Burkholderia pseudomallei is listed by the Centers for Disease Control as a potential bioterrorism agent (www.bt.cdc.gov/agent/agentlist.asp) because it causes melioidosis in humans, a potentially fatal septicemic infection following soil or water exposure (6, 41). Melioidosis usually takes one of three main courses: (i) a rapidly progressing septicemia with or without pneumonia, (ii) a localized soft-tissue infection, or (iii) a subclinical infection with delayed conversion to a clinically evident infection. Central-nervous-system infection has a particularly high mortality rate but is uncommon, especially outside Australia (9). In parts of Australia where melioidosis is endemic, histories of percutaneous-inoculation events can be determined in at least 25% of the cases (10). Exposure to B. pseudomallei by inhalation of aerosolized bacteria or by ingestion of contaminated water is a possible alternative route of infection (11, 28). Fatal disease occurs mainly in people with major comorbidities that involve metabolic acidosis, leading to the proposal that the progression of septicemic disease might be the result of substrate utilization by B. pseudomallei (31, 59). The minimum infective dose of B. pseudomallei has not been calculated for humans. The 50% lethal dose was between 103 and 105 bacteria when given by the intravenous route to C57BL/BALB/c mice (22). In Porton outbred mice and Syrian hamsters, the 50% lethal dose was only 19 CFU (65). Person-to-person transmission has been reported only rarely (38, 45), highlighting the relative importance of the environment in disease transmission.
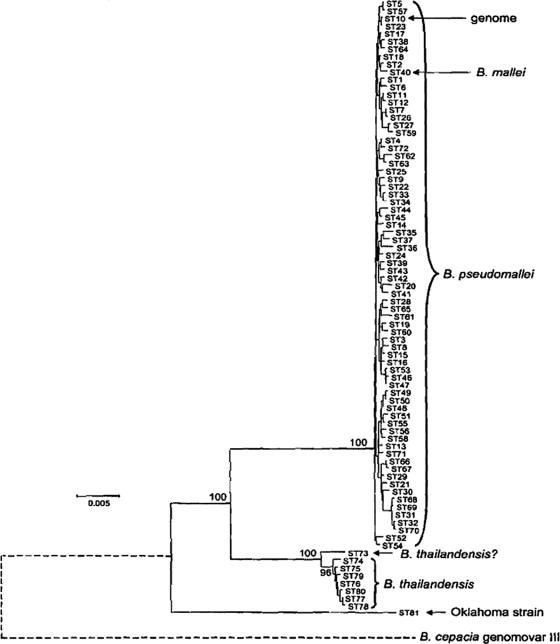
B. pseudomallei is an oxidase-negative, gram-negative bacillus that was grouped with other members of the former Pseudomonas RNA homology group II to form the genus Burkholderia (90). The published genome of B. pseudomallei (strain K96243) contains two chromosomes of 4.07 megabase pairs and 3.17 megabase pairs, respectively (20). The large chromosome encodes many core functions associated with central metabolism and bacterial growth. The small chromosome carries more genes associated with adaptation and survival in different niches. Multilocus sequence typing of B. pseudomallei suggests likely phylogenetic relationships between the first fully sequenced strain, other geographically distinct strains, and near-neighbor species (Fig. 1). The multilocus-sequence-typing result shown in Fig. 1 suggests that B. pseudomallei is more closely related to Burkholderia mallei than to Burkholderia thailandensis or Burkholderia cepacia. These phylogenetic studies indicate that B. pseudomallei has evolved more recently than the other members of the group and probably represents an adaptation to a more specialized habitat (17). B. mallei has been shown by multilocus sequence typing of several epidemiologically unrelated isolates to belong to a single clade within B. pseudomallei (17). However, unlike B. pseudomallei, B. mallei is immotile, does not persist in the environment, and has adapted to a more restricted ecological niche in close association with its mammalian hosts (54).
FIG. 1.
Phylogenic relationship. Minimum-evolution tree of B. pseudomallei and near-neighbor species (reproduced with permission from reference 17). The sequence types (STs) represent a unique allelic profile characterized from 147 isolates. The diagram was constructed from concatenated sequences of the multilocus sequence typing loci. The percentages for node recoveries in 1,000 bootstrap replicates are shown. The bar shows differences at 0.5% of nucleotide sites. The positions of B. pseudomallei ST10, whose genome was sequenced, and of B. mallei (ST40) are indicated by the arrows. The Oklahoma strain was isolated from a patient with suspected melioidosis in Oklahoma and was originally named B. pseudomallei. The position of B. cepacia genomovar III is indicated by a dotted line (see reference 17 and www.sanger.ac.uk/Projects/B_cepacia/).
