Abstract
In the laboratory, we documented large variation in the morphology, toxicity, and maximum population growth rates for 32 Microcystis aeruginosa strains isolated from 12 lakes. Growth rates and mean colony sizes varied significantly across strains and were positively correlated. However, growth rates were unrelated to toxin production.
Microcystis aeruginosa is an ubiquitous cyanobacterium that causes ecological and economic damage to freshwater ecosystems when it is abundant (2, 17, 21). Recent studies have highlighted significant genetic variation within and across Microcystis populations in nature (6, 20, 23). However, many laboratory-based physiological studies focus on only one or a few congeneric or conspecific cyanobacterial strains (9, 12), and this limits our understanding of the role that genetic or physiological variation among strains may play in determining growth, and potentially bloom formation, of Microcystis (14, 19). These types of laboratory studies have, however, documented considerable variation in physiological responses to temperature, light, salinity, and macronutrient manipulations (7, 10, 16).
To extend our current knowledge regarding the amount of physiological and morphological variation that exists across a large suite of conspecific Microcystis aeruginosa strains, we used a well-controlled laboratory experiment to measure morphological characteristics and maximum population growth rates of 32 axenic, genetically distinct strains isolated from 12 lakes in the lower peninsula of Michigan (23). The experiment was conducted under axenic conditions at 25°C under saturating light (250 μmol photons m−2 s−1 at an 18 h-6 h light-dark cycle) and nutrient (2,000 μM NaNO3, 180 μM K2HPO4; modified BG-11 medium [18]) conditions; at no point in the study were contaminating bacteria microscopically observed in the samples. Following a 5-week acclimation period to the experimental conditions, stock culture concentrations were determined with a Coulter Counter and approximately 1 × 103 μm3 biovolume ml−1 of each axenic clone was inoculated into replicate (n = 4 to 12 replicates clone−1) 250-ml flasks containing 150 ml of sterile medium. Mean particle biovolumes were similar (P = 0.755) across all clones on day 0. Flasks were hand stirred and rotated twice daily to homogenize light intensities.
Five-milliliter samples were collected on days 0, 2, 5, 7, and 9 and preserved in 1% Lugol's solution for cell, colony, and total biovolume measurements. All samples were gently agitated with a Vortex mixer to resuspend particles prior to sampling. This procedure did not affect colony size measurements, because our Microcystis colonies were not disrupted by Vortex mixing as applied in this study. After mixing, biovolume estimates were determined for two subsamples from each replicate by using a Coulter Counter. These estimates were averaged, and growth rates (μ [day−1]) for each Microcystis clone replicate were determined using the equation (ln Bt1 − Bt0)/time, where Bt0 and Btl are initial and later biovolume estimates, respectively. Doubling times (days) were calculated as ln(2)/μ, as has proved useful in previous studies (15). Cell diameters and colony surface areas (equal to combined areas after measuring the two longest dimensions for all colonial lobes) were measured with a compound microscope (at magnifications of ×1,000 and ×400, respectively) for all preserved samples collected on day 9 at the end of the experiment. The microcystin concentration (ng toxin [μg dry biomass]−1) for each strain was determined via an enzyme-linked immunosorbent assay (detection limit for extract, 0.07 μg liter−1 [1]). Analysis of variance evaluated differences among the strains and lakes. Pearson's correlation coefficient determined how well growth rates related to physiological features of the strains. Data were log transformed to reduce heteroscedasticity and standardize variance, when needed.
