Abstract
Cladobotryum spp. are responsible for cobweb disease of mushrooms. In two commercial and one experimental mushroom-growing room, Cladobotryum conidia were released into the air in direct response to physical disturbance of disease colonies during either crop watering or treatment by covering with salt to 10 mm. Conidia were detected using a Burkard spore trap or agar-based trap plates. A maximum concentration of ∼25,000 conidia m−3 was recorded in a small (75-m3) experimental growing room in the hour following the salting of 16 cobweb patches (0.55 m2). Concentrations of 100 and 40 conidia m−3 were recorded in the two larger commercial growing rooms in the hour following the salting of 18 and 11 patches of cobweb (diameter, approximately 50 to 200 mm), respectively. In controlled experiments, disturbed conidia were dispersed rapidly throughout a small growing room, with 91 to 97% of conidia settling out within 15 min. Eighty-five percent of conidia settled out within a 0.5-m radius when air-conditioning fans were switched off, consistent with airborne spore dispersal. Alternative methods for treating diseased areas to minimize conidial release and distribution were investigated and included covering disease colonies with damp paper tissue prior to salt application (tissue salting) and holding a dust extractor above disease colonies during salt application. Both methods resulted in no detectable airborne conidia, but the tissue paper salting technique was more convenient. Prevention of airborne conidial release and distribution is essential to avoid mushroom spotting symptoms, secondary colonies, and early crop termination.
Cobweb disease of the cultivated mushroom Agaricus bisporus (L) Sing. is caused by several species of Cladobotryum (13, 20) and is found in all mushroom-growing countries worldwide (5, 11, 14). The fungus produces a circular colony on the surface of the growing medium, enveloping any mushrooms in its path. Disease colonies produce masses of conidia (approximately 21 by 9 μm) that cause brown spotting symptoms if they land on mushroom caps and will reduce the quality and value of the product. During the mid-1990s, cobweb disease was considered the most problematic disease affecting mushroom cultivation in the United Kingdom and Ireland, with crop losses of up to 40% recorded due to severe spotting symptoms and early crop terminations at the height of the epidemic (7, 10). Many of the Cladobotryum isolates collected at that time were resistant to the benzimidazole fungicides previously used to control the disease, which increased the need for good hygiene measures to prevent and contain the disease (13, 19).
Conidial dispersal is a key factor in the spread of diseases within crops and is affected by water splash from rain or irrigation water and also by wind and air currents (16, 17, 21, 22). The environment in a mushroom-growing room is very uniform and considerably different from that in greenhouses or outdoors, since the crop is grown indoors, in closed rooms, under very specific conditions of temperature, humidity, and air speed, delivered through an air-conditioning system (2, 15). Growing rooms usually are either steamed or disinfected with approved biocides prior to each new crop, and terminated crops are usually killed either with steam or with an approved biocide. The air supply is filtered on intake to reduce the possibility of pathogens entering newly filled growing rooms. Disease outbreaks are associated primarily with either contaminated substrates or poor crop and farm hygiene.
Studies of the conidial dispersal of mushroom pathogens in the commercial mushroom environment are scarce. Gandy (6) showed that Verticillium malthousei conidia could be introduced into mushroom crops by contaminated airborne dust and could be locally dispersed from diseased areas by water splash. Dar (3) found that the spread of cobweb disease over short distances was affected by air movement, water splash, and water runoff.
The inadequacy of existing hygiene treatments for the control of cobweb disease in particular became apparent when, in Britain and Ireland, outbreaks of benzimidazole-resistant Cladobotryum failed to respond to hygiene measures (9, 13). Disease levels continued to rise within crops even when all of the cobweb colonies had been killed with salt and where hygiene practices had been implemented. The epidemiology and etiology of cobweb disease had not been extensively studied up to this time, although it was generally thought that cobweb disease was transmitted and spread by airborne conidia (3, 8, 9, 12, 18, 23, 24). There was a need to know how and when Cladobotryum conidia were moving within mushroom houses and how their distribution throughout growing rooms could be prevented.
