Abstract
We analyzed the communities of soil basidiomycetes in agroecosystems that differ in tillage history at the Kellogg Biological Station Long-Term Ecological Research site near Battle Creek, Michigan. The approach combined soil DNA extraction through a bead-beating method modified to increase recovery of fungal DNA, PCR amplification with basidiomycete-specific primers, cloning and restriction fragment length polymorphism screening of mixed PCR products, and sequencing of unique clones. Much greater diversity was detected than was anticipated in this habitat on the basis of culture-based methods or surveys of fruiting bodies. With “species” defined as organisms yielding PCR products with ≥99% identity in the 5′ 650 bases of the nuclear large-subunit ribosomal DNA, 241 “species” were detected among 409 unique basidiomycete sequences recovered. Almost all major clades of basidiomycetes from basidiomycetous yeasts and other heterobasidiomycetes through polypores and euagarics (gilled mushrooms and relatives) were represented, with a majority from the latter clade. Only 24 of 241 “species” had 99% or greater sequence similarity to named reference sequences in GenBank, and several clades with multiple “species” could not be identified at the genus level by phylogenetic comparisons with named sequences. The total estimated “species” richness for this 11.2-ha site was 367 “species” of basidiomycetes. Since >99% of the study area has not been sampled, the accuracy of our diversity estimate is uncertain. Replication in time and space is required to detect additional diversity and the underlying community structure.
Decomposer fungi are an important component of many terrestrial ecosystems, yet their presence and roles in nutrient cycling, plant succession, soil stabilization, and other ecosystem processes are often underrecognized (15, 21). We use the terms saprotrophic and saprobic for these organisms, in preference to saprophytic, since the latter refers to plants that feed on dead matter—although true saprophytes probably do not exist (29). Saprotrophic fungi include members of most phyla, but members of the phylum Basidiomycota (informally, basidiomycetes) are the main decomposers of recalcitrant components of plant litter through the production of lignin-modifying enzymes, such as lignin peroxidases, manganese-dependent peroxidases, and laccases (46). Approximately 8,500 described species of basidiomycetes are lignocellulose-degrading saprotrophs (24), and about half of these occur in soil and on fallen plant litter. Soil-inhabiting basidiomycetes also include a few important plant pathogens, such as Rhizoctonia (49), and at least 4,500 species that form mutualistic associations known as ectomycorrhizae with roots of vascular plants (24). Thus, approximately 9,000 described species of basidiomycetes are terrestrial, but how many might occur in any one locality is not well-known outside Europe.
Diversity estimates of soil-inhabiting basidiomycetes are usually based on surveys of their ephemeral fruiting bodies (“mushrooms”) (39, 62), since these fungi are difficult to isolate from soil and culture (59). Thus, detection and accurate identification of all members of the soil basidiomycete community have been difficult. DNA-based surveys of microbial communities provide a way of examining species composition without culturing and can potentially provide a more complete picture of mixed environmental samples (3, 16, 25, 44). Gardes and Bruns (19) used basidiomycete-specific primers to identify basidiomycetes from ectomycorrhizal roots and discovered a different community than the community known from above-ground fruiting bodies. However, most molecular studies of microbial community diversity have focused on prokaryotes.
Previous sequence-based surveys of fungal diversity in forested and nonforested ecosystems have usually been limited in scope and have detected only limited genetic and species diversity among soil basidiomycetes (4, 23, 26, 28, 50, 60). In a high-throughput study of fungi from forest soil and litter in North Carolina, O'Brien et al. (40) identified 412 sequence types among 863 internal transcribed spacer (ITS) sequences of fungi, 41% of which represented basidiomycetes. As species-effort curves were linear, they concluded that their samples underestimated the fungal diversity at those sites (40). Clearly, even with molecular techniques, we have not yet fully explored the diversity of soil fungi, especially basidiomycetes.
Basidiomycetes are important in soil ecology, but little is known of their species diversity in grassland and agricultural ecosystems outside of Europe. Of the ∼3,400 species of macrofungi in The Netherlands, 365 are grassland species, and 32 occur in arable fields (6). The macrofungal communities of European grasslands contain 4 to 135 species per plot (which ranged from 1 m2 to >25 ha) in unfertilized grasslands, 16 to 29 species in fertilized grasslands, and 2 to 21 species in weedy communities (6). These studies are based on collections of fruiting bodies, and 20 years or more is needed to approach a complete listing (39). Equivalent studies of grassland fungal communities in North America are lacking. In a culture-based study of lignin-degrading basidiomycetes from Michigan agricultural soils, 67 isolates representing 51 morphotypes were recovered (59), a larger diversity than is commonly found in such agroecosystems (6). This high yield of basidiomycete cultures was accomplished through a particle-washing technique followed by plating on a selective medium. However, the habitats present in the study site were not sampled systematically, and ectomycorrhizal basidiomycetes and some saprotrophic basidiomycetes would not grow using the method and medium chosen (59).
Our objective was to assess the diversity of basidiomycetes in Michigan agricultural soils. Our hypothesis was that a greater diversity would be detected using a method based on selective amplification of basidiomycete DNA than was obtained by a culture-based method. There are no prior intensive studies of the basidiomycete diversity in grassland or agricultural soils. We expect that such studies will lead to the discovery of new species and groups of basidiomycetes and will be of benefit to the fields of soil ecology and agronomy because of the functional importance of basidiomycetes in soil.
MATERIALS AND METHODS
Study site.