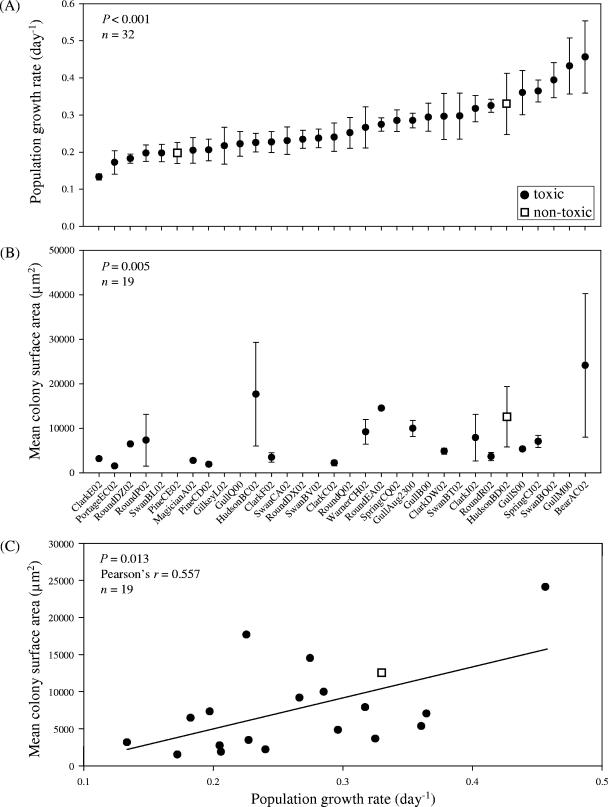
We documented significant variation in the maximum population growth rate and in the morphological characteristics of 32 Microcystis strains (Fig. 1). Maximum growth rates differed significantly among genotypes (Fig. 1A) (P < 0.001) but not among lakes (Table 1) (P = 0.148) and ranged from 0.13 to 0.46 day−1 (Fig. 1A) (doubling time, 5.2 to 1.5 days, respectively), with an average growth rate across all strains of 0.27 day−1 (Fig. 1A) (doubling time, 2.8 days). The growth rates of Microcystis observed in this study were similar to, but tended to be a bit lower than, rates observed in previous studies (range, 0.05 to 1.11 day−1) (4, 8, 14). However, many of the past studies incorporated single-celled strains. In our study, growth rates were similar (P = 0.944) for single-celled (0.27 ± 0.02 day−1 [mean ± standard error; n = 13]) and colonial (0.27 ± 0.02 day−1 [mean ± standard error; n = 19]) strains, suggesting that colony formation was not costly in terms of growth rate over 9 days.
FIG. 1.
Growth and physiological variation of 32 Microcystis aeruginosa strains from 12 Michigan lakes. (A) Maximum population growth rates for 32 M. aeruginosa strains. (B) Colony surface areas for the 19 of 32 M. aeruginosa clones that formed colonies throughout the experiment. The results in panel B exclude one outlier (studentized residual = −3.5; including outlier, P = 0.070). (C) Relationship between maximum population growth and colony surface area. Filled circles, toxic strains of M. aeruginosa (strains that contain the toxin gene [mcyA] and/or have been shown to produce microcystins). Empty squares, nontoxic strains of M. aeruginosa that lack the mcyA gene and do not produce microcystins. Error bars, ± 1 standard error; n, number of strains included in analysis.
TABLE 1.