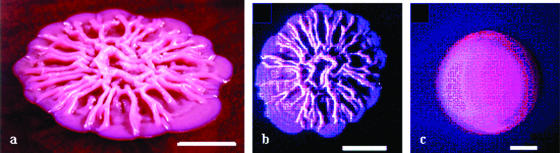
Mature colonies of B. pseudomallei on solid agar media often take on a wrinkled appearance after several days of incubation in solid media (23, 59) (Fig. 2a and b). Some strains do not show this wrinkling effect, which is more pronounced in solid agar formulations containing glycerol (23). Bacilli have one or more terminal flagella and are motile, particularly in the early stages of their growth cycle. Some strains of B. pseudomallei produce smooth colony growth on first culture (Fig. 2c), and occasional strains are overtly mucoid, with an appearance similar to that of Pseudomonas aeruginosa capsular polysaccharide overproducers (62). In stationary-phase culture, B. pseudomallei isolates consist of short bacilli measuring a spectrum of sizes around 1.2 μm (59). Precise measurement of the size distribution of B. pseudomallei cells has not been published.
FIG. 2.
Colony formation. Mature colonies of B. pseudomallei on solid media: (a) B. pseudomallei NCTC 13177 showing characteristic wrinkling on Ashdown's selective agar (angled shot), (b) NCTC 13177 on B. pseudomallei selective agar, and (c) persistently mucoid strain B. pseudomallei BCC 11 on B. pseudomallei selective agar. Scale bars measure 0.5 cm (23).
Adaptation to a wide variety of potential habitats is reflected in the species' metabolic repertoire since B. pseudomallei uses a wide range of substrates, including a variety of sugars (59). An important exception among these is l-arabinose, which the closely related but nonpathogenic Burkholderia thailandensis can metabolize (72). Small quantities of 2-hydroxymyristic acid (2-HMA) are present in B. pseudomallei derivatized extracts and are absent in B. thailandensis preparations (32). On the basis of work completed with Salmonella enterica serovar Typhimurium, the production of 2-HMA is thought to assist intracellular survival and thus pathogenesis (16).
B. pseudomallei accumulates polyhydroxybutyrate (PHB) in large, central granules to give a negative staining effect on Gram stains (59) and lucent zones on electron microscopic images (Fig. 3A) . The appearance is of bipolar staining, though this is not specific to the burkholderias and may be absent in young colonies. The accumulation of prominent granules of PHB as energy stores in each bacillus reflects a metabolism adapted to long-term survival (20). PHB accumulation is affected by carbon/nitrogen ratios. There is no ability to fix atmospheric nitrogen, a feature required by Burkholderia species recently noted to reside in leguminous root nodules (50). The metabolic activity of B. pseudomallei in its natural habitat (whether inside eukaryotic cells or in polymicrobial biofilms) is likely to differ from the metabolism exhibited under rapid-growth laboratory culture conditions.
FIG. 3.
Cellular appearance. Transmission electron microphotographs of B. pseudomallei showing prominent intracellular inclusions (white globules) of polyhydroxybutyrate (A) and a coccoid cell after being stressed at acid pH (pH 4) (B). The diameter of the cell is approximately 0.5 to 1.0 μm.
Laboratory identification of B. pseudomallei can be technically difficult (27). Laboratory identification systems that rely on substrate utilization profiles often erroneously label B. pseudomallei as B. cepacia (27, 34). Serological tests for melioidosis are more useful for epidemiological studies and possibly in following the course of disease than for diagnosis during the early stages of septicemia (34, 70, 80). Nucleic acid amplification of B. pseudomallei-specific sequences of the 16S-23S spacer region (39), the 2-hydroxymyristate synthesis operon based on a similar sequence in Salmonella enterica serovar Typhimurium (16, 48), or the groEL transcriptional regulator has been reported, the last target being capable of distinguishing B. pseudomallei and the nonpathogenic B. thailandensis (86, 87). Although a candidate vaccine that could provide some melioidosis protection by cross-reactivity is under trial for glanders (84), there is no commercial vaccine available for protection against melioidosis. The intracellular nature of melioidosis means that a vaccine is unlikely to provide complete immunological protection against melioidosis unless T-cell immunity is engaged. The combined lack of an effective vaccine and the unusual survival characteristics in the environment make B. pseudomallei an organism of significance in public health and biodefense.
The military connection.
Melioidosis was first reported by an army pathologist working in Burma in 1912 (85). Shortly afterwards, there were cases of disease in laboratory animals from Malaya, where an epizootic in a research animal house led to speculation that melioidosis was zoonotic (75). This focus on the role of animals in the disease and the need to distinguish it from glanders led veterinary scientists to document melioidosis cases in a series of domestic animal species. Given the lack of standardized bacterial nomenclature and the relative inaccessibility of preelectronic medical literature, it is difficult to assess the prevalence or distribution of the disease before military physicians recognized the infection during the conflict in French Indochina (4). Prior to the French conflict in Indochina, a synopsis of tropical medicine described the prevalence of the disease as “limited to Burma, Malaya, Ceylon, possibly Uganda. Beggars, rag-pickers and drug-addicts in Rangoon and Singapore” (43). Careful investigation highlighted the possible role of soil contaminated with B. pseudomallei as a source of infection (4). The frequency of melioidosis in Southeast Asia increased during the Vietnam conflict, when servicemen contracted the infection during their tours of duty. A particularly high incidence was noted among helicopter winch men, leading to the suspicion that inhalation of aerosols was an important means of transmission (26). There are reports of multiple cases of melioidosis in veterans of the Vietnam conflict manifesting as both severe and potentially fatal or as a more subacute infection (18, 74). Toward the end of the conflict, it became clear that melioidosis could remain dormant in patients for prolonged periods (68), the longest recorded being around 29 years after presumed exposure in the theater of operations (7). A late-onset case of cutaneous melioidosis following presumed exposure in the Pacific theater during the second world war has been reported, implying a disease-free interval of 62 years (53). Late-onset septicemic infections are thought to occur in patients with sequestered disease (6, 41). Interestingly, sero-epidemiology studies in British special forces deployed in the endemicity region during the Malayan emergency of 1948 to 1960 showed little evidence of residual infection (61). Moreover, late-onset melioidosis has not been observed in Australian veterans previously deployed in Vietnam. It is not clear why United States forces contracted melioidosis when allied units deployed on similar operations in the endemicity region, and close to locations where the native population currently experiences melioidosis, did not contract the disease. Melioidosis was among the differential diagnoses considered for the acute respiratory syndrome noted in armed forces serving in Iraq in mid-2003 (ProMed 20030810.1978).