The Kellogg Biological Station Long-Term Ecological Research site (KBS-LTER) is located near Battle Creek, Michigan (42° 24′ N, 85° 24′ W; elevation, 288 m). Soils on the main KBS-LTER are Kalamazoo loam (mesic Typic Hapludalfs; http://lter.kbs.msu.edu/Soil/characterization). The presettlement vegetation was an open oak savannah dominated by Quercus alba, Quercus muehlenbergii, and Quercus velutina (13), but there are now a series of replicate plots of varied cropping systems and successional plant communities on land that was farmed commercially until 1989 (http://lter.kbs.msu.edu/experimentalDesign.html).
Soil sample collection and DNA extraction.
One hundred sixty soil cores (2.5-cm diameter × 15-cm depth) were taken in June and October 2002 and stored at 4°C until processed. Five samples came from each of four replicate plots of conventional tillage, no-till, historically tilled succession, and never tilled succession treatments. The combined area of the plots sampled is ∼11.2 ha, of which our sample cores (a total of 785 cm2) represent ∼7 × 10−7. The litter horizon and top 5 cm were excluded to avoid surface effects and fungal species subsisting only on litter decomposing at the soil surface as well as basidiospores carried in the air from distant sources (8, 18) and for comparability with previous studies (59). A maize-soybean-wheat crop rotation is implemented at the KBS-LTER, with maize planted in the year of this study. Soil cores from agricultural treatments (conventional tillage and no-till) were sampled alternately from within and between rows. Subsamples of 10 g were suspended in 150 ml of 0.1 M sodium pyrophosphate, shaken for 5 min and washed with tap water through sieves of 1.18-, 0.25-, and 0.053-mm mesh to remove bacteria and most fungal spores and maximize hyphal yield. The sieves were rinsed with tap water and sterilized with 70% ethanol between samples. DNA was extracted with UltraClean soil DNA kits (Mo Bio Laboratories, Inc., Solana Beach, CA) from 250 μl of washed organic material collected on the 0.053-mm mesh sieve. The 53- to 250-μm size fraction was chosen for study, since previous culture-based studies found the greatest diversity of fungi, including basidiomycetes, in particles of this size range (8, 9). Particle washing removes most bacteria and spores of nontarget ascomycetes and zygomycetes, and the use of sodium pyrophosphate to disperse soil colloids reduces the amount of washing required to achieve this effect (59). Microscopic examination of soil particles washed with sodium pyrophosphate revealed fragments of plant debris, fungal hyphae, sclerotia, and rhizomorphs, but no spores (59). Cells were disrupted by bead beating in a FastPrep FP120 (Bio101; QBiogene, Carlsbad, CA) with four cycles of 30 s at a setting of 4.0, alternating with 5 min on ice.
PCR amplification and TA cloning.
Primers with specificity to conserved sequences in basidiomycetes (Basid-2R+ [5′-TACCGTTGTAGTCTTAACAG-3′] [modified from the primer in reference 54] and Basid001 [5′-GCTTTACCACATAAATCTGA-3′] [58]) were used to reduce amplification of other filamentous fungi, including ascomycetes, zygomycetes, and glomeromycetes. These primers yield DNA fragments ∼2.4 kb in length that span the nuclear ribosomal internal transcribed spacer regions and include ∼1,000 bp of both the nuclear small-subunit ribosomal DNA (nSSU rDNA) and nuclear large-subunit ribosomal DNA (nLSU rDNA) genes. Specificity and phylogenetic coverage of these primers were checked by searching for short nearly exact matches in BLAST (2; http://www.ncbi.nlm.nih.gov/BLAST/) and in comparisons with aligned reference sequences from GenBank.
PCR was performed with 30 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C, with 2 min of preheating at 95°C and a final extension of 7 min at 72°C. Results were inspected visually following agarose gel electrophoresis. Bands of expected size were extracted from the gel using QIAprep Miniprep (QIAGEN Inc., Mississauga, Ontario, Canada) and used as a template for TOPO TA cloning (Invitrogen Corp., Mississauga, Ontario, Canada).
Twelve transformed colonies were picked and subjected to restriction fragment length polymorphism (RFLP) screening following digestion with either RsaI or MspI (Fisher Scientific Ltd., Nepean, Ontario, Canada). RFLPs were compared within, but not between, sets of 12 from each cloning reaction. Clones with a unique RFLP banding pattern within a cloning reaction were sequenced by using the LR3 (61) sequencing primer in a CEQ 8000 capillary DNA analysis system (Beckman Coulter, Fullerton, CA). Sequences yielded ∼650 bp from the 5′ end of the nLSU rDNA gene, including the D1 and D2 divergent domains (21); 18S and ITS regions of the clones were not sequenced. Sequences were tested for chimeras resulting from PCR by using the Chimera Check program (14). KBS-LTER sequences were clustered by using the complete linkage (furthest-neighbor) algorithm in the DOTUR computer package (51) on the KBS-LTER sequences (to assess the number of “species” detected from KBS-LTER) and with named reference sequences from GenBank (to assign identities to the environmental sequences). Sequences with less than 99% nucleotide identity were considered to represent different “species,” following the definitions in other studies of 5′ nLSU rDNA in fungi (30, 33, 47). “Species” accumulation curves and estimates of total “species” richness were calculated with EstimateS (version 7.5; Robert K. Colwell, Department of Ecology and Evolutionary Biology, University of Connecticut [http://purl.oclc.org/estimates]) using the abundance-based coverage estimate nonparametric estimator on the basis of the number of encounters (clones) of each “species.”
Phylogenetic analysis.