Our original hypothesis was that some cobweb colonies were undetected and untreated prior to watering of the crop between flushes and that the dry airborne conidia were dislodged by watering and thereby spread the disease. As the study progressed, it became apparent that conidia were distributed throughout the growing room more rapidly and more uniformly than had been anticipated. We subsequently tested the hypothesis that the air circulation system in the growing rooms facilitated the rapid and uniform distribution of conidia. Finally, the ultimate objective was to develop an effective control measure for treating benzimidazole-resistant Cladobotryum strains, given the significant economic losses cobweb disease can inflict.
MATERIALS AND METHODS
Fungal culture.
A thiabendazole-resistant Cladobotryum strain, isolate 192B1 (IMI 372795), was used in these studies. Fresh cultures were prepared on 2% malt extract agar medium when required. The isolate was obtained from a mushroom farm in West Sussex, United Kingdom, in 1995, when cobweb disease was widespread in commercial mushroom farms (13). This strain originally was identified as Cladobotryum dendroides (Bull) W. Gams & Hooze. (type II) based on morphological characters, but genetic analysis suggests that it is more appropriately assigned to Cladobotryum mycophilum (Oudem.) W. Gams & Hooze., even though some morphological characters are not consistent with the published descriptions of that species (1, 20).
Spore trapping plates.
Agar spore trapping plates contained Oxoid (Basingstoke, United Kingdom) malt extract agar plus 100 μg ml−1 thiabendazole. Benzimidazole-resistant Cladobotryum conidia from isolate 192B1 can germinate and grow on this medium, but other fast-growing airborne fungi (e.g., Trichoderma spp.) found as contaminants cannot.
Burkard spore trapping.
A Burkard 7-day recording volumetric spore trap (Burkard Manufacturing Co. Ltd., Rickmansworth, United Kingdom) was used to detect the presence of Cladobotryum conidia in the growing rooms. The trap draws air through a 2- by 14-mm aperture, over a strip of adhesive cellophane tape (336 by 19 mm) attached to a cylinder that revolves once in 7 days (2 mm/h). The air flow was maintained at 10 liters/min. In all experiments the trap was positioned on the floor at the back of the house, away from the access door. Every 7 days, the recording tape was replaced with a fresh piece. Periodic marking of the recording tape through the aperture of the spore trap allowed verification of chronological accuracy. The recording tape was cut into seven daily sections (48 by 19 mm), permanently mounted, and microscopically examined. The mounting gel contained 100 ml distilled water, 50 ml glycerol, 35 g polyvinyl alcohol, and 2 g phenol.
Conidia were counted at 400× magnification along a series of 0.5-mm-wide passes of the 48-mm-long daily section. Each pass counted the number of Cladobotryum conidia in every 2-mm (1-h) section for the duration of an experiment. Several passes were examined per daily section, but the number of passes depended on the density of the conidial deposit. Where there were few conidia present, a greater number of passes was examined. A minimum of three passes per daily section were counted. The average number of conidia trapped for each hour was then calculated and converted into the number of conidia per cubic meter of air.
Mushroom production.
Experiments were conducted at the Warwick HRI experimental mushroom unit. The mushroom substrate, containing Agaricus bisporus inoculum or “spawn” (Sylvan A12 strain), was placed in 260-mm-diameter pots. The pots were placed on aluminum wire shelving (3 m long by 1.3 m wide and 2 m high), with three shelves 0.55 m apart, in a small growing room measuring 5.4 m long by 4 m wide and 4 m high at the apex. The total volume of the room was 75 m3. In most cases, the wire shelves were lined with polyethylene to prevent water runoff onto the lower shelves during watering. The pots were incubated at 25°C and 90% rH. When the substrate was fully colonized by A. bisporus mycelium, each pot was covered to a depth of 50 mm with a peat and sugar beet lime “casing” soil in preparation for fruit body development. The pots were maintained at 25°C and 90% rH for an additional 7 days, during which time they were drenched daily with 2 to 4 liters of water/m2. Between 7 and 10 days after casing, the temperature was slowly dropped to 18°C and 85% rH by the introduction of filtered fresh air through the air-conditioning system to stimulate fruit body initiation. These environmental conditions were maintained until the crop was terminated, usually ∼35 days after casing. Pots were watered with 2 to 4 liters/m2 per day for 2 to 3 days after the first flush of mushrooms was harvested and again after the second flush of mushrooms was harvested.
