Abstract
The α-proteobacterial strain Sphingobium japonicum UT26 utilizes a highly chlorinated pesticide, γ-hexachlorocyclohexane (γ-HCH), as a sole source of carbon and energy, and haloalkane dehalogenase LinB catalyzes the second step of γ-HCH degradation in UT26. Functional complementation of a linB mutant of UT26, UT26DB, was performed by the exogenous plasmid isolation technique using HCH-contaminated soil, leading to our successful identification of a plasmid, pLB1, carrying the linB gene. Complete sequencing analysis of pLB1, with a size of 65,998 bp, revealed that it carries (i) 50 totally annotated coding sequences, (ii) an IS6100 composite transposon containing two copies of linB, and (iii) potential genes for replication, maintenance, and conjugative transfer with low levels of similarity to other homologues. A minireplicon assay demonstrated that a 2-kb region containing the predicted repA gene and its upstream region of pLB1 functions as an autonomously replicating unit in UT26. Furthermore, pLB1 was conjugally transferred from UT26DB to other α-proteobacterial strains but not to any of the β- or γ-proteobacterial strains examined to date. These results suggest that this exogenously isolated novel plasmid contributes to the dissemination of at least some genes for γ-HCH degradation in the natural environment. To the best of our knowledge, this is the first detailed report of a plasmid involved in γ-HCH degradation.
γ-Hexachlorocyclohexane (γ-HCH) (also called γ-BHC and lindane) is a halogenated organic insecticide which was once used worldwide but has since been prohibited in most countries due to its toxicity and long persistence in upland soil. Although γ-HCH is a representative man-made xenobiotic, some γ-HCH-degrading bacterial strains have been isolated and characterized (30, 32, 35, 42, 43, 47). Genes and enzymes for γ-HCH degradation were first well characterized for an α-proteobacterial strain, Sphingobium japonicum UT26, which converts γ-HCH to β-ketoadipate through the action of six enzymes: LinA (dehydrochlorinase), LinB (halidohydrolase), LinC (dehydrogenase), LinD (reductive dechlorinase), LinE (ring cleavage dioxygenase), and LinF (reductase) (14, 40). The linA-to-linF genes in UT26 are dispersed on the three large circular replicons: the linA, linB, and linC genes on the 3.6-Mb chromosome I; the linF gene on the 670-kb chromosome II; and the linDE operon with its regulatory gene (linR) on a 185-kb plasmid, pCHQ1 (39). Nearly identical lin genes have also been identified in other HCH-degrading bacterial strains, such as Sphingobium indicum B90 (31) and B90A (12) from India and Sphingobium francense Sp+ from France (7); most of the lin genes in these strains are closely associated with an insertion sequence, IS6100 (7, 32). pCHQ1 is conjugally transferable from UT26 to another Sphingomonas paucimobilis strain (39), and a recent report showed that the linA and linB genes in other strains are also located on plasmids (7). These observations indicate that lin genes must be spread by mobile genetic elements (MGEs).
The recent determination of various bacterial genome sequences has revealed that the horizontal transfer of various phenotypic genes has played a significant role in the evolution of bacteria and in their adaptation to environmental changes (17, 45). There is no doubt that various MGEs have contributed greatly to horizontal gene transfer (44, 46, 48, 61), but the direct detection of such events in natural bacterial communities is rare. Exogenous isolation of MGEs, designated “exogenous plasmid isolation” when such MGEs are plasmids, has been developed to capture transferable plasmids directly from the natural microbial community by using bacterial conjugation systems (56). This technique involves conjugal mating of a suitable recipient with a natural microbial community and subsequent selection of transconjugants that have acquired a genetic marker. This approach has been used for the isolation of plasmids involved in resistance to mercury and in the degradation of 2,4-dichlorophenoxyacetic acid and naphthalene (3, 4, 54, 58, 60). Considering the fact that most bacterial cells in the environment are not easily culturable by conventional techniques (1), the exogenous plasmid isolation technique will provide novel insights into horizontal gene transfer in the natural environment.
In the present study, the exogenous plasmid isolation technique was applied to isolate a gene for LinB activity from HCH-contaminated soil. Considering the fact that LinB is a key enzyme in the degradation not only of γ-HCH but also of β-HCH (41), the linB gene is one of the most important genes in sites contaminated by a technical mixture of HCH (t-HCH) that consists of α, β, γ, and δ isomers. The present report provides a snapshot of the dynamism of the linB gene in the natural environment.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in the present study are listed in Table 1. Escherichia coli cells were grown at 37°C in Luria-Bertani (LB) broth (34), and Sinorhizobium meliloti and Mesorhizobium loti cells at 30°C in TY medium (6). Other strains were grown in 1/3LB broth (14) or W minimal medium (20) supplemented with γ-HCH as a carbon source at 30°C. The solid media were prepared by the addition of 1.5% agar. Antibiotics were used at final concentrations of 50 μg/ml for ampicillin and phosphomycin (Pho), 25 μg/ml for kanamycin (Km), 10 μg/ml for gentamicin (Gm), 15 μg/ml for tetracycline (Tc), 200 μg/ml for trimethoprim, and 1,000 μg/ml for streptomycin (Sm).
TABLE 1.
Bacterial strains and plasmids used in this study
| Species and strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1Δ(lacZYA-argF)φ80lacZΔM15 | 52 |
| S17-1λpir | pro thi recA hsdR λpir lysogen; Tpr Smr; chromosomally integrated RP4-2-Tc::Mu-Km::Tn7 | 64 |
| HB101 | hsdS20 recA13 ara-14 proA2 lacI1 galK2 rpsL20 xyl-5 mtl-1 supE44 | 34 |
| Pseudomonas putida | ||
| KT2440 | Wild-type strain | 2 |
| KT2440G | KT2440::TnMod-OGm; Gmr | This study |
| Sphingobium japonicum | ||
| UT26 | HCH+a; Nalr | 20 |
| UT26DB | UT26 linB::Kmr | 41 |
| UT26DBT1 | Transconjugant of UT26DB harboring pLB1 | This study |
| Sphingomonas paucimobilis | ||
| IAM12578 | Wild-type strain | 36 |
| IAM12578G | IAM12578::TnMod-OGm; Gmr | This study |
| Sinorhizobium meliloti1021 | Wild-type strain; Smr | 37 |
| Mesorhizobium lotiMAFF303099 | Wild-type strain; Phor | 23 |
| Burkholderia multivorans | ||
| ATCC 17616 | Wild-type strain | 57 |
| ATCC 17616G | ATCC17616::TnMod-OGm; Gmr | This study |
| Acidovorax sp. | ||
| KKS102 | Wild-type strain | 25 |
| KKS102G | KKS102::TnMod-OGm; Gmr | This study |
| Plasmids | ||
| pUC18 | pMB9 replicon; Apr | 62 |
| pTnMod-OGm | pMB1 replicon, Tn5 inverted repeat; Gmr | 11 |
| pK18mob | pMB1 replicon; Mob+ Kmr | 53 |
| pJP5608 | R6K replicon; Mob+ Tcr | 51 |
| pNIT6012 | pVS1 derivative, shuttle vector; Mob+ Tcr | 19 |
| pBBR1MCS-2 | pBBR1 replicon, broad host range; Mob+ Kmr | 28 |
| pRM11 | pUC18 derivative carrying EcoRI-BamHI fragment containing linB of pLB1 | This study |
| pRM14 | pNIT6012 derivative carrying BamHI-SphI fragment containing linB of pRM11 | This study |
| pRM15 | pNIT6012 derivative carrying BamHI fragment containing linB of pLB1 | This study |
| pRM26 | pJP5608 derivative carrying PstI fragment of pRM11 | This study |
| pK18OR | pK18mob derivative carrying HindIII-BamHI PCR fragment containing oriV and repA | This study |
| pK18BD3 | pK18mob derivative carrying DraI-BamHI fragment of pLB1 | This study |
| pLB1 | Exogenously isolated plasmid carrying linB | This study |
| pLB1Tc | pLB1::pRM26 | This study |
HCH+, growth on γ-HCH.