Phylogenetic trees were constructed by using all cloned sequences together with all nonredundant nLSU sequences of named basidiomycetes obtained from GenBank. Reference sequences that were phylogenetically related to cloned KBS-LTER sequences were retained, and others were deleted from the final analyses. Sequences were aligned by using ClustalX v.1.81 (57), and the alignments were improved manually. Models of DNA evolution were evaluated with Modeltest v.3.6 (45). Phylogenetic analysis was performed with PAUP* v4.0b10 (55). The neighbor-joining method, using the Tamura-Nei nucleotide substitution model (56), was used to determine general tree topology and clade composition, and bootstrap support was evaluated by using 1,000 replicates. The sequence alignment and tree files have been submitted to TreeBASE (http://www.treebase.org/ as M2839 and S1577, respectively).
Nucleotide sequence accession numbers.
All environmental sequences were deposited in GenBank as accession numbers DQ341602 to DQ342010.
RESULTS
Primer specificity and coverage.
A BLAST search for primer Basid2R+ yielded perfect matches (all 20 bases) in 1,621 nSSU basidiomycete rDNA sequences, including all groups for which sequences are available. Perfect matches were also found in nSSU rDNA sequences from three ascomycetes (Candida humicola, Escovopsis sp., and Taphrina californica), two zygomycetes (Rhizomucor miehei and Rhizomucor pusilus), and the animals Paraphanostoma (Nemertea) and Austropallene (Chelicerata). Several sequences of Geotrichum and Dipodascus (ascomycetes) differ only in having a C instead of a T at base 7 of the primer sequence. The next closest ascomycetes all lack the two bases (AG) at the 3′ end of the primer. A small number of basidiomycetes differ by a single base in the middle of the primer (position 11, 12, or 14). A BLAST search found perfect matches (20 of 20) to primer Basid001 in 1,329 homobasidiomycete nLSU rDNA sequences plus sequences from the zygomycetes Rhizopus, Rhizomucor, and Entomophthora. Perfect matches were found in seven of the eight major clades of homobasidiomycetes (24), whereas members of the cantharelloid clade and the Auriculariales typically have an A instead of a T at base 16 (five bases from the 3′ end), and a few homobasidiomycetes have a single base difference at one of the bases from position 18 to 20. An analysis of aligned reference sequences showed that most other fungi, including the remainder of the heterobasidiomycetes plus the ascomycetes, zygomycetes, glomeromycetes, and chytrids, have a complete match for the primer except at positions 9 (T) and 16 (A). Sequences of rDNA in plants and most animals differ by several bases at each end of both primers.
Species richness.
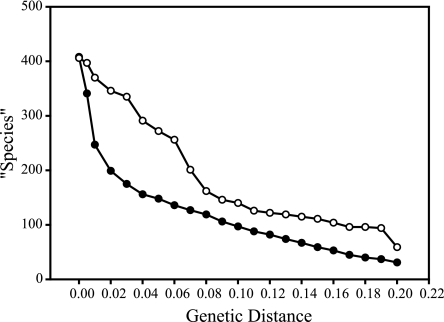
Successful PCR amplifications were obtained from 98 of 160 soil samples, and from these we recovered 1,146 clones, of which 472 had unique RFLP patterns within their cloning reaction. These 472 clones were sequenced, yielding 409 unique basidiomycete sequences, plus 11 sequences from zygomycetes or glomeromycetes and 52 sequences from ascomycetes. Two amplifications yielded only clones of ascomycetes, glomeromycetes, or zygomycetes, and one sample yielded no clones with inserts. When “species” were defined as organisms yielding PCR products with ≥99% identity in the 5′ 650 bases of the nuclear large-subunit ribosomal DNA, 241 “species” were detected among the environmental sequences (Fig. 1). Alternative definitions of ≥98% or ≥97% identity yielded 196 and 176 “species,” respectively. There was greater similarity among sequences from KBS-LTER than between these environmental sequences and reference sequences from GenBank (Fig. 1, lower and upper curves, respectively). The area between the curves indicates diversity that is not represented in GenBank. Only 24 of 241 “species” detected in our survey had matches of ≥99% with known reference sequences in GenBank (Table 1).
FIG. 1.
Clustering of basidiomycete sequences isolated from the Kellogg Biological Station Long-Term Ecological Research site in June and October 2002 with known GenBank sequences (open circles) and among themselves (solid circles). Clustering is based on genetic distance of 650 bases from the 5′ end of the nLSU ribosomal DNA region using the F84 model of DNA evolution and is calculated using the complete linkage (furthest-neighbor assignment) algorithm in the DOTUR computer package (51); “species” are defined as clusters differing by more than the genetic distance indicated at each point on the curves. The area between the two curves indicates diversity not represented in GenBank.
TABLE 1.
Named reference sequences from GenBank with 99% or greater sequence similarity to the environmental sequences recovered in this study and number of times encountered (cloned)
| Named reference sequence from GenBanka | Clade | GenBank accession no. | nb |
|---|---|---|---|
| Entolomasp. | Euagarics | AF261322 | 10 |
| Leptonia carnea | Euagarics | AF261313 | 1 |
| Lepista nuda | Euagarics | AF139963 | 1 |
| Nolanea sericea | Euagarics | AY207197 | 2 |
| Tetrapyrgos nigripes | Euagarics | AF261337 | 2 |
| Psilocybe inquilina | Euagarics | AF261598 | 1 |
| Coprinellus bisporus | Euagarics | AF041523 | 19 |
| Coprinellus domesticus | Euagarics | AY663837 | 3 |
| Coprinopsis cinerea | Euagarics | AF041494 | 4 |
| Mycena olivaceomarginata | Euagarics | AY207255 | 2 |
| Agaricus campestris | Euagarics | AF059221 | 8 |
| Fomes fomentarius | Polypores | DQ208420 | 3 |
| Irpex lacteus | Polypores | AF139961 | 6 |
| Uthatobasidium fusisporum | Ceratobasidiales | AF518664 | 10 |
| Waitea circinata | Ceratobasidiales | AY885164 | 1 |
| Cryptococcus luteolus | Tremellales | AF075482 | 1 |
| Cryptococcus podzolicus | Tremellales | AF459670 | 2 |
| Cryptococcus tephrensis | Tremellales | DQ000318 | 1 |
| Trichosporon dulcitum | Trichosporonales | AF075517 | 2 |
| Cryptococcus macerans | Cystofilobasidiales | AF459665 | 1 |
| Guehomyces pullulans | Cystofilobasidiales | AF105394 | 1 |
| Cryptococcus terreus | Filobasidiales | AF444692 | 2 |
| Cryptococcus terricola | Filobasidiales | AF459672 | 4 |
| Eocronartium muscicola | Urediniomycetes | AY512844 | 18 |
Reference sequences are listed in the order of major clades depicted in Fig. 3.