The controlled environment was maintained by continuous circulation of conditioned air delivered into the growing room through a central overhead polyethylene duct that ran the length of the room. The duct was perforated at 0.5-m intervals along both sides with holes (diameter, 50 mm). Conditioned air exited the holes in the direction of the side walls at an initial speed of 1.2 to 1.5 m s−1 and was eventually deflected downward and across the shelves at a speed of ∼0.1 m s−1. When the harvest of the crop was completed, the room was steamed out at 70°C (compost temperature) for 12 h.
Inoculation with Cladobotryum.
Pots of mushroom substrate were inoculated with Cladobotryum isolate 192B1 12 days after casing, by which time the A. bisporus mycelium had begun to produce mushroom initials on the casing surface. Three mycelial plugs (5 mm diameter) were taken from the edge of a fresh Cladobotryum culture, placed in the center of a pot, 15 to 20 mm beneath the casing layer surface, and covered with casing soil. Cobweb colonies were well developed within 8 to 10 days after inoculation, by which time the diameters of colonies ranged from 150 to 250 mm.
Statistical methods.
Where appropriate, the means and standard errors of conidial data were calculated and Student's t test was used to determine if the difference between two means was significant.
Conidial dispersal within an infected mushroom crop.
Cobweb-infected crops in separate growing rooms at a commercial mushroom farm were studied. The large growing rooms were 15.2 m long, 5.5 m high at the apex, and either 9.1 m or 14.6 m wide. The total volumes of the rooms were 570 and 915 m3, respectively. They each contained eight rows of mushroom-substrate-filled wooden trays stacked five high on either side of a central access area. A Burkard 7-day recording volumetric spore trap was placed on the floor at the far end of the room. Over a period of 7 days, cobweb patches, measuring 50 to 200 mm in diameter, were identified and treated with salt. The salt treatment covered disease patches with fine-grade table salt to a depth of ∼10 mm and to ∼30 mm beyond the edge of the colony. The crops were watered as required and the mushrooms picked as necessary.
A similar study of conidial dispersal was conducted at the Warwick HRI experimental mushroom unit. Conidial release data were obtained from an experiment testing the hypothesis that the casing matric potential and the growth of Cladobotryum were correlated (1). In that experiment, eight matric potential treatments were applied. Half the pots were inoculated with Cladobotryum on day 12 after casing, and half were left as uninoculated controls. Each treatment combination was replicated six times to give 96 pots in total. The treatments were arranged on three shelves in a mushroom-growing room according to a Trojan square design. After the first flush of mushrooms was harvested, all of the visible cobweb colonies were covered with salt as described above, prior to watering of the crop between flushes. Mushrooms were harvested and scored as healthy or diseased, i.e., with brown spots or lesions due to Cladobotryum infection. Burkard spore trap data were collected for 3 weeks following inoculation of the pots.
Spatial dispersal of Cladobotryum conidia following controlled release from a single source.
A single pot of mushroom substrate with a 150-mm-diameter cobweb colony was placed on the floor in a clean mushroom-growing room in front of a 3-tier shelving unit. Spore trap plates were positioned on each shelf at various positions along the length and width of the shelves. In addition, a pot of disease-free mushroom substrate with small developing mushrooms was placed at each of the four corners of each shelf and halfway along each side (six pots per shelf). The air-conditioning system within the mushroom-growing room was set to provide an air speed of ∼ 0.1 m s−1 across the shelves.
Conidia were released from the single Cladobotryum colony by watering over it from a height of 300 mm, using a watering lance calibrated to dispense 40 ml of water in 4 s. After the Cladobotryum colony was disturbed, fresh spore trap plates were exposed every 15 min for 3 h following the conidial disturbance, generating 12 trap plates for each position. Trap plates were incubated at 25°C, and Cladobotryum colonies were identified and recorded for each position. Mushrooms from the pots of substrate were assessed for spotting symptoms as they developed. This experiment was conducted twice.
Effect of air circulation on conidial dispersal patterns.