Mean growth rates, cell diameters, and colony surface areas for all Microcystis aeruginosa strains and microcystin concentrations for all toxigenic M. aeruginosa strains isolated from 12 lakes in the lower peninsula of Michigan
| Lake | Growth rate (day−1)
|
Cell diam (μm)
|
Colony surface area (μm2)
|
Microcystins (ng toxin [μg dry mass]−1)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| na | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Bear | 1 | 0.46 | 1 | 5.20 | 1 | 24,133 | 1 | 0.1173 | ||||
| Clark | 5 | 0.24 | 0.07 | 5 | 4.76 | 0.20 | 5 | 4,327 | 2215 | 5 | 0.3053 | 0.1253 |
| Gilkey | 1 | 0.22 | 1 | 4.41 | 0 | 1 | 0.9831 | |||||
| Gull | 5 | 0.32 | 0.08 | 5 | 4.60 | 0.42 | 2 | 7,673 | 3285 | 4 | 0.2375 | 0.1713 |
| Hudson | 2 | 0.28 | 0.07 | 2 | 4.78 | 0.11 | 2 | 15,132 | 3597 | 1 | 0.2426 | |
| Magician | 1 | 0.20 | 1 | 4.70 | 1 | 2,754 | 1 | 0.1746 | ||||
| Pine | 2 | 0.20 | 0.01 | 2 | 4.10 | 1.40 | 1 | 1,900 | 1 | 0.2436 | ||
| Portage | 1 | 0.17 | 1 | 4.98 | 1 | 1,524 | 1 | 0.1207 | ||||
| Round | 6 | 0.24 | 0.05 | 6 | 4.78 | 0.15 | 4 | 7,996 | 4617 | 6 | 0.0709 | 0.0410 |
| Spring | 2 | 0.32 | 0.06 | 2 | 4.61 | 0.13 | 1 | 7,052 | 2 | 0.4831 | 0.5773 | |
| Swan | 5 | 0.27 | 0.08 | 5 | 4.95 | 0.26 | 0 | 5 | 0.2533 | 0.1554 | ||
| Warner | 1 | 0.27 | 1 | 4.63 | 1 | 9,193 | 1 | 0.3319 | ||||
| Pvalue | 0.148 | 0.574 | 0.013 | 0.054 | ||||||||
n, number of genetically distinct M. aeruginosa strains per lake.
Cell diameters (P < 0.001) and colony surface areas (Fig. 1B) (P = 0.005) also differed across the distinct Microcystis strains. Colony surface areas differed among lakes (Table 1) (P = 0.013), while cell diameters did not (Table 1) (P = 0.574). Interestingly, there was a significant positive correlation between maximum population growth rate and average colony surface area for the colonial strains of Microcystis (Fig. 1C) (P = 0.013; Pearson's r = 0.557). This finding is counterintuitive given that nutrient transfer efficiency predicts that smaller colonies should grow more rapidly than larger colonies due to their greater surface-to-volume ratios (3, 13). Also, larger colonies should suffer more from self-shading than smaller colonies, potentially lowering biomass-specific growth rates in large forms. Our results suggest that there is no growth rate cost for colony formation and growth over the 9-day duration of this experiment; however, other ecological consequences could occur as a result of colony size. For example, large cyanobacterial morphologies could be too large for some grazers to handle and ingest (22), or larger colonies may have better migratory capabilities, enabling them to outcompete smaller phytoplankters for light (13).
Microcystin concentrations across all clones ranged from below the detection limit to 0.98 ng (μg dry mass)−1 and averaged 0.24 ng (μg dry mass)−1. There was a nearly significant (P = 0.054) variance in microcystin concentrations for toxic Microcystis strains from different populations (Table 1). Additionally, there was no correlation of toxin content with growth rate for toxic strains (P = 0.945; Pearson's r = −0.013), suggesting no tradeoff of growth rate with increased toxin production for Microcystis.
Large genetically based trait variation within species could influence competitive and trophic interactions by introducing variance in how a species interacts with its consumers and competitors. Thus, future theoretical and empirical studies might consider extending our observations to evaluate the ecological and evolutionary consequences of intraspecific variation of harmful bloom-forming cyanobacteria on community and ecosystem dynamics. Such variation may help explain the dominance of cyanobacteria over other phytoplankters in some situations (5, 11).
Acknowledgments
We thank J. Rose and M. Morgan for analyzing microcystins, O. Sarnelle and E. Litchman for advice about the experimental design, and the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology for access to their Coulter Multisizer III. O. Sarnelle, E. Litchman, J. Montoya, T. Snell, and two anonymous reviewers provided comments that improved the manuscript.
Support was provided by EPA's Science to Achieve Results graduate fellowship program, NSF's IGERT program, and the Harry and Linda Teasley endowment to Georgia Tech.
Footnotes
Published ahead of print on 8 September 2006.