Methods of DNA manipulation.
Established methods were used for the preparation of plasmid DNA, its digestion with restriction endonucleases, ligation, agarose gel electrophoresis, and the transformation of E. coli cells (34). Large plasmids from α-proteobacterial strains were extracted using the method described by Kado and Liu (22), and the transformation of bacterial cells by electroporation was performed as described previously (27). Nucleotide sequencing was performed with an ABI PRISM model 310 sequencer (Applied Biosystems, Foster City, CA). Southern blot analysis was carried out using the conventional protocols and a digoxigenin system (Roche Diagnostics, Mannheim, Germany). For the preparation of the linB probe, a linB gene fragment was amplified by PCR with UT26 total DNA as a template and the primers Bgl-linB-Xho.R (5′-GGGCTCGAGGATTATGCTGGGCGCAATC-3′) and linB.F (5′-TAAGGAGGAATATCGATGAGCCTC-3′). For the repA probe of pLB1, we used plasmid pLB1Tc (see below) as a template and the primers Hin_pLB1_65868.F (5′-CCCAAGCTTGTGCCACCGAAGTGAGC-3′) and pLB1_894_Bam.R (5′-CCCGGATCCGAACTTCTTCCGTCAACG-3′).
Microbiological methodology for a soil sample.
The clay soil sample used in the present study was obtained from a field which had been contaminated with HCH isomers in Miyagi Prefecture, Japan. To determine the most probable number (MPN) of γ-HCH-degrading cells in the soil, 1-ml portions of serially diluted soil bacterial suspensions were inoculated into 9 ml of 1/10 W [containing the following per liter: KH2PO4, 170 mg; Na2HPO4, 980 mg; (NH4)2SO4, 100 mg; MgSO4, 48.7 mg; FeSO4, 0.52 mg; MgO, 10.75 mg; CaCO3, 2.0 mg; ZnSO4, 0.81 mg; CuSO4, 0.16 mg; CoSO4, 0.15 mg; and H3BO3, 0.06 mg) containing 10 ppm of γ-HCH and were incubated at 30°C for 2 weeks with shaking. An equal volume of ethyl acetate was mixed with each suspension to extract the solution that was used for the analysis of γ-HCH degradation with a Shimadzu GC-17A gas chromatograph equipped with an electron capture 63Ni detector (Shimadzu, Kyoto, Japan) as described previously (41).
Exogenous plasmid isolation was performed using a modified version of the procedure described by Top et al. (60). The bacterial fraction in 10 g of soil sample was suspended in phosphate-buffered saline (PBS) and filtered through 7-μm-pore-size filter paper (Advantec MFS, Inc., Tokyo, Japan) to remove nonbacterial materials. The filtered fraction was centrifuged at 10,000 × g for 20 min, and the cell pellet was resuspended in 0.5 ml of PBS buffer and mixed with 1 ml of an overnight culture of the recipient (UT26DB) cells. This mating mixture was concentrated by centrifugation at 10,000 × g for 2 min and spotted onto 1/3LB agar plates supplemented with cycloheximide (100 μg/ml). After overnight incubation at 30°C, cells were suspended in 1 ml of PBS, diluted, and spread on W agar plates supplemented with γ-HCH and Km.
Sequencing and computer analysis of pLB1.
A 1.9-kb PstI fragment of pLB1 (bp 20285 to 22160) was cloned into the PstI site of an R6K-based Tc-resistant (Tcr) plasmid, pJP5608. The resulting plasmid, pRM26, was conjugally transferred from E. coli S17-1λpir to UT26DBT1 (see below), and selection was made for resistance to Tc. One such transconjugant carried the pLB1 derivative (pLB1Tc) in which pRM26 was integrated into pLB1 by single-crossover-mediated homologous recombination between the common PstI fragment shared by the two plasmids. To avoid contamination of endogenous plasmids in UT26DB, pLB1Tc was conjugally transferred from UT26DB to E. coli S17-1λpir and purified by a standard alkaline lysis method (34). Shotgun sequencing of pLB1Tc was performed, the DNA fragments not covered by shotgun sequencing were directly amplified by PCR, and the nucleotide sequences of the resulting PCR products were determined.
Sequence assembly and calculation of G+C contents were performed using Genetyx 13 software (SDC, Tokyo, Japan). Annotation was performed using the GenBank CDS translations/PDB/SwissProt/PIR/PRF protein databases and the DDBJ/EMBL/GenBank DNA databases with the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). The conserved domains were searched using the InterProScan (http://www.ebi.ac.uk/InterProScan/) and the Pfam (http://www.sanger.ac.uk/Software/Pfam/search.shtml) programs. The putative amino acid sequences were used to generate a neighbor-joining tree by running the CLUSTALW program (http://www.ddbj.nig.ac.jp/search/clustalw-j.html).
Replication analysis of pLB1.
The 2-kb DNA fragment containing the repA gene and its upstream region (bp 64908 to 894) was amplified by PCR using pLB1Tc as a template and the primers pLB1_64908.F (5′-TAAAAGCTTTCATCGCTTTCTCC-3′) and pLB1_894_Bam.R (see above). The amplicon was cloned into the multiple cloning site of a narrow-host-range vector, pK18mob (Kmr), to obtain pK18OR as a minireplicon. Insertion of the 3.3-kb BamHI-DraI fragment of pLB1 (bp 36846 to 40144) (see Fig. 2 and Table 2) into pK18mob generated pK18BD3, which was used as a negative control. A broad-host-range vector, pBBR1MCS-2 (Kmr), was used as a positive control. pK18OR, pK18BD3, and pBBR1MCS-2 (150 ng each) were introduced into the UT26 cells by electroporation to obtain Kmr transformants.
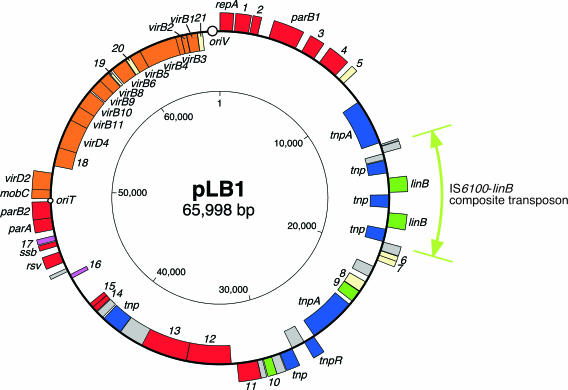
FIG. 2.
Circular map of pLB1. CDSs or gene remnants outside the circle are transcribed in the clockwise direction and those inside in the counterclockwise direction. The putative functions of genes are shown by the following colors: red, replication and stable inheritance; orange, conjugative transfer; blue, transposase and resolvase; magenta, regulation; yellow, unknown; and green, others. Gene remnants are shown in gray. Large and small circles indicate the putative oriV and oriT regions, respectively. The region of the IS6100-linB cluster is shown by the light green arrow. The positions of tnp in the IS6100-linB cluster are almost equal to those of IS6100 (Table 2).