A single pot of mushroom substrate with a 130-mm-diameter cobweb colony was placed in the center of the middle shelf of a clean growing room. Spore trap plates were put into position at 0.25, 0.5, 1.0, and 1.5 m from the cobweb colony down the center of the middle shelf, and four further plates were put on each of the top, middle, and bottom shelves at the front, back and two side locations. The cobweb colony was watered as described above to release conidia into the air. Spore trap plates were changed every 15 min within a 60-min period. This experiment was performed three times with the air-conditioning system set for standard mushroom-growing conditions and three times with the air-conditioning fans switched off, with a gap of 4 h between each two experiments. Exposed trap plates were incubated at 25°C and the number of Cladobotryum colonies counted for each position.
Effects of different cobweb disease treatments on conidial dispersal patterns.
Four different cobweb disease treatment techniques were studied to determine their influence on the dispersal of Cladobotryum conidia. (i) In salting, cobweb colonies are covered with fine-grade domestic salt to a depth of ∼10 mm and to ∼30 mm beyond the edge of the colony. (ii) In tissue salting, cobweb colonies are first covered with a strong damp paper tissue or paper towel (e.g., a paper kitchen roll or paper hand towels) to ∼30 mm farther in all directions than the colony area, and salt is then applied around the edges of the paper tissue to seal in the diseased area before the whole central area of the tissue is finally covered with salt. (iii) In salting and air extraction, salting is conducted as in technique i, but during treatment the air above the salted area is drawn through a hazardous dust extractor (Draper, Eastleigh, Hants., United Kingdom). (iv) In tissue salting and air extraction, tissue salting is conducted as in technique ii, but during treatment the air above the salted area is drawn through a hazardous dust extractor as in technique iii.
An additional treatment was superimposed on the four disease treatment techniques; this consisted of the standard air-conditioning system in the mushroom-growing room being switched on (fans on) or off (fans off) in order to determine the effect of air movement within the room on the pattern of conidial dispersal during disease treatment operations.
For each treatment combination, a fresh pathogen colony, with a diameter of 160 to 165 mm, was positioned in the center of the middle shelf of the three-tiered, polyethylene-lined shelving unit in the growing room. Trap plates were arranged along the length and width of the middle shelf only. One set of trap plates was exposed for 20 min following each disease treatment. A period of 2 h was left between the different disease treatments (these times were known to be satisfactory from the results of the previous experiments). Each treatment combination was replicated three times.
RESULTS
Conidial dispersal within an infected mushroom crop.
Cladobotryum conidia were detected by the Burkard spore trap in the cobweb-infected commercial mushroom-growing rooms at very specific times during the 7-day experimental period. The smaller room had more severe cobweb disease, with 17, 18, and 12 patches of cobweb salted on days 2, 3, and 4, respectively, while in the larger room, cobweb disease was less severe, with 11, 12, and 8 patches salted in the same period. The crop in the smaller room was terminated early due to continued high levels of disease. Conidial peaks usually occurred when cobweb patches were salted or crops were watered. Conidia were detected more frequently, and at higher concentrations, in the smaller room (14 conidial peaks ranging from 20 to 100 conidia m−3) than in the larger (six conidial peaks ranging from 20 to 40 conidia m−3). Few to no conidia were detected for long periods when the crops were being harvested or at night time.
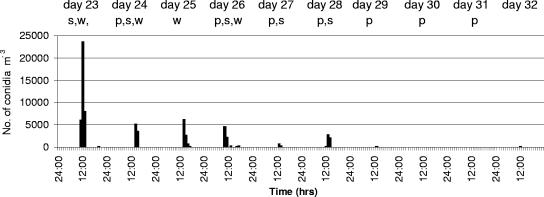
In the controlled experiment at the Warwick HRI experimental mushroom unit, a similar pattern was observed, with peaks of Cladobotryum conidia occurring only when diseased areas were salted or the crop was watered (Fig. 1). Conidial concentrations in the air reached a peak of just under 25,000 conidia m−3 on the day when the first patches of cobweb were salted (16 patches with an area of 0.55 m2). Conidial concentrations dropped to highs of ∼5,000 conidia m−3 following subsequent salting and watering operations, and, as in the commercial rooms, there were long periods when no conidia were detected in the air. Brown spotting symptoms were present on 21% of the mushrooms from the first flush, before any salting of diseased areas was done, while 100% of the second flush mushrooms developed spotting symptoms. The crop was terminated at this point, although normally a third flush would also be harvested.