GEOGRAPHICAL DISTRIBUTION
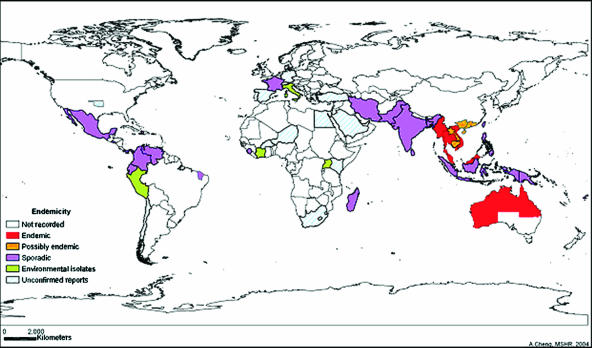
The disease distribution shown in Fig. 4 indicates that the principal burden of the disease is between latitudes 20oN and 20oS. There are only two evidences for local acquisition of melioidosis outside the laboratory in North America: one infection in Oklahoma, following a farming accident, and another infection in Georgia, following a motor vehicle accident (46, 55). The Oklahoma isolate belongs to a distinct clade of B. pseudomallei (Fig. 1) and possibly represents a distinct species (17, 91). Melioidosis has been reported in Puerto Rico (15), suspected in El Salvador (5), and considered to be under-diagnosed in other areas of the Caribbean and South America (49, 58). Melioidosis has recently been recognized as an emerging infectious disease in Brazil, where a case cluster in Northern Brazil was associated with the onset of unusually heavy rainfall (63).
FIG. 4.
Melioidosis world distribution. The incidence of melioidosis is represented by the color code shown on the map (reviewed in reference 6). Epidemiology in Thailand as reported by Vuddhakul et al. (82) is represented. Areas showing nonrecorded cases (white regions) should not be assumed to be free of melioidosis since identification and reporting in the Americas, parts of Southeast Asia, and most of Africa are incomplete (6). Zones with higher incidences (red regions) may correlate with a higher awareness of melioidosis and more-developed laboratory diagnostic and surveillance infrastructure.
The epidemiology of melioidosis in Africa remains uncertain and may reflect a lack of diagnostic laboratory infrastructure in parts of Africa where the disease is most likely to be prevalent. There were cases of human melioidosis in Iran in the 1970s, when B. pseudomallei was isolated from Iranian rice fields (60). In Asia, there are documented cases of human melioidosis in the Hong Kong SAR and parts of southern China, including Hainan island (Fig. 4) (6, 17). A first case of melioidosis was recently reported from South Korea, following possible occupational exposure in Indonesia (40), but the status of the disease in North Korea is unknown. In Thailand, a correlation indicating a higher frequency of specific HLA-DR alleles in patients with the more severe forms of melioidosis was observed (14).
The highest prevalence of melioidosis is observed in rice farmers, servicemen, miners, adventure travelers, and indigenous peoples in the main endemicity zone of northern Australia and Southeast Asia (41). The sharp increase in acute septicemic cases in the Darwin region in 1990 and 1991 mainly affected Australian indigenous aboriginal people (47). After a higher incidence of comorbidities, such as diabetes and chronic renal failure, and a high level of exposure to moist surface soil were taken into account, there was no independent risk attributable to ethnic factors of the indigenous affected population. The indigenous population of the Australian endemicity region has a high prevalence of antibodies to B. pseudomallei (1), probably reflecting a high level of exposure to contaminated soil and water. It is possible that low-level exposure to environmental B. pseudomallei may render the indigenous population relatively resistant to melioidosis.
Water supply-related melioidosis has been documented (11, 28, 29). Sporadic cases in Australia and seasonal peaks of septicemic disease are usually associated with soil exposure, while case clusters appear to be linked with water contamination. The peak risk of septicemic disease in Australia occurs at 2 weeks following the onset of summer rainfall in the tropical north (12). Whether this is due to a change in the hydration of B. pseudomallei-contaminated soil, increased aerosol or percutaneous-inoculation risk, or B. pseudomallei washed out of rain clouds has yet to be systematically investigated. Careful prospective environmental sampling across northern Australia targeted around the location of septicemic cases has shown that the environment is not widely or heavily contaminated throughout the endemicity region, despite assertions repeatedly made by public health authorities. Culture-positive environmental samples of both surface soil and water were surprisingly rare (33). However, PCR-positive samples have been obtained from sites where cultures were persistently negative, indicating the limits of sensitivity for culture-based detection (3).