TABLE 2.
Genes, sites, and specific fragments on pLB1
| Gene | Positiona | Determined or estimated function | % Amino acid identityb | Source | Protein identification no. of closest relative |
|---|---|---|---|---|---|
| repA | 1-783 | Replication protein | 33 (83/245) | Agrobacterium tumefaciens K84 plasmid pAgK84 | YP_086770.1 |
| orf1 | 895-1776 | Putative primase | 38 (111/291) | Novosphingobium aromaticivorans DSM 12444 | ZP_00873452.1 |
| orf2 | 1863-2360 | Putative nuclease | 29 (45/151) | Nitrobacter hamburgensis X14 | ZP_00626575.1 |
| parB1 | 3079-4854 | Putative plasmid stabilization protein | 29 (190/638) | A. tumefaciens K84 plasmid pAgK84 | YP_086769.1 |
| orf3 | 5293-6165 | ArdC-like antirestriction protein | 51 (152/294) | Nitrobacter hamburgensis X14 | ZP_00627826.1 |
| orf4 | 6577-7857 | Fic-like filamentation protein | 37 (70/187) | Xyielia fastidiosa Dixon | ZP_00653050.1 |
| orf5 | 8186-8575 | Conserved hypothetical protein | 35 (47/132) | Rhodobacter sphaeroides 2.4.1 | ABA81614.1 |
| tnpA | 10065-12956C | Tn3 family transposase | 92 (888/964) | Acetobacter aceti plasmid pAC5 | AAF20014.1 |
| —c | 13082-13186 | Resolvase N-terminal fragment | 94 (33/35) | Xanthomonas campestris pv. Vesicatoria strain 85-10 | YP_364058.1 |
| — | 13220-13639 | IS6100 transposase N-terminal fragment | 100 (140/140) | Salmonella entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| — | 13694-14074C | IS6100 transposase C-terminal fragment | 100 (126/126) | S. entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| tnp | 14131-14925C | IS6100 transposase | 100 (264/264) | S. entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| linB | 15264-16154 | 1,4-TCDNd halidohydrolase | 100 (296/296) | Sphingobium indicum B90A | AAR05978.1 |
| tnp | 16314-17108C | IS6100 transposase | 100 (264/264) | S. entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| linB | 17447-18337 | 1,4-TCDN halidohydrolase | 100 (296/296) | S. indicum B90A | AAR05978.1 |
| tnp | 18497-19291C | IS6100 transposase | 100 (264/264) | S. entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| — | 19324-19866 | Resolvase C-terminal fragment | 75 (131/174) | Zymomonas mobilis subsp. mobilisZM4 | AAV90079.1 |
| orf6 | 19898-20206 | Putative inner membrane protein | 56 (55/97) | S. entericaserovar Typhimurium LT2 | NP_463387.1 |
| orf7 | 20187-20519 | Hypothetical protein | 52 (45/86) | Escherichia coli B7A | ZP_00714508.1 |
| — | 20981-21688C | IS4 transposase gene remnant | 67 (161/238) | N. hamburgensis X14 | ZP_00627545.1 |
| orf8 | 22076-22762C | Hypothetical protein | 34 (29/85) | Synechococcus sp. strain CC9605 | YP_381146.1 |
| orf9 | 22790-23551C | Short-chain dehydrogenase | 53 (136/253) | S. indicum B90A | AAR05964.1 |
| tnpA | 23816-26773C | Tn3 family transposase | 69 (682/985) | Gluconobacter oxydans 621H plasmid pG0X1 | YP_190365.1 |
| tnpR | 26832-27506 | Resolvase | 70 (141/199) | Uncultured bacterium plasmid pTB11 | YP_112363.1 |
| — | 27519-28349C | Putative ring-hydroxylating dioxygenase gene remnant | 41 (110/265) | Burkholderia sp. strain 383 | YP_366455.1 |
| tnp | 28381-29175 | IS6100 transposase | 100 (264/264) | S. entericaserovar Typhimurium G8430 plasmid pU302L | AAR05758.1 |
| — | 29230-29751 | Resolvase gene remnant | 89 (150/168) | Pseudomonas syringae pv. Syringae A2 plasmid pPSR1 | NP_940689.1 |
| orf10 | 29754-30278 | Putative acetyltransferase | 29 (47/158) | P. fluorescens Pf-5 | YP_260527.1 |
| — | 30344-30655 | LexA gene remnant | 47 (47/100) | Rhodoferax ferrireducens DSM 15236 | ZP_00695279.1 |
| orf11 | 30666-31928 | Putative UV protection protein | 41 (176/422) | Magnetococcus sp. strain MC-1 | ZP_00607746.1 |
| orf12 | 32290-35097C | Hypothetical protein | 68 (641/935) | Aurantimonas sp. strain SI85-9A1 | ZP_01227946.1 |
| orf13 | 35106-38039C | Putative Type III restriction-modification enzyme | 45 (447/972) | Thermoplasma volcanium GSS1 | NP_111994.1 |
| — | 38039-39613C | Adenine-specific DNA methylase C-terminal fragment | 50 (270530) | Azotobacter vinelandii AvOP | ZP_00416345.1 |
| tnp | 39630-40952C | IS5 family transposase | 62 (260/418) | Desulfovibrio desulfuricans G20 | YP_390215.1 |
| — | 41057-41551C | Adenine-specific DNA methylase N-terminal fragment | 49 (90/183) | A. vinelandii AvOP | ZP_00416345.1 |
| orf14 | 41607-41945C | Plasmid maintenance system antidote protein | 85 (87/102) | Rhodospirillum rubrum | ZP_00270380.1 |
| orf15 | 41949-42239C | Plasmid maintenance system killer protein | 82 (79/96) | N. hamburgensisX14 | ZP_00624977.1 |
| orf16 | 44188-44445C | XRE family transcriptional regulator | 41 (25/60) | Rhizobium sp. strain NGR234 plasmid pNGR234a | NP_443819.1 |
| — | 44697-44897 | RNA polymerase sigma 70 factor fragment | 44 (30/67) | Thermosynechococcus elongatus BP-1 | NP_681289.1 |
| rsv | 45325-46023 | Resolvase | 70 (153/217) | Plasmid RP4 | AAA26414.1 |
| ssb | 46417-46731 | Single-strand binding protein | 50 (52/103) | N. hamburgensis X14 | ZP_00627857.1 |
| orf17 | 46789-47091 | Putative transcriptional regulator | 40 (34/85) | Sphingopyxis alaskensis RB2256 | ZP_00579357.1 |
| parA | 47479-48237 | ParA-like partition protein | 28 (67/235) | Sinorhizobium meliloti MBA19 plasmid pMBA19a | AAX19280.1 |
| parB2 | 48234-49253 | ParB-like partition protein | 30 (73/242) | Bradyrhizobium sp. strain BTAi1 | ZP_00864437.1 |
| oriT | 49254-49443 | Putative oriT region | |||
| mobC | 49444-49995 | MobC-like protein | 28 (47/163) | Bradyrhizobium sp. strain BTAi1 | ZP_00864438.1 |
| virD2 | 49982-51049 | Relaxase | 32 (104/320) | Aeromonas punctata HGB5 plasmid pFBAOT6 | YP_067824.1 |