FIG. 1.
Concentrations of Cladobotryum conidia in the air on an hourly basis in a cobweb-infected mushroom crop from day 23 to day 32 after casing as detected by a Burkard spore trap. Letters across the top of the graph indicate the operations carried out in the room on each day: s, salting event; w, watering event; p, mushroom picking.
Conidial dispersal following a controlled release of Cladobotryum conidia from a single source.
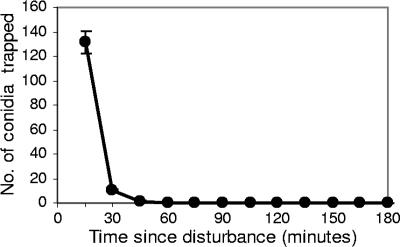
Cladobotryum conidia were distributed fairly uniformly on all three shelves throughout the mushroom-growing room following the controlled disturbance of a cobweb colony at one end of the room by watering (Table 1). Conidia settled out very rapidly, with 91% settling out within the first 15 min, 98% settling after 30 min, and almost 100% after 45 min (Fig. 2). An occasional conidium was detected for up to 2.5 h after disturbance. Pots of mushroom substrate placed on shelves at various locations in the room all produced mushrooms with 2 to 5 brown lesions per 100 mm2 of the mushroom surfaces. The concentration of conidia in the air ranged from 2,800 to 3,300 conidia m−3 in the hour following the disturbance of the cobweb colony, but the Burkard spore trap was not sensitive enough to detect changes over a short (<1-h) time interval. The trap plates, exposed at 15-min intervals, were more sensitive and gave a more accurate representation of the Cladobotryum conidial load in the air immediately following the disturbance of the colony.
TABLE 1.
Average numbers of Cladobotryum conidia on trap plates on different shelves in a mushroom-growing room and at various distances from a Cladobotryum colony disturbed by wateringa
| Shelf | Location on shelf | Avg no. of conidia at the following distance from the Cladobotryum colony:
|
||
|---|---|---|---|---|
| 0.75 m | 1.95 m | 3.2 m | ||
| Top | Left | 157 | ||
| Center | 210 | 141 | 159 | |
| Right | 152 | |||
| Middle | Left | 132 | ||
| Center | 135 | 121 | 129 | |
| Right | 124 | |||
| Bottom | Left | 137 | ||
| Center | 163 | 155 | 130 | |
| Right | 123 | |||
Values are means from two experiments and are not significantly different by analysis of variance.
FIG. 2.
Average number of Cladobotryum conidia per trap plate settling out at 15-min intervals following a controlled disturbance of a sporulating cobweb colony by watering. Each point is the mean ± standard error for 30 plates per time interval.
Effect of air circulation on conidial dispersal patterns.
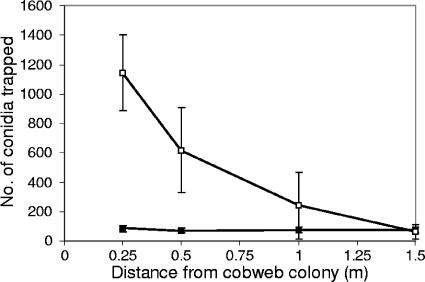
When a Cladobotryum colony was positioned in the center of the middle shelf of a growing room and then watered, more conidia were trapped on the shelf containing the pathogen than on the other shelves. An even greater number of conidia were trapped on the shelf containing the pathogen when the air-conditioning fan was switched off. The majority of conidia (97%) settled within 15 min, whether or not the fan was operating, confirming the results obtained in the previous experiment. Conidial dispersal decreased rapidly with increasing distance from the source when the fans were off (still air), with 85% deposited within a radius of 0.5 m, but conidia were uniformly distributed along the shelf when the air-conditioning fan was switched on (Fig. 3). The concentration of conidia in the hour following the controlled release was 1,500 conidia m−3 when the air conditioning fans were on and 600 conidia m−3 when the fans were off, but the difference was not significant (Student's t test on square root-transformed data) due to a large variance in the number of conidia recorded for each experiment.