The possible association of heavy rainfall and melioidosis was first recognized during an outbreak in captive marine mammals in a Hong Kong oceanarium (19), where infection peaked following heavy summer rainfall and subsequent soil contamination of their water. The apparent lack of melioidosis in marine mammals in the wild, combined with the poor growth of B. pseudomallei under conditions of high osmotic stress, suggests that these bacteria are unlikely to survive in the open sea and pose an infection risk there. If the Hong Kong findings apply more widely, then infection in coastal waters where there has been significant soil runoff remains a possibility.
ENVIRONMENTAL SURVIVAL CHARACTERISTICS
The unusual ability of B. pseudomallei to survive for months to years in the environment can be inferred from the persistence of the bacterium in the melioidosis endemicity zone throughout the dry season (78). It is notable that the ability to survive an adverse soil or water environment appears to parallel the ability of B. pseudomallei to sequester within human macrophages and lympho-reticular organs in a dormant or quiescent state for periods of many years. Useful long-term survival work has been undertaken on B. pseudomallei in water (89). The inactivation kinetics of B. pseudomallei in culture broth appear complex (32), while other matrices, such as soil or laboratory media, have not been studied in the same detail or for as long as culture broth.
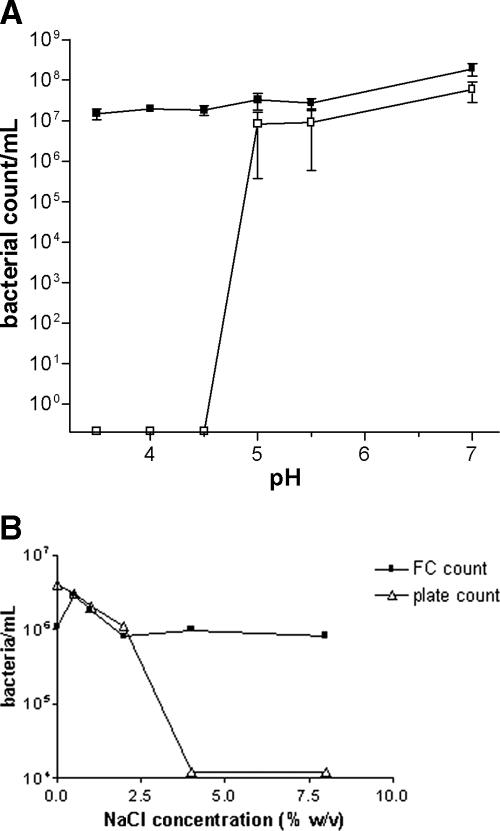
The bacterial responses in liquid medium to the different physical and chemical stresses seem to follow broadly similar patterns, as shown, for example, by the effects of pH or salt (Fig. 5). The number of culturable bacteria generally falls very quickly with increasing stress levels (see data on rapid bacterial inactivation at acid pHs or salt concentrations above 2.5% [wt/vol] in Fig. 5). Available data on the survival of B. pseudomallei under various physical conditions are summarized in Table 1. Although Table 1 highlights the need for further research on the effects of physical and chemical variables, the data demonstrate the ability of B. pseudomallei to survive for months or even years in moist and warm environments with pHs between 5 and 8.
FIG. 5.
Effect of pH and salt. Bacteria were grown in trypticase-peptone-glycerol, overnight cultures were washed twice by centrifugation as described by Howard and Inglis (25), and the cells in the pellet were resuspended in cold sterile water at the indicated pH or NaCl concentrations. Panel A shows the divergence between B. pseudomallei colony counts and viable cells as detected by flow cytometry and supravital stains produced by incubation at low pHs. Panel B shows the same divergence between culturable cells and viable cells counted by flow cytometry of B. pseudomallei NCTC 13177 at various NaCl concentrations. Open boxes indicate bacterial counts in numbers of CFU/ml. Closed boxes indicate viable bacterial counts as detected by flow cytometry.
TABLE 1.