| orf18 | 51526-52734C | Putative DNA primase | 28 (58/202) | X. fastidiosa 9a5c plasmid pXF51 | NP_061673.1 |
| virD4 | 52724-54640C | VirD4 type IV secretion protein | 45 (257/569) | Bradyrhizobium sp. strain BTAi1 | ZP_00864242.1 |
| virB11 | 54618-55610C | VirB11 type IV secretion protein | 62 (201/324) | Bradyrhizobium sp. strain BTAi1 | ZP_00864241.1 |
| virB10 | 55607-56782C | VirB10 type IV secretion protein | 38 (143/371) | Mesorhizobium sp. strain BNC1 | ZP_00614245.1 |
| virB9 | 56877-57719C | VirB9 type IV secretion protein | 38 (108/279) | R. etli CFN42 plasmid p42d | NP_659886.1 |
| virB8 | 57716-58399C | VirB8 type IV secretion protein | 44 (97/220) | S. meliloti 1021 plasmid pSymA | NP_435958.1 |
| orf19 | 58447-58620C | Hypothetical protein | |||
| virB6 | 58740-59756C | VirB6 type IV secretion protein | 28 (95/336) | A. tumefaciensstrain C58 plasmid AT | NP_535541.1 |
| orf20 | 59797-60102C | Hypothetical protein | |||
| virB5 | 60105-60818C | VirB5 type IV secretion protein | 36 (80/217) | R. etli CFN42 plasmid p42d | NP_659890.1 |
| virB4 | 60832-63216C | VirB4 type IV secretion protein | 51 (402/777) | R. etli CFN42 plasmid p42d | NP_659891.1 |
| virB3 | 63203-63547C | VirB3 type IV secretion protein | 43 (42/96) | Bartonella henselae strain Houston-1 | YP_034052.1 |
| virB2 | 63554-63895C | VirB2 type IV secretion protein | 38 (38/99) | Mesorhizobium sp. strain BNC1 | ZP_00614254.1 |
| virB1 | 63917-64582C | VirB1 type IV secretion protein | 47 (80/169) | X. fastidiosa 9a5c plasmid pXF51 | NP_061658.1 |
| orf21 | 64597-64920C | Hypothetical protein | 48 (38/79) | S. meliloti 1021 plasmid pSymA | NP_435966.1 |
| oriV | 64921-65998 | Putative oriV region |
The letter C indicates that the gene is carried on the complementary strand.
Values in parentheses refer to numbers of identical amino acids per the number examined.
—, probable gene remnant.
TCDN, 1,3,4,6-tetrachloro-1,4-cyclohexadiene.
Filter matings between pure cultures.
The donor and recipient cells grown overnight were harvested by centrifugation, washed with TY broth, and resuspended in fresh TY broth. They were then mixed and subsequently spotted on a sterile 0.45-μm-pore-size cellulose acetate filter (Advantec) placed on a TY agar plate. After incubation at 30°C for 13 h, the cells on the filter were suspended in TY broth, diluted, and plated on selective agar plates. S. meliloti 1021, M. loti MAFF303099, and E. coli HB101 were selected with Sm, Pho, and Sm, respectively. The chromosomes of other recipient strains (Pseudomonas putida KT2440, S. paucimobilis IAM12578, Burkholderia multivorans ATCC 17616, and Acidovorax sp. strain KKS102) were marked with the TnMod-OGm-derived Gmr gene. The introduction of this gene into the genomes of the parental strains was carried out by electroporation-mediated mutagenesis using pTnMod-OGm (11).
Nucleotide sequence accession number.
The complete sequence for the circular plasmid pLB1 has been deposited in DDBJ/EMBL/GenBank under accession number AB244976.
RESULTS
Exogenous isolation of a plasmid involved in γ-HCH degradation.
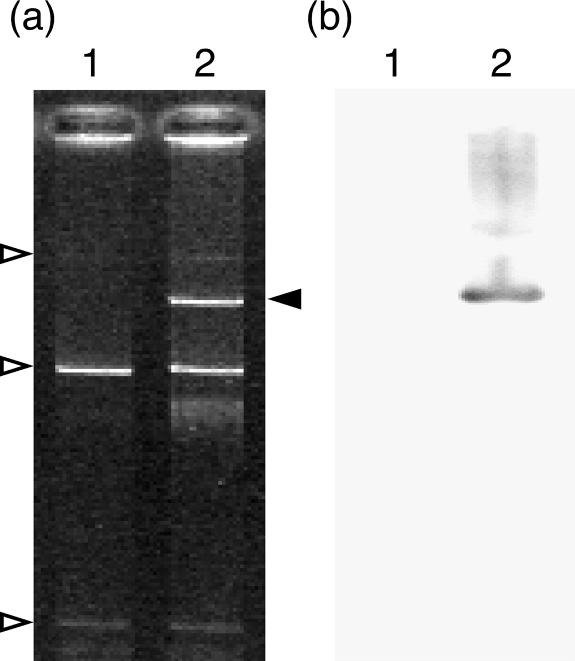
In the present study, we used a clay soil sample from Miyagi Prefecture, Japan. The soil had been contaminated by t-HCH, and still contained low levels of HCH isomers (0.03 to 0.1 mg/liter, mainly α-HCH). The number of culturable bacterial cells on each 1/100 NB (30 mg of beef extract per liter and 50 mg of peptone per liter) agar plate was 1.4 × 107 CFU/g of soil. The number of indigenous γ-HCH-degrading bacterial cells was estimated to be at least 103 MPN/g of soil. The bacterial fraction collected from the soil was mated with a linB mutant of UT26 (UT26DB) (41), using the protocol described in Materials and Methods. We obtained one putative transconjugant, UT26DBT1, that was able to utilize γ-HCH as a sole carbon source on the W agar plate plus Km. The control experiments without UT26DB did not give rise to such transconjugants. We further confirmed that UT26DBT1 was indeed a transconjugant of UT26DB by the following three results: (i) the 16S rRNA gene of UT26DBT1 was completely identical to that of UT26; (ii) linB::Km-specific DNA fragments were amplified by PCR using the UT26DBT1 colony as a template; and (iii) three UT26-endogenous plasmids, pCHQ1 (185 kb), pUT1 (30 kb), and pUT2 (5 kb), were also observed in UT26DBT1 (Fig. 1). UT26DBT1 harbored one additional plasmid, designated pLB1. Southern blot analysis with the linB gene as a probe indicated that pLB1 carries the linB homologue(s) (Fig. 1).
FIG. 1.
Plasmids residing in UT26DB and its transconjugant UT26DBT1. Gel electrophoresis of plasmids (a) and Southern blot analysis with the linB gene as a probe (b) are shown. Lanes 1, UT26DB; lanes 2, UT26DBT1. The white arrowheads indicate endogenous plasmids pCHQ1 (185 kb), pUT1 (30 kb), and pUT2 (5 kb), respectively. The black arrowhead indicates pLB1.
Sequence analysis of pLB1.
Our determination of the entire nucleotide sequence of pLB1Tc purified from E. coli S17-1λpir cells revealed that its parental plasmid, pLB1, is 65,998 bp in size. The average G+C content of pLB1 is 60.2%, and this score approximately corresponds to those of plasmids from other α-proteobacterial strains, such as pNL1 (62%) from Sphingomonas aromaticivorans F199, pSymA (60%) from S. meliloti 1021, and pMLa (59%) from M. loti MAFF303099. pLB1 was found to carry 50 coding sequences (CDSs) (Fig. 2 and Table 2), which represent 72.1% of the total plasmid DNA. The remaining 18,385-bp regions consist of partial gene fragments and intergenic regions. The putative products of 24 of 50 CDSs showed low similarity (less than 48% identity) to those deposited in public databases.