n, number of times encountered (cloned).
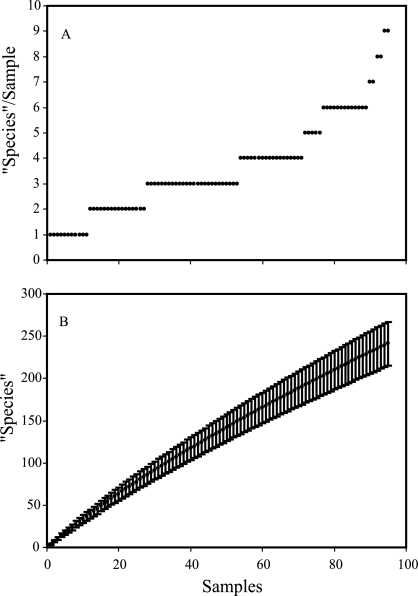
Individual 10-g samples yielded one to nine “species” (Fig. 2A), indicating high “species” density as well as high diversity. The “species” accumulation curve for combined samples (Fig. 2B) and the low number of “species” found in more than one sample indicate that much diversity remains to be discovered with additional sampling. The abundance-based coverage estimate algorithm of EstimateS estimated total “species richness” as 367 “species,” suggesting that our sample includes approximately two-thirds of the “species” estimated to be present in the 11.2-ha site. Use of the classic Chao1 algorithm in EstimateS provided a similar estimate of 375 “species.”
FIG. 2.
Numbers of basidiomycete “species” detected in soil samples from KBS-LTER in June and October 2002. A. Number of “species” per sample (with “species” defined as having ≥99% sequence identity over the 5′ nLSU rDNA region), with samples arranged in order from those with fewest to most “species” (65 samples yielded no basidiomycetes [not shown]). B. Species accumulation curve for the combined samples from all replicates and treatments sampled (conventional tillage, no-till, historically tilled successional community, and never-tilled successional community). Points on the “species” accumulation curve represent the sample-based rarefaction curve derived using the Mau Tau algorithm in EstimateS (version 7.5; Robert K. Colwell, Department of Ecology and Evolutionary Biology, University of Connecticut [http://purl.oclc.org/estimates]), and the error bars depict the 95% confidence interval of the estimate.
Identification of sequences.
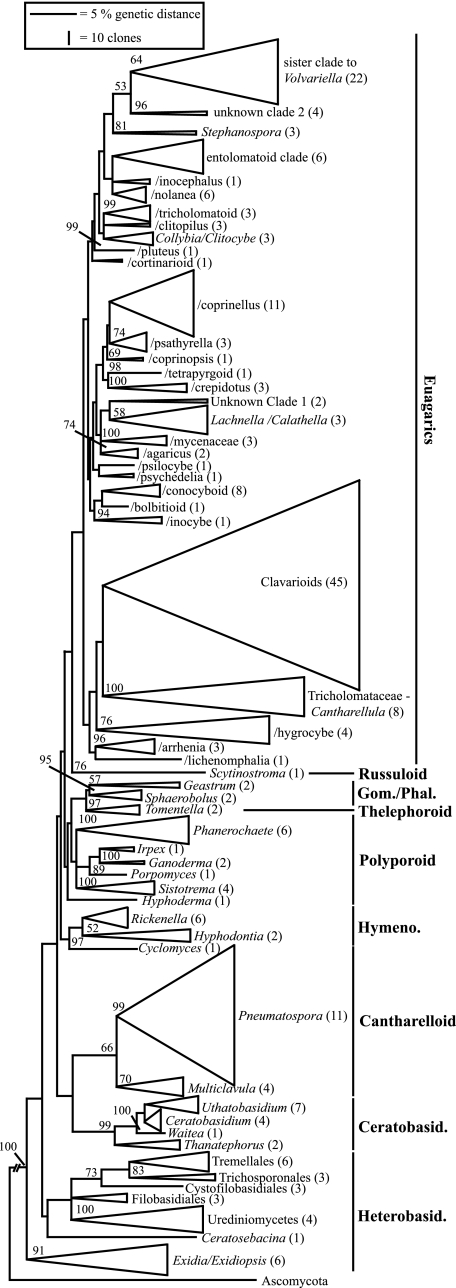
On the basis of phylogenetic analysis of the nLSU rDNA sequences, all 241 “species” detected from KBS-LTER were assigned to an order, and all but 26 “species” were assigned to terminal clades at the genus or family level (Fig. 3). Both the assignment of genera to major clades and the overall topology of the tree are generally in good agreement with the few available broad phylogenetic analyses of basidiomycetes (10, 17, 34). Sequences from KBS-LTER represented both heterobasidiomycetes (sensu reference 27) and homobasidiomycetes (24, 27) or, alternatively, urediniomycetes and hymenomycetes (sensu reference 54). No sequences of ustilaginomycetes (smuts and their relatives) were detected. Heterobasidiomycetes were represented by basidiomycetous yeasts (Tremellales, Cystofilobasidiales, Filobasidiales, and Trichosporonales), jelly fungi (Auriculariales) and rusts related to Eocronartium (Urediniomycetes: Platygloeales). The majority of basidiomycete sequences (215 species) were placed among the homobasidiomycetes and represented most clades corresponding to the classification of Hibbett and Thorn (24) (Fig. 3). Groups with multiple genera included the polyporoids, ceratobasidiales, hymenochaetoids, cantharelloids, and euagarics. More than half of the “species” (155 of 241) and terminal clades (30 of 56) in this analysis were euagarics. Sequences of euagarics included representatives of many saprotrophic taxa well-known from grasslands and agroecosystems, such as Agaricus, Bolbitius, Conocybe, Coprinellus, Entoloma, Hygrocybe, Nolanea, and Psilocybe.