Effect of environmental factors on the survival of B. pseudomallei
| Environmental factor | Condition | Duration of expt | Outcome |
|---|---|---|---|
| Triple-distilled watera | 10 CFU/ml6 | >3 yr | Rise to 108 CFU/ml after 1 mo then fall to 104 CFU/ml after 2 yr |
| Water contentb | 0% | 30 days | No later survival |
| 5% | 40 days | No later survival | |
| 10% | 70 days | No later survival | |
| 20% | 439 days | No later survival | |
| 40% | >726 days | Survival for expt duration | |
| 80% | >726 days | Survival for expt duration | |
| Tempb | 0°C | <42 days | No later survival |
| 8°C | <190 days | No later survival | |
| 16°C | <477 days | No later survival | |
| 24 °C | >720 days | Survival for expt duration | |
| 32°C | >720 days | Survival for expt duration | |
| 40°C | <42 days | No later survival | |
| 48°C | <12 days | No later survival | |
| pH | 2b | 1 day | No later survival |
| 3b | 7 days | No later survival | |
| 4b | 231 days | No later survival | |
| 5-8b | >726 days | Survival for expt duration | |
| 9b | 26 days | No later survival | |
| 10b | 1 day | No later survival | |
| 3.5-7.0c | 24 h | 7 log10 reduction in CFU/ml at pHs of <4.5 | |
| Chlorine | 1 mg/literc | 30 min | 4 log10 reduction in CFU/ml |
| 0.5-4.0 mg/literd | 0-60 min | 1-6 log10 reduction (at pHs of 9 and 6, respectively) | |
| 4 mg/liter (pH 6-9)d | Up to 30 min | 7 log10 reduction in CFU/ml after 30 min | |
| Chloraminec | 1 mg/liter | 24 h | 2.5 log10 reduction in CFU/ml |
| 1 mg/liter | 48 h | 3 log10 reduction in CFU/ml | |
| UV light (254 nm) | 4.65 W/m2b | 7.75 min | Shorter survival time than control bacteria |
| 44 J/m2c | NAe | 1 log10 reduction in CFU/ml | |
| 120 J/m2c | 4 log10 reduction in CFU/ml |
Survival measured by colony formation alone may be misleading since low pH or high osmolarity produces bacterial cells that seem viable as determined by flow cytometry and supravital stains (32) but that cannot be cultured by conventional means. These stressed bacteria are considered viable but nonculturable cells (VBNC) (for details on this type of cell, see the review of characteristics observed in stressed Legionella pneumophila in reference 2). B. pseudomallei does not appear to persist in a culturable form in environmental samples for more than a matter of hours or days, with the exception of bacteria in distilled water, in which this species can persist for over 3 years (89). In contrast, nonculturable B. pseudomallei in the environment must persist for at least 1 year in a form that can survive through the dry season.
(i) Survival in liquid suspension.
Given the ability of B. pseudomallei to survive for long periods in moist soil, the species' ability to survive in water has been studied at length, though the mechanism of prolonged survival is still not understood. Cultures of B. pseudomallei inoculated into triple-distilled water have remained culture positive for over 3 years (89). The rise, fall, and stabilization of the number of B. pseudomallei bacteria in water are summarized in Table 1. The experiment was continued for several years beyond the publication date, and still, the cultures remained positive (V. Wuthiekanun, personal correspondence). The number of cells of the B. pseudomallei strain implicated in the Western Australian outbreak of 1997 dropped from 6 log10 to around 4 log10 CFU/ml in the 24 h after inoculation into filter-sterilized potable water (circa 1 ppm chlorine) from the affected community but returned to the original count (near 6 log10) 4 weeks after inoculation. The capacity to survive in distilled water for prolonged periods implies a bacterial lifestyle in water where long-term carbon storage in bacterial cells powers a low-rate metabolism. The PHB granules present in mature, stationary-phase bacilli may be the energy storage unit. Since all the burkholderias, including nonpathogenic species, produce PHB, it is unlikely that PHB synthase is a virulence factor, albeit this substance may enhance the environmental survival of the species. A putative polyhydroxybutyrate synthase gene for B. pseudomallei, PhaC, was recently identified (GenInfo Identifier number DQ083762).
(ii) Survival in soil.
Periodic soil sampling for B. pseudomallei showed that the persistence of culturable bacteria depends on soil type, hydration, and the nature of the starting inoculum (78). Well-drained, light, sandy soils are less able to support the prolonged persistence of B. pseudomallei. Waterlogged, heavy clay soils are much better at supporting bacterial persistence (33). A faster decline in bacterial count has been inferred for drier soil environments from the available qualitative data. Samples taken from greater depths (>30 cm) in field studies were more likely to contain culturable B. pseudomallei, possibly reflecting the higher residual water content at greater depths (78). Samples recovered from a depth of 90 cm in softer soils of Thailand yielded up to 105 CFU of B. pseudomallei per ml of soil supernatant, with a median count of 250 CFU/ml in soil from the main endemicity region of northeast Thailand (71). A rising water table at the onset of the tropical summer rain season was cited as an explanation for the reappearance of B. pseudomallei in the more superficial layers of soil prior to human exposure. Soil with a water content of less than 10% led to the death of B. pseudomallei within 70 days, while soil with a water content of more than 40% maintained bacterial life for 726 days (79). It is possible that the attraction of B. pseudomallei to oxygen (aerotaxis) leads to preferential colonization of the water/air interface bridging soil particles in well-aerated moist soils. The cellular effects of desiccation on bacterial survival and recovery have not been studied. Although quantitative data are lacking, an increase in B. pseudomallei soil half-life would be expected with increased water content. Other physical characteristics of soil, such as particle size, organic content, and hydrophilic/hygroscopic properties, are also expected to affect B. pseudomallei survival. Given the capacity of B. pseudomallei to survive from months to years in distilled water, the survival kinetics of this species in soils of various water content levels should help determine the risk of melioidosis according to regional and seasonal soil conditions.
(iii) Survival in dry environments.