(i) The IS6100-linB cluster.
pLB1 carried two directly oriented copies of genes whose nucleotides were identical to linB from S. indicum B90A. To confirm the functionality of the two pLB1-specified linB genes, pRM14 and pRM15 were constructed such that each carried one of the two linB genes (bp 17148 to 18531 and 14965 to 16348, respectively) (Table 1). The introduction of these plasmids into UT26DB gave rise to transformants able to grow on a W agar plate containing γ-HCH as a carbon source, indicating that both linB genes on pLB1 are functional. The two linB genes and three complete and directly oriented copies of IS6100 were clustered in the order IS6100-linB-IS6100-linB-IS6100, and this cluster was preceded by two truncated versions of this IS element. This 6-kb region including the IS6100 remnants (bp 13187 to 19323) was designated the IS6100-linB cluster. Although the transposition of IS6100 usually generates 8-bp direct repeats of its target site (8), no obvious duplication of such a target site was observed around this cluster.
(ii) Genes for replication.
The predicted pLB1 replication initiator protein, RepA, is unique because only three homologues with homology at a low level were found in databases: RepA of Agrobacterium radiobacter K84 plasmid pAgK84 (33% identity), Rep of Agrobacterium tumefaciens 1D1422 plasmid pTAR (31% identity), and a hypothetical protein of a Zymomonas mobilis ZM4 plasmid (27% identity). An InterProScan search revealed that the pLB1 RepA protein contains in its middle part a putative winged-helix motif that would promote the binding to DNA. Although the upstream region of the repA gene (bp 64921 to 65998) showed no similarity to other well-characterized replication origin (oriV) sequences, this region was relatively A+T rich (55.8% of G+C content) and was found to contain seven 9-bp direct repeats (5′-kCwAwCwsd-3′) and one inverted repeat (Fig. 3). The direct repeats may serve as iterons (9), which are the interaction sites of iteron-type replication initiator proteins. Additionally, two putative DnaA boxes (38) were also located in this region (bp 64945 to 64953 and 65134 to 65142).
FIG. 3.
Structure of the putative oriV region of pLB1. The 9-bp direct repeats (5′-kCwAwCwsd-3′), which may serve as iterons, are shown by solid arrows, and one inverted repeat is indicated by dashed arrows. Putative DnaA-binding sequences are boxed, and parts of repA and orf21 are shaded. Putative ribosome-binding sites and start codons of orf21 and repA are shown in boldface.
(iii) Genes for stable inheritance.
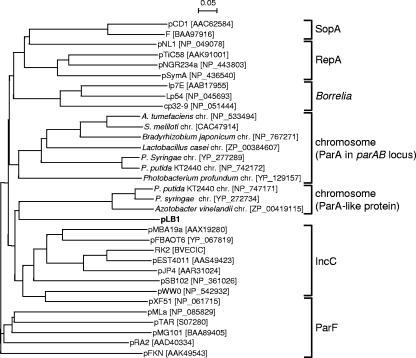
The parA-parB2 cluster (bp 47479 to 49253) is considered to be involved in the partitioning of pLB1. The putative ParA protein shows 28% identity to an IncC-like protein of S. meliloti MBA19 plasmid pMBA19a (Table 2) and belongs to the Walker-type ATPases (63). A phylogenetic tree of partitioning ATPases, in which each clade is thought to coincide with the genetic organization of the par loci (15, 16), demonstrated that ParA of pLB1 belongs to a novel clade (Fig. 4). The putative ParB2 protein has a functional domain (IPR003115) required for its binding to a specific DNA sequence (partition site). Another putative partitioning gene, parB1 (bp 3079 to 4854), was found far away from the parA-parB2 cluster. Although ParB1 also had the predicted functional domain, its C-terminal region, which was postulated to be involved in dimerization, showed a very low level of similarity to that of ParB2.
FIG. 4.
Phylogenetic tree of putative Walker-type partition ATPases. Lengths of horizontal lines reflect relative evolutionary distances among the 33 Walker-type partition ATPases encoded by various plasmids or chromosomes. The GenBank accession numbers of respective proteins are shown in brackets. Groups of similar sequences are labeled. pLB1 is shown in boldface. The scale bar indicates 0.05 substitution per site.
Two CDSs, rsv and orf2, encode a putative resolvase (site-specific serine recombinase) and nuclease, respectively. Their predicted products show homology with ParA and ParB of plasmid RP4, respectively (49), and they are considered to be involved in multimer resolution (13, 21). (Note that ParA and ParB of RP4 are different from ParA, ParB1, and ParB2 of pLB1.) Therefore, the Rsv and Orf2 proteins might contribute to the stable maintenance of pLB1 in the host cells by the site-specific resolution and subsequent decatenation of the multimer form of pLB1. The putative products of orf14 and orf15 showed high similarity (82 to 85% identity) to antitoxin and toxin, respectively, of the classical proteic killer system for plasmid maintenance (26), and they may be involved in the genetic addiction system of pLB1.
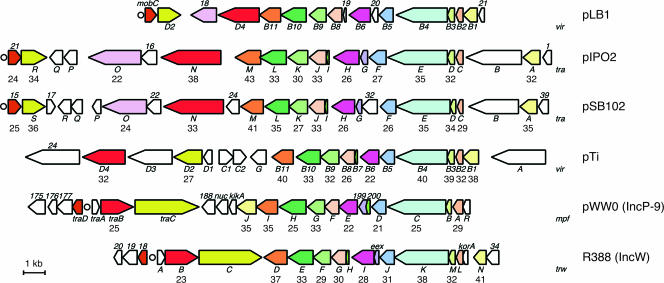
(iv) Genes for conjugative transfer.
Putative genes for conjugative DNA transfer, constituting the DNA transfer and replication (Dtr) system and mating pair formation (Mpf) system, were found in a 15-kb region of pLB1 (Fig. 2). The gene organization suggested that this region consists of two transcriptional units (mobC-virD2 and orf21-orf18) (Fig. 5). The putative origin of transfer (oriT; bp 49254 to 49443) was deduced on the basis of typical features of other previously well-characterized oriT sequence (65). This region, which had a relatively low G+C content (52.6%), contained inverted repeats (bp 49302 to 49327 and 49386 to 49425) and the putative nic site (5′-TATCCCGC-3′), which showed significant similarity to the RP4-type site (49).
FIG. 5.
Genetic organization of the putative transfer region of pLB1 and comparison with related systems. Genes encoding similar functions are displayed in the same color. Circles indicate the putative oriT regions of the respective plasmids. The numbers below the genes indicate the percent amino acid identity (>20%) to the corresponding gene product from pLB1. The lengths of TraO homologues are various, because only their C-terminal regions are conserved, which contain a putative functional domain for primase. The GenBank accession numbers of the respective nucleotide sequences are as follows: broad-host-range cryptic plasmid pIPO2, NC_003213; mercury resistance plasmid pSB102, NC_003122; A. tumefaciens strain C58 plasmid pTi, NC_003065; catabolic plasmid pWW0, NC_003350; and antibiotic resistance plasmid R388, BR000038.