FIG. 3.
Phylogenetic identification of basidiomycete sequences amplified from KBS-LTER soils. Neighbor-joining tree of the 241 environmental sequences together with 665 named reference sequences from GenBank, after which the reference sequences were pruned to depict the placement and identity of the environmental sequences. Numbers in parentheses following terminal clade names (38) indicate the numbers of “species” recovered, based on a definition of ≥99% sequence similarity. Numbers at nodes are bootstrap values indicating support for nodes including reference sequences, based on a neighbor-joining bootstrap analysis with 1,000 replicates. Vertical bars at right indicate seven of the eight major clades of Homobasidiomycetes recognized by Hibbett and Thorn (24) plus the Ceratobasidiales and Heterobasidiomycetes (Gom./Phal., gomphoid/phalloid; Hymeno., hymenochaetoid; Ceratobasid., Ceratobasidiales; Heterobasid., Heterobasidiomycetes). The height of a clade triangle is proportional to the number of clones recovered, and the width is proportional to terminal branch lengths (scale bars at upper left). Blastocladiella (Chytridiomycota), Blumeria, Neolecta, and Taphrina (Ascomycota) were used as the outgroup.
DISCUSSION
Species richness.
For territorial organisms whose mycelia range in area from square meters to hectares (20, 52), a species density averaging 3.4 (up to 9) species per 10-g sample comes as a surprise. Similarly, grassland and especially agricultural habitats are regarded as depauperate in basidiomycetes (6), but in our study we detected 94 “species” of basidiomycetes in agricultural plots (conventional tillage and no-till maize), 182 “species” in successional plots (historically tilled and never-tilled succession), and “241” species total. The total “species” richness predicted by EstimateS, 367 species, is equivalent to the total number of species of grassland macromycetes known from The Netherlands (6). The majority of “species” detected were not represented by reference sequences in GenBank, and several poorly known clades contained multiple species (clavarioids with 45 species, the sister group to Volvariella with 22 species, and Pneumatospora with 11 species). Similar studies of basidiomycetes in grasslands and agricultural soils from other locations may also detect unanticipated diversity in these fungi. Some of the “missing species” of fungi (22) may well be beneath our feet.
Molecular studies of microbial diversity could overestimate “species” diversity due to DNA sequence errors introduced by Taq polymerase, chimeras formed during PCR amplification, and the presence of heteroduplex DNA after PCR (1, 44). Acinas et al. (1) started with many more colonies (1,000) from two sites, and their results would therefore be more susceptible to overestimation. In our study, individual samples were not studied in as much detail, and all sequences were tested for the presence of chimeras. Even if some sequence diversity results from errors in sequencing or amplification, Fig. 1 and 3 show that the clade diversity is high. The many divergent clades detected, most with a relatively small number of “species,” suggests that overestimation of diversity caused by PCR artifacts was not a major problem in our study.
Clade diversity and ecology.
Primers Basid2R+/Basid001 yielded a broad array of basidiomycetes from Michigan agricultural soils, including representatives of all major groups except the smuts (Ustilaginomycetes). Out of 1,146 clones recovered, only 63 yielded sequences of ascomycetes, zygomycetes, and glomeromycetes, primarily from soil samples that yielded few or no basidiomycetes. The basidiomycete specificity of Basid2R+ is primarily at the 3′ end of the primer, which is particularly important in primer specificity (32). Although primer Basid001 would not be expected to contribute much basidiomycete specificity except in high-stringency PCR, the combination of Basid2R+ and Basid001 appears to give broad coverage and moderately good specificity for basidiomycetes. The broad taxonomic range of basidiomycete sequences recovered (Fig. 3) confirms the utility of these primers, although we cannot exclude the possibility that a few taxa or even major unknown lineages of basidiomycetes possess novel mismatches in the targeted regions and were not detected in our sampling for this reason.
The 5′ end of nLSU rDNA is moderately conserved, and basidiomycetes are relatively well represented in GenBank for this gene region, a combination that allowed us to resolve the identity of all 241 basidiomycete sequences recovered at least to an order. In contrast, in a similar study of fungi from forest soils (40), only 60% of 169 novel sequences among cloned fungal ITS sequences could be assigned to an order. ITS sequences of basidiomycetes are not yet as well represented in GenBank, and the high variability of this region means that a poor match (<90%) is phylogenetically uninformative. The ITS region and the highly conserved 3′ nSSU rDNA region are contained in our clones but were not sequenced in this study. For a few sequences, the phylogenetic placement to a genus was unstable in our analyses, depending upon the reference sequences used as clustering points. Their closest matches in GenBank are other unidentified environmental sequences from soil (40), and their identities are currently unresolved (Fig. 3, unknown clades 1 and 2).