Though not as prodigious as its ability to survive in water, the ability of B. pseudomallei to resist desiccation is greater than that of many other gram-negative bacilli (79). B. pseudomallei can be cultured from some dry soils 4 weeks after inoculation, provided that suitable resuscitation methods are used in the preliminary stages of bacterial isolation (34). B. pseudomallei will freeze dry with an approximately 1 log10 decrease in viable cells per freeze-dry cycle. This is a relatively low proportion of the high numbers (several log10s) that can be recovered from environmental samples. An explanation for the relatively high resistance of B. pseudomallei to desiccation has yet to be found.
(iv) Temperature.
Storing B. pseudomallei in a refrigerator (between 0 and 4°C) converts a proportion of the bacteria to a nonculturable state. Loss of apparent viability can be 80 to 90% or more after 24 h at 0°C (Table 1). At 42°C, B. pseudomallei will grow rapidly in liquid media, exhaust the nutrients within 48 h, and form a sediment consisting of approximately 80% VBNC cells (59). Analysis of B. pseudomallei even grown at 37°C shows a significant discrepancy between the numbers of culturable cells and VBNC (as identified by flow cytometry with supravital stains). While the effects of different temperatures on bacterial growth have been studied, the effect of long-term exposure to low or high temperatures on survival in the environment or in the laboratory has not been characterized.
(v) UV exposure.
Little work has been done on the B. pseudomallei response to sunlight or other UV sources. One questionable study reports that the susceptibility of B. pseudomallei to UV is greater than that of other bacterial species. In this study, B. pseudomallei (in a starting inoculum of 107 CFU/ml) was inactivated after 7.75 min of exposure to 4.65 W/m2 of UV radiation at a wavelength of 254 nm (79). This result is puzzling since, in the same study, other permanent soil bacteria survived a dose of 18.60 W/m2 for 31 min (79). In a more quantitative study, the dose required to reduce the number of B. pseudomallei bacteria by 1 log or by 4 logs was 44 or 120 J/m2, respectively (25), well within the range for other bacteria. At present, there is little to suggest that B. pseudomallei is significantly different from other bacterial species in the ability to resist UV light or repair the damage that UV light induces. The use of UV treatment units for potable water supply systems should prompt a rigorous evaluation of the effect of UV on B. pseudomallei in liquid suspension.
(vi) pH changes.
The soils from which B. pseudomallei was most commonly isolated in the high-endemicity region of northeast Thailand were found to be unusually acid (35). These conditions enhanced the production of a bacterial acid phosphatase (13). The enzyme was subsequently identified as a tyrosine kinase and thought to be a virulence factor (37). The optimal pH range for B. pseudomallei has been reported to be between pH 5 and 8, with rapid bacterial inactivation below pH 4.5 (79) (Fig. 5A). The abrupt reduction observed in colony-forming numbers suggests that the damage produced by pH occurs after reaching a threshold. Below this threshold (pH 4.5), the reduction in bacterial cells able to produce colonies parallels an increase in VBNC (see above). These VBNC take on a gram-positive-coccoid shape as shown in Fig. 3B. The VBNC revert to the conventional gram-negative-bacillus appearance (Fig. 3A) after removal of the acidic medium and its replacement with fresh media at neutral pH. More light on this interesting change in appearance and colony-forming ability will have to wait until data on the detailed survival kinetics of B. pseudomallei as a function of acid exposure become available.
(vii) Osmotic stress.
B. pseudomallei survives exposure to solutions containing less than 2.5% NaCl (wt/vol) with no significant reduction in survival, as shown by the similar numbers of cells able to form colonies and VBNC (Fig. 5B). Only at concentrations above 2.5% (wt/vol) does NaCl become an apparent physiological stress similar to that seen under low-pH conditions (Fig. 3B). VBNC were observed at higher but not at lower osmotic pressures, within osmolarities produced by varying the NaCl concentration between 0.1 and 8% (wt/vol) (Fig. 5B). Little is known about survival in seawater because, until recently, only a few cases of melioidosis had been associated with near-drowning in seawater. The reports of melioidosis following the 2004 Indian Ocean tsunami may provide epidemiological clues to the effect of salinity on marine B. pseudomallei survival.
(viii) Chemical stress.
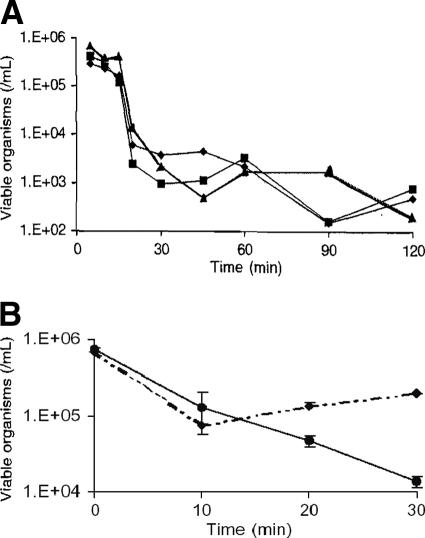
The activity of chlorine against B. pseudomallei is of particular interest since the chemical is widely used to treat drinking water supplies (at concentrations around 1 ppm, or 1 mg/liter). Under laboratory conditions, a concentration of 103 ppm free chlorine (that is, 1 g per liter, formulated as a hypochlorite solution and measured by colorimetry) decontaminated 106 CFU of B. pseudomallei in stationary phase (strains NCTC 13177 and NCTC 10276) (24). Interestingly, the data in Fig. 6A (24) show that the inactivation kinetics of B. pseudomallei by chlorine occurs at several different inactivation rates. There is a lag time after initial exposure to chlorine (a latent phase extending approximately up to 15 min) where the cells seem to tolerate 1 ppm chlorine, as indicated by little inactivation. Following is a short phase of rapid-inactivation kinetics, after which (at 30 min) the bacteria stabilize and persist at relatively constant levels for the reminder of the experiment. A similar inactivation kinetics by chlorine, consisting of a rapid inactivation followed by a relatively constant level of survival, was reported for bacillus spores and related to consumption of chlorine and not to a heterogeneous population including resistant organisms (66).
