multitemplate PCR. Appl. Environ. Microbiol. 64:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossolini. G. M., S. Cresti, A. Ingianni, P. Cattani, M. L. Riccio, and G. Satta. 1994. Use of deoxyinosine-containing primers vs degenerate primers for polymerase chain reaction based on ambiguous sequence information. Mol. Cell. Probes 8:91-98. [DOI] [PubMed] [Google Scholar]
- 21.Saito, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Sarkar, G., J. Cassady, C. D. K. Bottema, and S. S. Sommer. 1990. Characterization of polymerase chain reaction amplification of specific alleles. Anal. Biochem. 186:64-68. [DOI] [PubMed] [Google Scholar]
- 24.Sekiguchi, Y., Y. Kamagata, K. Syutsubo, A. Ohashi, H. Harada, and K. Nakamura. 1998. Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 144:2655-2665. [DOI] [PubMed] [Google Scholar]
- 25.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 26.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe, K., Y. Kodama, and S. Harayama. 2001. Design and evaluation of PCR primers to amplify 16S ribosomal DNA fragments used for community fingerprinting. J. Microbiol. Methods 44:253-262. [DOI] [PubMed] [Google Scholar]
- 28.Watkins, N. E., Jr., and J. SantaLucia, Jr. 2005. Nearest-neighbor thermodynamics of deoxyinosine pairs in DNA duplexes. Nucleic Acids Res. 33:6258-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White, B. A. 1993. PCR protocols: current methods and applications. Humana Press, Totowa, N.J.