Most of the “species” (155 of 241) and clades (30 of 56) detected are members of the euagarics (Agaricales), which are well-known as saprotrophs on litter and in soil. The clavarioid clade (coral fungi related to Clavaria) was particularly well represented with 45 species, or more than 20% of the total basidiomycete diversity detected. Although many species of Clavaria are recognized as grassland taxa and 21 are listed as threatened in Europe (7), their true diversity has apparently gone unrecognized. Other saprotrophs detected include Exidia/Exidiopsis (Auriculariales, Heterobasidiomycetes), Scytinostroma (russuloid clade), Geastrum and Sphaerobolus (gomphoid/phalloid clade), Phanerochaete, Sistotrema and other members of the polyporoid clade, Hyphodontia and Cyclomyces (hymenochaetoid clade), and Pneumatospora (cantharelloid clade). Pneumatospora obcoronata (= Tricellulortus peponiformis) has been isolated from fresh and fallen leaves in Malaysia (31), Japan (37), and Barbados (41). These KBS-LTER sequences are the first instance of this (or a closely related) species found outside of tropical regions. The presence of 11 “species” in this clade from KBS-LTER indicates that this portion of the phylogenetic tree has not yet been adequately sampled.
A number of clades include fungal symbionts of plants or algae. Three species were detected in a clade with Rickenella pseudogrisella (hymenochaetoid clade), a small mushroom associated with thallose liverworts (36). This clade, part of the group formerly known as Omphalina (48), contains a number of bryophilous mushrooms, and our results suggest that more diversity is present in this group than has been recognized. Four “species” were detected in a clade with Multiclavula mucida (cantharelloid clade), a species with minute club-shaped fruiting bodies that is associated with green algae (36, 42). Four “species” were detected in a clade together with Eocronartium muscicola (Urediniomycetes), which is biotrophic on weedy ephemeral bryophytes. The Ceratobasidiales includes the teleomorphs of Rhizoctonia species that cause damping-off diseases and blights in many wild and cultivated plants (49). Clades corresponding to Thanatephorus, Ceratobasidium, Waitea, and Uthatobasidium were detected in this analysis. The basidiomycetous yeasts detected include multiple “species” of the polyphyletic genus Cryptococcus, which is common in soils in association with plant roots (43, 53).
The absence of boletes and the low number of “species” detected in the gomphoid/phalloid, russuloid, and thelephoroid clades may be due, at least in part, to insufficient sampling, but many of the boletes and rususloids and most or all thelephoroids are ectomycorrhizal and would not be expected in agroecosystems that lack suitable host plants (11, 24). Nonetheless, we found two “species” of Tomentella (thelephoroid clade) and one “species” each in Inocybe and Cortinarius (euagarics clade) that are undoubtedly ectomycorrhizal. Saplings of ectomycorrhizal host trees (Populus and Quercus) are present in the successional plots at KBS-LTER and even occur sporadically in the plots of maize-soybean-wheat rotation. These scattered saplings are apparently sufficient to host a small number of what Arnolds (6) terms “alien ectomycorrhizal fungi” within the grassland and agroecosystem communities. These “aliens” contributed very little to the total diversity detected, which appears to consist mainly of species that are native to grasslands and agroecosystems. Similarly, our list was not dominated by basidiomycetes, such as Calvatia (giant puffballs), Ganoderma (massive polypores), or Puccinia (stem and leaf rusts), which are known to produce trillions of airborne basidiospores in the region (12, 35). Thus, we think that the species we detected are resident in KBS-LTER soils.
Within agroecosystems that have been traditionally regarded as impoverished in basidiomycete diversity, we detected 241 “species” in 56 clades of basidiomycetes in just 95 samples taken on two occasions in 1 year, and we estimate that at least another third of the total diversity remains to be discovered with additional sampling. This unexpected diversity consists of species that lack fruiting bodies, e.g., basidiomycetous yeasts, those with inconspicuous fruiting bodies that would be easily overlooked, e.g., Ceratobasidiales, Hyphoderma and Sistotrema, and mushrooms with conspicuous but ephemeral fruiting bodies, e.g., Agaricus, Entoloma, Hygrocybe, and Geastrum. Experience from Europe (5) suggests that it would take years to observe and collect even the species with conspicuous fruiting bodies, and those without such structures would still be missed. A culture-based study of the same location (59) detected 51 morphospecies of basidiomycetes in 64 soil samples; however, their sampling included several treatments (e.g., perennial alfalfa, old field, conifer forest, and hardwood forest) that were not sampled in our study, and few of the morphospecies could be identified by their morphology in culture. Thus, our method based on selective amplification of basidiomycete DNA detected a greater diversity with much better identification than was obtained by a culture-based method and yielded results with far less time and effort than would be required for sampling and identification of fruiting bodies. Since we sampled <1% of the study area, we are uncertain of the total diversity present. There is a large amount of uncharacterized diversity of closely related species and major clades. Replication in time and space is required to detect additional diversity and the underlying community structure. The recognition and further characterization of this diversity will have value to soil ecology, sustainable agriculture, and fungal conservation.
Acknowledgments
This research was supported by funding to R. G. Thorn from NSERC (discovery grant 238464), with additional support provided by the NSF Long-Term Ecological Research Program at the Kellogg Biological Station and by the Michigan Agricultural Experiment Station.
We thank S. Petropoulos and D. Salgado for assistance in soil sampling, A. Corbin for logistics and guidance at the site, J. M. Moncalvo for provision of aligned sequence files, and M. A. Lachance and several reviewers for improvements to the manuscript.