FIG. 3.
Number of Cladobotryum conidia settling out on trap plates with increasing distance from a point source following the controlled disturbance of a sporulating cobweb colony located at the center of the middle shelf, and with the air-conditioning fan switched on (▪) or off (□). Values are means ± standard errors from three replicate experiments.
Effects of disease treatment methods on conidial dispersal patterns.
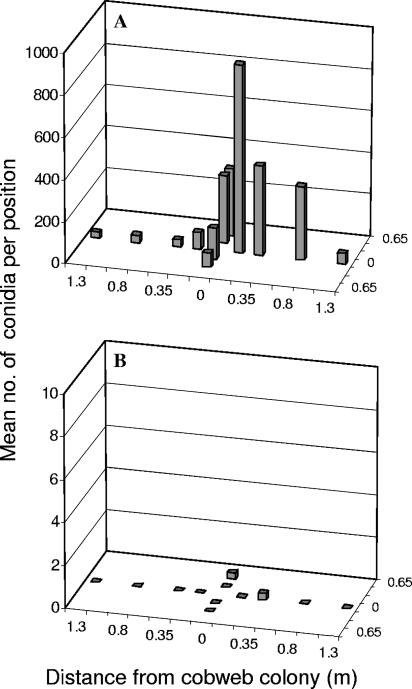
Applying salt to kill a sporulating colony of Cladobotryum resulted in the release of a large number of conidia and their dispersal along the length and width of the shelf, primarily in the direction in which the salt was applied, i.e., left to right and front to back. If the colonies were covered with damp paper tissue prior to salting, conidial release was almost completely prevented (Fig. 4). The use of a dust extractor while salting without damp tissues was as effective as tissue salting alone, but using the dust extractor while salting is a very cumbersome task. If the fans were switched off during the salting operation, the number of conidia and their dispersal were both reduced by ∼75%, but a considerable number of conidia (1 to 20 per trap plate) were still detected at 1.3 m from the treated colony.
FIG. 4.
Numbers of Cladobotryum conidia (vertical bars) settling out on trap plates along the length (eight positions) and width (four positions) of a growing shelf following the treatment of a centrally located cobweb colony with different salting techniques. (A) Salting; (B) salting plus paper tissue. Data are means from three replicate experiments. Note the different y-axis scales and the many zero values in panel B.
DISCUSSION
In a number of cobweb-infected mushroom crops, the release of Cladobotryum conidia from sporulating colonies occurred consistently at times when colonies were physically disturbed by either salting or watering. No other movements or operations within the mushroom house, e.g., the daily picking of the mushrooms, were associated with any significant release of conidia. Dar (3) showed that only a small number of Cladobotryum conidia were dislodged from a sporulating colony by an electric fan, and then only to a distance of 0.6 m. Thus, only the physical disturbance of a Cladobotryum colony results in the release of significant numbers of conidia.
Once airborne, Cladobotryum conidia were transported throughout the mushroom-growing rooms by the constant air circulation systems. In experiments with spore trap plates, 91 to 97% of the Cladobotryum conidia that became airborne following disturbance of a single colony by watering settled out within 15 min. Within that 15-min interval, they were dispersed to the maximum distance evaluated (3 m). In commercial mushroom houses, as in the experimental mushroom house at Warwick HRI, there usually are four to eight complete air changes per hour, depending on the design of the air-handling system, the volume of the growing rooms, and the area of the crop. In large commercial growing rooms, therefore, Cladobotryum-laden air could be completely recirculated through the air-handling system within 15 min. This rapid and uniform distribution of conidia throughout growing rooms following the disturbance of a cobweb colony explains why spotting symptoms were severe during the 1994-to-1995 epidemic in the United Kingdom and Ireland relative to the amount of cobweb growth on the mushroom beds (7) and also helps explain the high level of spotting symptoms reported in some cobweb experiments (12). Switching the air-circulating fans off in the mushroom house reduced the distribution of conidia to the area near the source, producing a steep conidial deposition gradient with respect to distance from the inoculum source. Similar gradients have been reported for spores that are splash dispersed in still air (4, 22). Thus, switching fans off during watering and disease treatment operations could be a useful strategy to adopt if there were insufficient time or personnel available to treat disease areas before watering a crop.