REFERENCES
- 1.An, J., and W. W. Carmichael. 1994. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 32:1495-1507. [DOI] [PubMed] [Google Scholar]
- 2.Chorus, I., and J. Bartram (ed.). 1999. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. E & FN Spon, London, United Kingdom.
- 3.Fogg, G. E. 1991. The phytoplanktonic ways of life. New Phytol. 118:191-232. [DOI] [PubMed] [Google Scholar]
- 4.Hesse, K., and J.-G. Kohl. 2001. Cyanotoxins: occurrence, causes, consequences, p. 104-115. Springer-Verlag, Berlin, Germany.
- 5.Hutchinson, G. E. 1961. The paradox of the plankton. Am. Nat. 95:137-145. [Google Scholar]
- 6.Janse, I., W. E. A. Kardinaal, M. Meima, J. Fastner, P. M. Visser, and G. Zwart. 2004. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl. Environ. Microbiol. 70:3979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, D.-Y., and G.-Y. Rhee. 1999. Kinetics of growth and death in Anabaena flos-aquae (cyanobacteria) under light limitation and supersaturation. J. Phycol. 35:700-709. [Google Scholar]
- 8.Long, B. M., G. J. Jones, and P. T. Orr. 2001. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 67:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyck, S., and K. Christoffersen. 2003. Microcystin quota, cell division and microcystin net production of precultured Microcystis aeruginosa CYA 228 (Chroococcales, Cyanophyceae) under field conditions. Phycologia 42:667-674. [Google Scholar]
- 10.Oh, H.-M., S. J. Lee, M.-H. Jang, and B.-D. Yoon. 2000. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl. Environ. Microb. 66:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter, K. G. 1977. The plant-animal interface in freshwater ecosystems. Am. Sci. 65:159-170. [Google Scholar]
- 12.Repka, S., J. Mentonen, J. Vaitomaa, L. Saari, and K. Sivonen. 2001. Effects of nutrients on growth and nodularin production of Nodularia strains GR8b. Microb. Ecol. 42:606-613. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds, C. S. 1984. The ecology of freshwater phytoplankton. Cambridge University Press, Cambridge, United Kingdom.
- 14.Robarts, R. D., and T. Zohary. 1987. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 21:391-399. [Google Scholar]
- 15.Sherr, B. F., E. B. Sherr, and T. Berman. 1983. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl. Environ. Microbiol. 45:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivonen, K. 1990. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production of Oscillatoria agardhii strains. Appl. Environ. Microbiol. 56:2658-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivonen, K. 1996. Cyanobacterial toxins and toxin production. Phycologia 35:12-24. [Google Scholar]
- 18.Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel, and T. F. Nalepa. 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can. J. Fish Aquat. Sci. 58:1208-1221. [Google Scholar]
- 19.Vézie, C., J. Rapala, J. Vaitomaa, J. Seitsonen, and K. Sivonen. 2002. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 43:443-454. [DOI] [PubMed] [Google Scholar]
- 20.Via-Ordorika, L., J. Fastner, R. Kurmayer, M. Hisbergues, E. Dittmann, J. Komarek, M. Erhard, and I. Chorus. 2004. Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: detection of microcystins and microcystin genes in individual colonies. Syst. Appl. Microbiol. 27:592-602. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe, M. F., K.-I. Harada, W. W. Carmichael, and H. Fujiki. 1996. Toxic Microcystis. CRC Press, Boca Raton, Fla.
- 22.Webster, K. E., and R. H. Peters. 1978. Some size-dependent inhibitions of larger cladoceran filterers in filamentous suspensions. Limnol. Oceanogr. 23:1238-1245. [Google Scholar]
- 23.Wilson, A. E., O. Sarnelle, B. A. Neilan, T. P. Salmon, M. M. Gehringer, and M. E. Hay. 2005. Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms. Appl. Environ. Microbiol. 71:6126-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]