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Acinas, S. G., V. Klepac-Ceraj, D. E. Hunt, C. Pharino, I. Ceraj, D. L. Distel, and M. F. Polz. 2004. Fine-scale phylogenetic architecture of a complex bacterial community. Nature 430:551-554. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, I. C., and J. W. G. Cairney. 2004. Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques. Environ. Microbiol. 6:769-779. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, I. C., C. D. Campbell, and J. I. Prosser. 2003. Potential bias of fungal 18S rDNA and internal transcribed rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ. Microbiol. 5:36-47. [DOI] [PubMed] [Google Scholar]
- 5.Arnolds, E. 1992. The analysis and classification of fungal communities with special reference to macrofungi, p. 7-47. In W. Winterhoff (ed.), Handbook of vegetation science: fungi in vegetation science, vol. 19. Kluwer Academic, Dordrecht, The Netherlands. [Google Scholar]
- 6.Arnolds, E. 1992. Macrofungal communities outside forests, p. 113-149. In W. Winterhoff (ed.), Handbook of vegetation science: fungi in vegetation science, vol. 19. Kluwer Academic, Dordrecht, The Netherlands. [Google Scholar]
- 7.Arnolds, E., and B. de Vries. 1993. Conservation of fungi in Europe, p. 211-230. In D. N. Pegler, L. Boddy, B. Ing, and P. M. Kirk (ed.), Fungi of Europe: investigation, recording and mapping. Royal Botanic Gardens, Kew, United Kingdom.
- 8.Baath, E. 1988. A critical examination of the soil washing technique with special reference to the effect of the size of the soil particles. Can. J. Bot. 66:1566-1569. [Google Scholar]
- 9.Bills, G. F., and J. D. Polishook. 1994. Abundance and diversity of microfungi in leaf litter of a lowland rain forest in Costa Rica. Mycologia 86:187-198. [Google Scholar]
- 10.Binder, M., D. S. Hibbet, K.-H. Larsson, E. Larsson, E. Langer, and G. Langer. 2005. The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Syst. Biodivers. 3:113-157. [Google Scholar]
- 11.Brundrett, M. C. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytol. 154:275-304. [DOI] [PubMed] [Google Scholar]
- 12.Buller, A. H. R. 1909. Spore deposits—the number of spores, p. 79-88. In Researches on fungi, vol. 1. Longmans, Green and Co., London, United Kingdom. [Google Scholar]
- 13.Burbank, D. H., K. S. Pregitzer, and K. L. Gross. 1992. Vegetation of the W. K. Kellogg Biological Station, Kalamazoo County, Michigan. Research Report 510. Michigan State University Agricultural Experimental Station, East Lansing, Mich.
- 14.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer, W., L. B. Folman, R. C. Summerbell, and L. Boddy. 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 29:795-811. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar, J., S. Takala, S. M. Barns, J. A. Davis, and C. R. Kuske. 1999. Levels of bacterial community diversity in four arid soils compared by cultivation and 16S rRNA gene cloning. Appl. Environ. Microbiol. 65:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fell, J. W., T. Boekhout, A. Fonseca, and J. P. Sampiao. 2001. Basidiomycetous yeasts, p. 3-35. In D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke (ed.), The mycota VII. Part B: systematics and evolution. Springer, Berlin, Germany.
- 18.Gams, W., and K. H. Domsch. 1967. Beitrage zur Anwendung der Bodenwaschtechnik fur die Isolierung von Bodenpilzen. Arch. Mikrobiol. 58:134-144. [Google Scholar]
- 19.Gardes, M., and T. D. Bruns. 1996. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can. J. Bot. 74:1572-1582. [Google Scholar]
- 20.Gow, N. A. R., G. D. Robson, and G. M. Gadd (ed.). 1999. The fungal colony. Cambridge University Press, Cambridge, United Kingdom.
- 21.Hassouna, N., B. Michot, and J. P. Bachellerie. 1984. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 12:3563-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawksworth, D. L. 2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol. Res. 105:1422-1432. [Google Scholar]
- 23.He, J. Z., Z. H. Xu, and J. Hughes. 2005. Analyses of soil fungal communities in adjacent natural forest and hoop pine plantation ecosystems of subtropical Australia using molecular approaches based on 18S rRNA genes. FEMS Microbiol. Lett. 247:91-100. [DOI] [PubMed] [Google Scholar]
- 24.Hibbett, D. S., and R. G. Thorn. 2001. Basidiomycota: Homobasidiomycetes, p. 121-168. In D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke (ed.), The mycota VII. Part B: systematics and evolution. Springer, Berlin, Germany.
- 25.Hughes, J. B., J. J. Hellmann, T. H. Ricketts, and B. J. M. Bohannan. 2001. Counting the uncountable: statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 67:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt, J., L. Boddy, P. F. Randerson, and H. J. Rogers. 2004. An evaluation of 18S rDNA approaches for the study of fungal diversity in grassland soils. Microb. Ecol. 47:385-395. [DOI] [PubMed] [Google Scholar]
- 27.Julich, W. 1981. Higher taxa of Basidiomycetes. Bibl. Mycol. 85:1-485. [Google Scholar]
- 28.Jumpponen, A. 2003. Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol. 158:569-578. [DOI] [PubMed] [Google Scholar]
- 29.Kirk, P. M., P. F. Cannon, J. C. David, and J. A. Stalpers. 2001. Dictionary of the fungi, 9th ed. CAB International, Wallingford, United Kingdom.