The release of dry airborne Cladobotryum conidia into the air following watering events was expected, but the high level of conidial release resulting from salt application to diseased areas was unexpected. Salting is a standard technique used during mushroom cultivation to treat areas of disease caused by pathogens such as Verticillium, Mycogone, and Trichoderma spp. (5). However, these pathogens have conidia that are held together in mucilage and that are more likely to adhere to salt and water droplets if they are disturbed by salting or watering than they are to become airborne. In hindsight, perhaps it should have been obvious that the physical application of salt to Cladobotryum colonies would disturb the dry conidia and make them airborne. Since salting was a major cause of the spread of cobweb disease, an alternative method to treat disease colonies was needed. Since the physical contact of the salt dropping onto the colony was causing the conidia to be released, we tested the hypothesis that covering the colony with damp paper tissue prior to salting, or extracting the air above a colony while salting, would reduce the numbers of conidia being released into the air. Both techniques effectively prevented the conidia from becoming airborne, but the tissue paper method is much easier to execute. Further work is needed to establish how widely conidia would be dispersed from a single point source by air-handling systems in a commercial-size growing room, but circumstantial evidence obtained during the course of these experiments from a medium-sized (9.5 m by 7 m) room suggests that they would be disseminated to a considerable distance. Effective control of cobweb disease will therefore depend on rapid identification and tissue salting of initial outbreaks of the disease within a crop. Reports from industry to the corresponding author indicate that where this strategy is currently in use, it is very effective.
This work has provided data for the temporal and spatial distribution of fungal propagules within a contained and controlled environment. It has clearly demonstrated that dry fungal conidia are easily and rapidly spread via air-handling systems with air speeds as low as 0.1 m s−1. This work is highly relevant to analogous situations where fungal cultures may be found in similar contained environments with air-handling systems, such as greenhouses, animal-rearing units, postharvest storage facilities for food crops and animal feed, hospital laboratories and wards, and commercial food preparation areas. Inadvertent disruption of sporulating fungal colonies in any of these facilities could occur for a variety of reasons and would result in rapid and widespread dispersal of conidia within the facility. The tissue-salting technique described here, or a similar containment-without-disturbance method, would be suitable for treating discrete fungal cultures that may occur, for example, in greenhouses or on foodstuffs; however, treatment of fungal cultures that occur in moist water films inside air-handling ducts would be more difficult. Further work is required to determine the spread of fungal spores with different characteristics such as size, surface charge, and mucilage under similar conditions.
Acknowledgments
This work was funded by the Horticultural Development Council of the United Kingdom.
We thank Andrew Mead for advice on experimental design and statistical analyses, Neil Willoughby for assistance with growing mushroom crops, and Richard Gaze for help and advice on the industry perspective of mushroom pathology.
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Adie, B. A. T. 2000. The biology and epidemiology of the cobweb disease pathogen (Cladobotryum spp.) infecting the cultivated mushroom (Agaricus bisporus). Ph.D. thesis. University of London, London, United Kingdom.
- 2.Arkenbout, J. 1988. Air-conditioning as a technical procedure, p. 145-177. In L. J. L. D. Van Griensven (ed.), The cultivation of mushrooms. Darlington Mushroom Laboratories Ltd., Sussex, United Kingdom.
- 3.Dar, G. M. 1997. Studies on the dispersal of cobweb disease of cultivated white button mushroom. Res. Dev. Reporter 14:43-48. [Google Scholar]
- 4.Fitt, B. D. L., P. J. Walklate, H. A. McCartney, A. Bainbridge, N. F. Creighton, J. M. Hirst, M. E. Lacey, and B. J. Legg. 1986. A rain tower and wind tunnel for studying the dispersal of plant pathogens by rain and wind. Ann. Appl. Biol. 109:661-671. [Google Scholar]
- 5.Fletcher, J. T., P. F. White, and R. H. Gaze. 1989. Mushrooms—pest and disease control. Intercept, Newcastle upon Tyne, United Kingdom.