We believe that the four putative gene products (MobC, VirD2, Orf18, and VirD4) play a role in the Dtr system. MobC contained at its C terminus the MobC domain (PF05713) that is believed to act as a molecular wedge for the relaxosome-induced melting of oriT DNA (66), and thus MobC may facilitate relaxosome function. VirD2 has been predicted to serve as a relaxase capable of site-specific nicking of plasmid DNA at oriT, because it contains each of the three defined motifs that are conserved in the relaxases of IncP-1 and Ti plasmids (50). As is the case with TraC of IncP-1 plasmids (49), the orf18 product is assumed to be transported to the recipient cells during the conjugative transfer of single-stranded plasmid DNA and is thought to subsequently initiate the synthesis of the complementary strand. The presence of the nucleotide-binding Walker motifs A and B in VirD4 allowed us to categorize this protein as being isofunctional with TrwB from an IncW plasmid, TraG from IncP-1 plasmids, and VirD4 from the Ti plasmid, all of which are necessary for conjugal DNA transfer.
The products of the 10 pLB1-specified genes (virB1 to virB6 and virB8 to virB11) putatively involved in the Mpf system showed 28% (VirB6) to 62% (VirB11) identity with the products of the virB gene clusters of symbiotic plasmids from rhizobial strains (Table 2). The level of identity was relatively low, but the functional domains of the putative products were conserved, suggesting that they are functional as the Mpf system. The gene order of the virB cluster on pLB1 was fundamentally the same as that in the Mpf gene clusters in various conjugative plasmids (Fig. 5) (55). The position of the Dtr-related gene cluster relative to that of the Mpf-related cluster on pLB1 was furthermore conserved in pIPO2 and pSB102, although several CDSs (such as traB, traP, and traQ) are located within the Mpf-related gene clusters on the latter two plasmids. This indicates that the putative transfer region of pLB1 is more compact than those of other plasmids. The VirB7 protein of Ti plasmid and its homologues in other Mpf systems of various plasmids encode outer-membrane-anchored lipoproteins to stabilize VirB9 and its homologues (18, 29). The virB7 homologues are flanked by virB6 and virB8 homologues in various plasmids. Although orf19 of pLB1 had a size similar to that of other virB7-related genes, the orf19 product showed no similarity with other VirB7-related proteins. The functions of the two genes, orf20 and orf21, in the pLB1 virB cluster remain unknown.
Identification of the region essential for replication.
The most probable region essential for the replication of pLB1 is the repA gene and its upstream region (bp 64908 to 894). To confirm this possibility, this 2-kb region of pLB1 was cloned into a narrow-host-range vector, pK18mob (Kmr), to obtain a minireplicon, pK18OR. Electroporation of UT26 cells with pK18OR gave rise to the Kmr transformants. Southern blot analysis of such transformants with the repA gene as a probe confirmed that pK18OR exists as an autonomously replicating unit in UT26 (data not shown). Control experiments using pK18BD3, a pK18mob variant into which another part of pLB1 was inserted, gave no Kmr transformant at all. These results demonstrate that the repA gene and its upstream region of pLB1 are functional as a replication unit in UT26.
Conjugal transferability of pLB1.
To investigate the self-transmissibility of pLB1, the donor strain UT26DB harboring pLB1Tc, a Tcr variant of pLB1 with a replication origin of plasmid R6K (R6Kγori), was separately mated with seven recipient strains: S. paucimobilis IAM12578G, S. meliloti 1021, M. loti MAFF303099, B. multivorans ATCC 17616G, Acidovorax sp. strain KKS102G, P. putida KT2440G, and E. coli HB101. The Tcr transconjugants of IAM12578G, 1021, and MAFF303099 were obtained at frequencies ranging from 10−7 to 10−5 per donor (Table 3). Gel electrophoresis and Southern blot analysis of plasmids residing in the transconjugants led to the confirmation of the successful transfer of pLB1Tc without any obvious structural changes in the three α-proteobacterial strains (data not shown). Because transconjugants are thought to have no pir gene, which is necessary for the replication of plasmids carrying the R6Kγori sequence, we concluded that pLB1Tc replicated by using the pLB1-derived replication machinery in the transconjugants. On the other hand, no Tcr transconjugants were obtained when ATCC 17616G, KKS102G, KT2440G, and HB101 were used as recipients (<10−9 per donor).
TABLE 3.
Conjugal transfer of pLB1Tc from UT26DBa
| Recipientb | Conjugation frequencyc |
|---|---|
| Sphingomonas paucimobilis IAM12578G | (4.1 ± 1.3) × 10−5 |
| Sinorhizobium meliloti 1021 | (4.4 ± 5.1) × 10−7 |
| Mesorhizobium loti MAFF303099 | (5.6 ± 0.6) × 10−6 |
| Burkholderia multivorans ATCC 17616G | <3.3 × 10−9 |
| Acidovorax sp. strain KKS102G | <6.0 × 10−9 |
| Pseudomonas putida KT2440G | <9.3 × 10−9 |
| Escherichia coli HB101 | <1.8 × 10−9 |
The donor cells were grown overnight at 30°C in 1/3LB medium containing Tc.
The optical density of the recipient cells at 660 nm was 0.9. Counterselection was made for resistance to Pho in the case of Mesorhizobium, to Sm in the cases of Sinorhizobium and Escherichia coli, and to Gm in the cases of the other recipient strains.
Conjugation frequency is expressed as the number of transconjugants per donor. The data represent the range from three independent experiments.
DISCUSSION
Our previous observation that the six structural lin genes are dispersed on the UT26 genome suggested that each lin gene is separately distributed in other environmental bacteria (39). Based on this suggestion, the exogenous plasmid isolation technique was successfully employed in the present study to capture pLB1 that carried only linB genes. Since other lin genes have been reported to be located on plasmids (7, 39), there may be other indigenous plasmids carrying other lin genes, such as pCHQ1, whose self-transmissibility has been demonstrated (39), and it should be possible to isolate them by techniques similar to that used here. However, we have not yet succeeded in isolating plasmids carrying other lin genes by using linA and linRED mutants of UT26 as recipients (data not shown). Endogenous plasmids in UT26 might have inhibited the capturing of exogenous plasmids. Plasmid-free recipient strains are desirable for exogenous plasmid isolation, and the use of such strains will allow us to conduct a more comprehensive analysis of the dynamism of lin genes in the natural environment.
The linB genes on pLB1 were found to be organized as the IS6100-linB cluster. It is known that IS6100 is closely associated with lin genes (32). IS6100 belongs to the IS6 family and has been found in various bacterial chromosomes and plasmids (8), such as pZWL0 from Pseudomonas sp. strainWBC-3 (33), pOAD2 from Arthrobacter sp. strain KI72 (24), and pTET3 from Corynebacterium glutamicum LP-6 (59). On these plasmids, two copies of IS6100 form a composite transposon carrying catabolic or antibiotic resistance genes (24, 33, 59). The organization of the IS6100-linB cluster on pLB1 suggests that this cluster also behaves as a composite transposon, giving rise to the potential to exhibit highly efficient dissemination. Considering the fact that the IS6100-linB cluster on pLB1 is flanked by divided gene remnants (bp 13082 to 13186 and 19324 to 19866), which might have formerly encoded a resolvase, we believe that the cluster has recently been disseminated into archetypal pLB1.