- 30.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 73:331-371. [DOI] [PubMed] [Google Scholar]
- 31.Kuthubutheen, A. J., and S. Muid. 1984. Pneumatospora obcoronata gen. et sp. nov. from Malaysia. Trans. Br. Mycol. Soc. 83:423-429. [Google Scholar]
- 32.Kwok, S., D. E. Kellogg, N. McKinney, D. Spasic, L. Goda, C. Levenson, and J. J. Sninsky. 1990. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 18:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landeweert, R., P. Leeflang, T. W. Kuyper, E. Hoffland, A. Rosling, K. Wernars, and E. Smit. 2003. Molecular identification of ectomycorrhizal mycelium in soil horizons. Appl. Environ. Microbiol. 69:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson, K. H., E. Larsson, and U. Koljalg. 2004. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 108:983-1002. [DOI] [PubMed] [Google Scholar]
- 35.Levetin, E. 1995. Fungi, p. 87-120. In H. A. Burge (ed.), Bioaerosols. Lewis Publishers, Boca Raton, Fla.
- 36.Lutzoni, F., and M. Pagel. 1997. Accelerated evolution as a consequence of transitions to mutualism. Proc. Natl. Acad. Sci. USA 94:11422-11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushima, T. 1995. Matsushima mycological memoirs. Matsushima Mycol. Mem. 8:1-44. [Google Scholar]
- 38.Moncalvo, J. M., R. Vilgalys, S. A. Redhead, J. E. Johnson, T. Y. James, C. M. Aime, V. Hofstetter, S. J. W. Verduin, E. Larsson, T. J. Baroni, R. G. Thorn, S. Jacobsson, H. Clemencon, and O. K. Miller, Jr. 2002. One hundred and seventeen clades of euagarics. Mol. Phylogenet. Evol. 23:357-400. [DOI] [PubMed] [Google Scholar]
- 39.Mueller, G. M., G. F. Bills, and M. S. Foster. 2004. Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, New York, N.Y.
- 40.O'Brien, H., J. L. Parrent, J. A. Jackson, J. M. Moncalvo, and R. Vilgalys. 2005. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 71:5544-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peláez, F., J. Collado, G. Platas, T. Diez, A. Basilio, A. González, F. Vincente, P. Dai, G. Harris, M. Roxenbach, S. Dreikorn, and J. Thompson. 2001. Studies on Tricellulortus pepoformis, a tropical mitosporic fungus. Nova Hedwigia 72:217-230. [Google Scholar]
- 42.Petersen, R. H. 1967. Notes on clavarioid fungi. VII. Redefinition of the Clavaria vernalis-C. mucida complex. Am. Midl. Nat. 77:205-221. [Google Scholar]
- 43.Phaff, H. J., and W. T. Starmer. 1987. Yeasts associated with plants, insects and soil, p. 123-180. In A. H. Rose and J. S. Harrison (ed.), The yeasts, 2nd ed., vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 44.Polz, M. F., S. Bertilsson, S. G. Acinas, and D. Hunt. 2003. A(r)ray of hope in analysis of the function and diversity of microbial communities. Biol. Bull. (Woods Hole) 204:196-199. [DOI] [PubMed] [Google Scholar]
- 45.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 46.Rayner, A. D. M., and L. Boddy. 1988. Fungal decomposition of wood, its biology and ecology. John Wiley and Sons, Ltd., Chichester, United Kingdom.
- 47.Redberg, G. L., D. S. Hibbett, J. F. Ammirati, and R. J. Rodriguez. 2003. Phylogeny and genetic diversity of Bridgeoporus nobilissimus inferred using mitochondrial and nuclear rDNA sequences. Mycologia 95:836-845. [PubMed] [Google Scholar]
- 48.Redhead, S. A., J.-M. Moncalvo, R. Vilgalys, and F. Lutzoni. 2002. Phylogeny of agarics: partial systematics solutions for bryophilous omphalinoid agarics outside of the Agaricales (euagarics). Mycotaxon 82:151-168. [Google Scholar]
- 49.Roberts, P. 1999. Rhizoctonia-forming fungi. The Herbarium, Royal Botanic Gardens, Kew, United Kingdom.
- 50.Schadt, C. W., A. P. Martin, D. A. Lipson, and S. K. Schmidt. 2003. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 301:1359-1361. [DOI] [PubMed] [Google Scholar]
- 51.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, M. L., J. N. Bruhn, and J. B. Anderson. 1992. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356:428-431. [Google Scholar]
- 53.Spencer, J. F. T., and D. M. Spencer. 1997. Ecology: where yeasts live, p. 33-58. In J. F. T. Spencer and D. M. Spencer (ed.), Yeasts in natural and artificial habitats. Springer, New York, N.Y.
- 54.Swann, E. A., and J. W. Taylor. 1993. Higher taxa of basidiomycetes: an 18S rRNA perspective. Mycologia 85:923-936. [Google Scholar]
- 55.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), v4.0b10. Sinauer, Sunderland, Mass.
- 56.Tamura, K., and M. Nei. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10:512-526. [DOI] [PubMed] [Google Scholar]
- 57.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorn, R. G., J. Gaskill, C. A. Reddy, and E. A. Paul. 1995. PCR primers for selective amplification of partial 18S and 25S nuclear rDNA from Basidiomycetes. CME Research Findings Spring 1995, p. 42-44. Center for Microbial Ecology, Michigan State University, East Lansing, Mich.
- 59.Thorn, R. G., C. A. Reddy, D. Harris, and E. A. Paul. 1996. Isolation of saprophytic Basidiomycota from soil. Appl. Environ. Microbiol. 62:4288-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandenkoornhuyse, P., S. L. Baldauf, C. Leyval, J. Straczek, and J. P. W. Young. 2002. Extensive fungal diversity in plant roots. Science 295:2051. [DOI] [PubMed] [Google Scholar]
- 61.Vilgalys, R., and M. Hester. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172:4238-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watling, R. 1995. Assessment of fungal diversity—macromycetes, the problems. Can. J. Bot. 73:S15-S24. [Google Scholar]