FIG. 6.
Effect of chlorine. Overnight cultures were washed twice by centrifugation and resuspended in cold sterile water before exposure to chlorine (24). (A) The longer-term survival of three strains of B. pseudomallei (diamonds, NCTC 13177; squares, NCTC10276; and triangles, BCC11) with exposure to 1 ppm chlorine in water. (B) Survival of B. pseudomallei NCTC 13177 with no prior (⧫) and prior (•) exposure to 100 ppm chlorine in water after subsequent exposure to 1 ppm chlorine. Error bars indicate standard errors of the means (24).
A later assessment of the susceptibility of B. pseudomallei to chlorine, using a most-probable-number method, confirmed the persistence of bacteria at commonly used chlorine concentrations but showed that chlorine was a more effective agent than monochloramine. Each of the three tested strains (NCTC 13177, NCTC 10276, and BCC 11) showed an initial decrease to undetectable levels by conventional colony count, with a rebound within 2 days. These same three strains were all recovered in viable form after exposure to 1,000 ppm free chlorine. Tolerance was not a cumulative phenomenon, but prior exposure to 100 ppm available chlorine produced the small additive survival effect shown in Fig. 6B, which resembles that observed with Pseudomonas aeruginosa (24, 25, 67). Clinical B. pseudomallei isolates from the Northern Territory of Australia were even more chlorine tolerant than the standard strains from culture collections tested as described above (24). After 30 min of incubation, survival of clinical strains increased, on average, 12% over that of standard control cells per 1 ppm free chlorine in the treatment mixture. The limitations of chlorine as a biodefense disinfectant were independently confirmed with other bacterial select agents of interest (64). Quicklime (CaO) has been tried as a decontaminant for B. pseudomallei in soil, but without complete success (52).
The identification of the drinking water supply as a likely source of B. pseudomallei in fatal melioidosis outbreaks highlights the importance of adequate chlorine treatment in preventing melioidosis (11, 28) and raises questions about the effectiveness of generally used concentrations of free chlorine for completely inactivating B. pseudomallei in water. Moreover, the presence of B. pseudomallei at high concentrations or the additional presence of free-living freshwater amoebae should easily overwhelm or neutralize the disinfectant effect of chlorine. Culture-based detection of B. pseudomallei in environmental specimens may fail to detect B. pseudomallei inadequately treated with chlorine, since these cells will be nonculturable but still able to cause disease.
SURVIVAL HABITATS OR NICHES PROVIDING ENHANCED SURVIVAL
B. pseudomallei may not persist in a mono-dispersed suspension in the wild, but data on its preferred microhabitat are lacking. The normal location where B. pseudomallei can be found is in surface water, soil, or a mixture of the two. B. pseudomallei has been shown to form biofilms and internalize in amoebic cysts (30, 81), properties that may be critical to the increased persistence of this pathogen in the environment.
(i) Biofilms.
The normal location for B. pseudomallei in soil is the rhizosphere or root zone where a complex biota exists. The interface between liquids and solids is a preferred habitat for biofilm formation by B. pseudomallei (30, 42). B. pseudomallei also forms a film at the liquid/air interface in a container of broth after incubation at 37°C for 24 h or more. Biofilms contain slowly growing bacteria and extracellular polysaccharide in complex three-dimensional structures, and their growth or lack thereof is controlled by the release of quorum-sensing substances (acyl homoserine lactones) (73). Slow-growing bacterial consortia are inherently more resistant to the actions of disinfectants and antibiotics. This form of growth has been cited as a possible explanation for in vivo resistance to antibiotics used to treat B. pseudomallei infections (81).
(ii) Protozoa.