Almost all putative genes of pLB1 involved in its replication, stable inheritance, and conjugative transfer functions showed low levels of identity to the homologues so far reported, indicating that the basic plasmid mechanisms of pLB1 (replication, stable inheritance, and conjugation) are novel. pLB1 seems to belong to a new incompatibility group of plasmids, at least on the basis of RepA sequence similarity. Our present results provide a novel insight into the evolution of bacterial catabolic plasmids, because most well-characterized catabolic plasmids are categorized into the IncP-1, IncP-2, IncP-7, and IncP-9 groups of Pseudomonas origin (10). Interestingly, ParA of pLB1 was found to be related to the chromosomal ParA-like proteins from P. putida, Pseudomonas syringae, and Azotobacter vinelandii, whose genes are solely separated from their canonical parAB loci (Fig. 4). The ParA homologues in the parAB loci of bacterial genomes are believed to be ATPases essential for the partitioning of replicons and thus have been investigated in recent studies (16), while the ParA homologues separately encoded alone at the other loci have not been studied. The present findings suggest that the latter group of parA-like genes have some unknown functions for the partitioning of chromosomes and/or endogenous plasmids.
In the present study, pLB1 was found to be able to be conjugally transferred to α-proteobacterial strains but not to β- or γ-proteobacterial strains. The frequency of conjugation of pLB1Tc to IAM12578G was higher than that to the other two α-proteobacterial strains, indicating that pLB1 is optimally transferable to Sphingomonadaceae family strains. To the best of our knowledge, there has been no previous indication of the transfer of catabolic plasmids from sphingomonads to bacterial strains not belonging to the Sphingomonadaceae family (5). Therefore, our present results are the first demonstration that a catabolic plasmid from sphingomonads is transferable to strains outside the order.
In conclusion, the features of pLB1 indicate that it is a novel class of plasmids. In the present study we demonstrated that genes for the degradation of recently released xenobiotics can be distributed by a novel plasmid in the natural environment. Further modification of this work will enable us to access novel genetic sinks in the environment and to make significant observations related to bacterial evolution via MGEs.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Agriculture, Forestry, and Fisheries (HC-06-2323), Japan. R.M. is supported by a Japan Society for the Promotion of Science research fellowship.
We thank Akiho Ankai and Yasuyuki Terui from the National Institute of Technology and Evaluation for technical advice on sequencing.
Footnotes
Published ahead of print on 8 September 2006.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 3.Bale, M. J., J. C. Fry, and M. J. Day. 1987. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J. Gen. Microbiol. 133:3099-3107. [DOI] [PubMed] [Google Scholar]
- 4.Bale, M. J., J. C. Fry, and M. J. Day. 1988. Transfer and occurrence of large mercury resistance plasmids in river epilithon. Appl. Environ. Microbiol. 54:972-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta, T., A. Keck, J. Klein, and A. Stolz. 2004. Detection and characterization of conjugative degradative plasmids in xenobiotic-degrading Sphingomonas strains. J. Bacteriol. 186:3862-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 7.Ceremonie, H., H. Boubakri, P. Mavingui, P. Simonet, and T. M. Vogel. 2006. Plasmid-encoded γ-hexachlorocyclohexane degradation genes and insertion sequences in Sphingobium francense (ex-Sphingomonas paucimobilis Sp+). FEMS Microbiol. Lett. 257:243-252. [DOI] [PubMed] [Google Scholar]
- 8.Chandler, M., and J. Mahillon. 2002. Insertion sequences revisited, p. 305-366. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 9.Chattoraj, D. K. 2000. Control of plasmid DNA replication by iterons: no longer paradoxical. Mol. Microbiol. 37:467-476. [DOI] [PubMed] [Google Scholar]
- 10.Dennis, J. J. 2005. The evolution of IncP catabolic plasmids. Curr. Opin. Biotechnol. 16:291-298. [DOI] [PubMed] [Google Scholar]
- 11.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dogra, C., V. Raina, R. Pal, M. Suar, S. Lal, K. H. Gartemann, C. Holliger, J. R. van der Meer, and R. Lal. 2004. Organization of lin genes and IS6100 among different strains of hexachlorocyclohexane-degrading Sphingomonas paucimobilis: evidence for horizontal gene transfer. J. Bacteriol. 186:2225-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberl, L., C. S. Kristensen, M. Givskov, E. Grohmann, M. Gerlitz, and H. Schwab. 1994. Analysis of the multimer resolution system encoded by the parCBA operon of broad-host-range plasmid RP4. Mol. Microbiol. 12:131-141. [DOI] [PubMed] [Google Scholar]
- 14.Endo, R., M. Kamakura, K. Miyauchi, M. Fukuda, Y. Ohtsubo, M. Tsuda, and Y. Nagata. 2005. Identification and characterization of genes involved in the downstream degradation pathway of γ-hexachlorocyclohexane in Sphingomonas paucimobilis UT26. J. Bacteriol. 187:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funnel, B. E., and R. A. Slavcev. 2004. Partition systems of bacterial plasmids, p. 81-103. In B. E. Funnel and G. J. Phillips (ed.), Plasmid biology. ASM Press, Washington, D.C.
- 16.Gerdes, K., J. Moller-Jensen, and R. Bugge Jensen. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455-466. [DOI] [PubMed] [Google Scholar]
- 17.Gogarten, J. P., and J. P. Townsend. 2005. Horizontal gene transfer, genome innovation and evolution. Nat. Rev. Microbiol. 3:679-687. [DOI] [PubMed] [Google Scholar]
- 18.Harris, R. L., V. Hombs, and P. M. Silverman. 2001. Evidence that F-plasmid proteins TraV, TraK and TraB assemble into an envelope-spanning structure in Escherichia coli. Mol. Microbiol. 42:757-766. [DOI] [PubMed] [Google Scholar]
- 19.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol. Plant Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 20.Imai, R., Y. Nagata, K. Senoo, H. Wada, M. Fukuda, M. Takagi, and K. Yano. 1989. Dehydrochlorination of γ-hexachlorocyclohexane (γ-BHC) by γ-BHC-assimilating Pseudomonas paucimobilis. Agric. Biol. Chem. 53:2015-2017. [Google Scholar]
- 21.Johnson, E. P., T. Mincer, H. Schwab, A. B. Burgin, and D. R. Helinski. 1999. Plasmid RK2 ParB protein: purification and nuclease properties. J. Bacteriol. 181:6010-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 24.Kato, K., K. Ohtsuki, H. Mitsuda, T. Yomo, S. Negoro, and I. Urabe. 1994. Insertion sequence IS6100 on plasmid pOAD2, which degrades nylon oligomers. J. Bacteriol. 176:1197-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimbara, K., T. Hashimoto, M. Fukuda, T. Koana, M. Takagi, M. Oishi, and K. Yano. 1989. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J. Bacteriol. 171:2740-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi, I. 2004. Genetic addiction: a principle of symbiosis in a genome, p. 105-144. In G. J. Phillips and B. E. Funnel (ed.), Plasmid biology. ASM Press, Washington, D.C.