- 6.Gandy, D. G. 1972. Observations on the development of Verticillium malthousei in mushroom crops and the role of cultural practices in its control. Mushroom Sci. 8:171-181. [Google Scholar]
- 7.Gaze, R. H. 1995. Dactylium or Cobweb. Mushroom J. 546:23-24. [Google Scholar]
- 8.Gaze, R. H. 1995. Dactylium or Cobweb II. Mushroom J. 548:13. [Google Scholar]
- 9.Gaze, R. H. 1995. Dactylium or Cobweb, in conclusion. Mushroom J. 549:26. [Google Scholar]
- 10.Gaze, R. H. 1996. The past year. Mushroom J. 552:24-25. [Google Scholar]
- 11.Geels, F. P., J. van de Geijn, and A. J. Rutjens. 1988. Pests and diseases, p. 361-422. In L. J. L. D. Van Griensven (ed.), The cultivation of mushrooms. Darlington Mushroom Laboratories Ltd., Sussex, United Kingdom.
- 12.Grogan, H. 2006. Fungicide control of mushroom Cobweb disease caused by Cladobotryum strains with different benzimidazole-resistance profiles. Pest Manag. Sci. 62:153-161. [DOI] [PubMed] [Google Scholar]
- 13.Grogan, H., and R. H. Gaze. 2000. Fungicide resistance among Cladobotryum spp.—causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol. Res. 104:357-364. [Google Scholar]
- 14.Harvey, C. L., P. J. Wuest, and L. C. Schisler. 1982. Diseases, weed molds, indicator molds and abnormalities of the commercial mushroom, p. 19-33. In P. J. Wuest and G. D. Bengston (ed.), Penn State handbook for commercial mushroom growers. The Pennsylvania State University, State College.
- 15.Hermens, C. 1988. Climate and cultivation technique, p. 213-248. In L. J. L. D. Van Griensven (ed.), The cultivation of mushrooms. Darlington Mushroom Laboratories Ltd., Sussex, United Kingdom.
- 16.Holb, I. J., B. Heijne, J. C. M. Withagen, and M. J. Jeger. 2004. Dispersal of Venturia inequalis ascospores and disease gradients from a defined inoculum source. Phytopathology 152:639-646. [Google Scholar]
- 17.Ingold, C. T. 1971. Fungal spores: their liberation and dispersal. Clarendon Press, Oxford, United Kingdom.
- 18.Lane, C. R., R. C. Cooke, and L. J. Burden. 1991. Ecophysiology of Dactylium dendroides—the causal agent of cobweb mould, p. 365-372. In T. J. Elliott (ed.), Science and cultivation of edible fungi, vol. 1. Proceedings of the 14th International Congress on the Science and Cultivation of Edible Fungi. Balkema, Rotterdam, The Netherlands. [Google Scholar]
- 19.McKay, G. J., D. Egan, E. Morris, and A. E. Brown. 1998. Identification of benzimidazole resistance in Cladobotryum dendroides using a PCR-based method. Mycol. Res. 102:671-676. [Google Scholar]
- 20.McKay, G. J., D. Egan, E. Morris, C. Scott, and A. E. Brown. 1999. Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Appl. Environ. Microbiol. 65:606-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meredith, D. S. 1973. Significance of spore release and dispersal mechanisms in plant disease epidemiology. Annu. Rev. Phytopathol. 11:313-342. [Google Scholar]
- 22.Pedersen, E. A., R. A. A. Morrall, H. A. McCartney, and B. D. L. Fitt. 1994. Dispersal of conidia of Ascochyta fabae f. sp. lentis from infected lentil plants by simulated wind and rain. Plant Pathol. 43:50-55. [Google Scholar]
- 23.Sinden, J. W. 1971. Ecological control of pathogens and weed-molds in mushroom culture. Annu. Rev. Phytopathol. 9:411-432. [Google Scholar]
- 24.Sinden, J. W., and E. Hauser. 1953. Nature and control of three mildew diseases of mushrooms in America. Mushroom Sci. 2:177-180. [Google Scholar]