- 27.Komatsu, H., Y. Imura, A. Ohori, Y. Nagata, and M. Tsuda. 2003. Distribution and organization of auxotrophic genes on the multichromosomal genome of Burkholderia multivorans ATCC 17616. J. Bacteriol. 185:3333-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 29.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar, M., P. Chaudhary, M. Dwivedi, R. Kumar, D. Paul, R. K. Jain, S. K. Garg, and A. Kumar. 2005. Enhanced biodegradation of β- and δ-hexachlorocyclohexane in the presence of α- and γ-isomers in contaminated soils. Environ. Sci. Technol. 39:4005-4011. [DOI] [PubMed] [Google Scholar]
- 31.Kumari, R., S. Subudhi, M. Suar, G. Dhingra, V. Raina, C. Dogra, S. Lal, J. R. van der Meer, C. Holliger, and R. Lal. 2002. Cloning and characterization of lin genes responsible for the degradation of hexachlorocyclohexane isomers by Sphingomonas paucimobilis strain B90. Appl. Environ. Microbiol. 68:6021-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lal, R., C. Dogra, S. Malhotra, P. Sharma, and R. Pal. 2006. Diversity, distribution and divergence of lin genes in hexachlorocyclohexane-degrading sphingomonads. Trends Biotechnol. 24:121-130. [DOI] [PubMed] [Google Scholar]
- 33.Liu, H., J. J. Zhang, S. J. Wang, X. E. Zhang, and N. Y. Zhou. 2005. Plasmid-borne catabolism of methyl parathion and p-nitrophenol in Pseudomonas sp. strain WBC-3. Biochem. Biophys. Res. Commun. 334:1107-1114. [DOI] [PubMed] [Google Scholar]
- 34.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Manickam, N., M. Mau, and M. Schlomann. 2006. Characterization of the novel HCH-degrading strain, Microbacterium sp. ITRC1. Appl. Microbiol. Biotechnol. 69:580-588. [DOI] [PubMed] [Google Scholar]
- 36.Masai, E., Y. Katayama, S. Kawai, S. Nishikawa, M. Yamasaki, and N. Morohoshi. 1991. Cloning and sequencing of the gene for a Pseudomonas paucimobilis enzyme that cleaves β-aryl ether. J. Bacteriol. 173:7950-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messer, W., and C. Weigel. 1997. DnaA initiator—also a transcription factor. Mol. Microbiol. 24:1-6. [DOI] [PubMed] [Google Scholar]
- 39.Nagata, Y., M. Kamakura, R. Endo, R. Miyazaki, Y. Ohtsubo, and M. Tsuda. 2006. Distribution of γ-hexachlorocyclohexane-degrading genes on three replicons in Sphingobium japonicum UT26. FEMS Microbiol. Lett. 256:112-118. [DOI] [PubMed] [Google Scholar]
- 40.Nagata, Y., K. Miyauchi, and M. Takagi. 1999. Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 23:380-390. [DOI] [PubMed] [Google Scholar]
- 41.Nagata, Y., Z. Prokop, Y. Sato, P. Jerabek, A. Kumar, Y. Ohtsubo, M. Tsuda, and J. Damborsky. 2005. Degradation of β-hexachlorocyclohexane by haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. Appl. Environ. Microbiol. 71:2183-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalin, R., P. Simonet, T. M. Vogel, and P. Normand. 1999. Rhodanobacter lindaniclasticus gen. nov., sp. nov., a lindane-degrading bacterium. Int. J. Syst. Bacteriol. 49:19-23. [DOI] [PubMed] [Google Scholar]
- 43.Nawab, A., A. Aleem, and A. Malik. 2003. Determination of organochlorine pesticides in agricultural soil with special reference to γ-HCH degradation by Pseudomonas strains. Bioresour. Technol. 88:41-46. [DOI] [PubMed] [Google Scholar]
- 44.Normark, B. H., and S. Normark. 2002. Evolution and spread of antibiotic resistance. J. Intern. Med. 252:91-106. [DOI] [PubMed] [Google Scholar]
- 45.Ochman, H., E. Lerat, and V. Daubin. 2005. Examining bacterial species under the specter of gene transfer and exchange. Proc. Natl. Acad. Sci. USA 102(Suppl. 1):6595-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1099. [DOI] [PubMed] [Google Scholar]
- 47.Okeke, B. C., T. Siddique, M. C. Arbestain, and W. T. Frankenberger. 2002. Biodegradation of γ-hexachlorocyclohexane (lindane) and α-hexachlorocyclohexane in water and a soil slurry by a Pandoraea species. J. Agric. Food Chem. 50:2548-2555. [DOI] [PubMed] [Google Scholar]
- 48.Osborn, A. M., K. D. Bruce, P. Strike, and D. A. Ritchie. 1997. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol. Rev. 19:239-262. [DOI] [PubMed] [Google Scholar]
- 49.Pansegrau, W., E. Lanka, P. T. Barth, D. H. Figurski, D. G. Guiney, D. Haas, D. R. Helinski, H. Schwab, V. A. Stanisich, and C. M. Thomas. 1994. Complete nucleotide sequence of Birmingham IncPα plasmids. Compilation and comparative analysis. J. Mol. Biol. 239:623-663. [DOI] [PubMed] [Google Scholar]
- 50.Pansegrau, W., W. Schroder, and E. Lanka. 1994. Concerted action of three distinct domains in the DNA cleaving-joining reaction catalyzed by relaxase (TraI) of conjugative plasmid RP4. J. Biol. Chem. 269:2782-2789. [PubMed] [Google Scholar]
- 51.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 54.Schneiker, S., M. Keller, M. Droge, E. Lanka, A. Puhler, and W. Selbitschka. 2001. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 29:5169-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroder, G., and E. Lanka. 2005. The mating pair formation system of conjugative plasmids: a versatile secretion machinery for transfer of proteins and DNA. Plasmid 54:1-25. [DOI] [PubMed] [Google Scholar]
- 56.Smalla, K., A. M. Osborn, and E. M. H. Wellington. 2000. Isolation and characterization of plasmids from bacteria, p. 207-248. In C. M. Thomas (ed.), The horizontal gene pool—bacterial plasmids and gene spread. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 57.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 58.Stuart-Keil, K. G., A. M. Hohnstock, K. P. Drees, J. B. Herrick, and E. L. Madsen. 1998. Plasmids responsible for horizontal transfer of naphthalene catabolism genes between bacteria at a coal tar-contaminated site are homologous to pDTG1 from Pseudomonas putida NCIB 9816-4. Appl. Environ. Microbiol. 64:3633-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tauch, A., S. Gotker, A. Puhler, J. Kalinowski, and G. Thierbach. 2002. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet 33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 48:117-129. [DOI] [PubMed] [Google Scholar]
- 60.Top, E. M., W. E. Holben, and L. J. Forney. 1995. Characterization of diverse 2,4-dichlorophenoxyacetic acid-degradative plasmids isolated from soil by complementation. Appl. Environ. Microbiol. 61:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Top, E. M., and D. Springael. 2003. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 14:262-269. [DOI] [PubMed] [Google Scholar]
- 62.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 63.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO. J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson, K. J., A. Sessitsch, J. C. Corbo, K. E. Giller, A. D. Akkermans, and R. A. Jefferson. 1995. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology 141:1691-1705. [DOI] [PubMed] [Google Scholar]
- 65.Zechner, E. L., F. de la Cruz, R. Eisenbrandt, A. M. Grahn, G. Koraimann, E. Lanka, G. Muth, W. Pansegrau, C. M. Thomas, B. M. Wilkins, and M. Zatyka. 2000. Conjugative-DNA transfer processes, p. 87-174. In C. M. Thomas (ed.), The horizontal gene pool—bacterial plasmids and gene spread. Harwood Academic publishers, Amsterdam, The Netherlands.
- 66.Zhang, S., and R. Meyer. 1997. The relaxosome protein MobC promotes conjugal plasmid mobilization by extending DNA strand separation to the nick site at the origin of transfer. Mol. Microbiol. 25:509-516. [DOI] [PubMed] [Google Scholar]