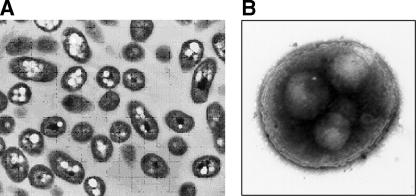
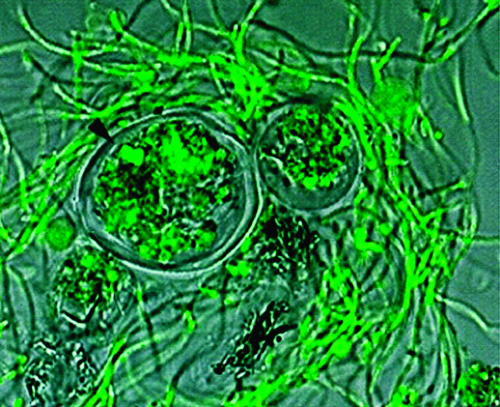
Persistence in amoebic cysts represents another means of surviving very hostile environmental conditions. Amoebic species in which B. pseudomallei can survive include Acanthamoeba astronyxis, Acanthamoeba castellanii, Acanthamoeba palestiniensis, and Acanthamoeba polyphaga. By analogy with Legionella, B. pseudomallei should also survive in Hartmannella vermiformis and a variety of other free-living species (83). Many of these protozoa graze on the polymicrobial biofilms that form in wet habitats. B. pseudomallei employs the coiling phagocytosis mechanism to penetrate free-living amoebae (similarly to other intracellular bacteria, such as Legionella and Listeria) (30). Entry into Acanthamoeba trophozoites, amoebae that live in surface water and the rhizosphere, forms vacuoles full of bacteria and cysts containing live bacteria (Fig. 7). Bacillus-filled amoebic vacuoles are about 5 to 15 μm in diameter, which is close to the ideal size for aerosolized particles that can efficiently infect humans via the respiratory tract. As in the case of Legionnaires’ disease, the infective particle for melioidosis and the normal intracellular habitat may be the same entity (2). The effect of amoebic passage on the virulence of B. pseudomallei has yet to be studied, but amoebic passage is known to enhance the virulence of Legionella (8). Current evidence from work with other bacterial species suggests that internalization in amoebic cysts should render B. pseudomallei more resistant to chlorine (36, 44).
FIG. 7.
Persistence in amoeba. The arrow indicates a small cluster of SYTO-stained B. pseudomallei bacteria inside an Acanthamoeba astronyxis cyst. The two cysts in this picture are surrounded by a matted web of elongated B. pseudomallei cells. This preparation was photographed after 24 h of coculture (30).
(iii) Fungi.
Another environmental cellular habitat identified for B. pseudomallei is the cytoplasm of arbuscular mycorrhizal fungi belonging to the genus Gigaspora, where the penetration, persistence, and survival of B. pseudomallei in culturable form within fungi were all demonstrated (42). Mycorrhizal fungi form endosymbioses with plant roots and therefore provide an intracellular habitat for bacteria, inside another eukaryotic habitat. This double layer of protection both shields bacteria from the external environment and provides a potential genetic crossroads for traffic between different biological kingdoms. The periodic acquisition of genetic material from the host fungus or plant by the bacteria that it plays host to could explain the genetic complexity of the burkholderias. This is supported by the discovery of an endosymbiont of environmental fungi whose DNA sequence has strong homology to that of B. pseudomallei (57).
(iv) Legumes.
Legumes are nitrogen-fixing plants whose root nodules contain symbiotic bacteria that are usually associative (atmospheric) nitrogen fixers. B. pseudomallei has been recovered from soil adhering to the roots of a native wattle, Acacia colei (29). This finding is consistent with root surface colonization but does not support the establishment of a symbiotic or commensal relationship.
(v) Other plants (rice, rubber, cassava).
The persistence of B. pseudomallei in rice fields has been extensively studied in Thailand following the observation that rice farmers were at a higher risk for infection (51, 76, 77). There has been very little work on the survival of B. pseudomallei in the rice rhizosphere. With the exception of the original observations on rice fields in Iran, this work has been conducted only in Southeast Asia (56, 60, 71, 88). Rice farming does not provide an occupational-exposure explanation for the melioidosis risk in other places such as northern Australia, where rice is not currently grown. Another environmental niche occupied by B. pseudomallei can be inferred from the propensity of rubber workers for developing melioidosis (51). This observation has not been followed up with detailed environmental sampling similar to that in work with rice fields, despite the close proximity of rubber plantations to rice fields in parts of Southeast Asia. It is notable that rubber is grown in many locations where melioidosis is endemic. The main plantation rubber tree, Hevea brasiliensis, was imported from northern Brazil to Malaya and other parts of Southeast Asia via Sri Lanka during the 19th century. Interestingly, a lesser-known source of latex, Manihot glaziovii, or Ceara rubber, was imported to the Darwin region in the Australian Northern Territory around the same time (21). M. glaziovii was used to improve the stock of the closely related Manihot esculenta, or cassava, before its introduction to parts of Africa and Southeast Asia, including the region of Thailand where melioidosis is most prevalent (69). As some Manihot spp. support growth of Burkholderia spp., and the Brazilian state of Ceara has had an outbreak of melioidosis caused by a strain of B. pseudomallei that is genotypically similar to the Western Australian outbreak strain (63), it is possible that B. pseudomallei could have been transferred from the Americas to Southeast Asia and northern Australia in rubber or cassava plants or the soil surrounding them. Geographic analysis of genotypic data from B. pseudomallei and near-neighbor species isolated from environmental investigations will be required to develop or refute this hypothesis.
CONCLUSIONS
Understanding the environmental biology of B. pseudomallei should lead to improvements in the control and prevention of melioidosis. The key to protecting civilians and military personnel from melioidosis is prevention by avoiding exposure. The use of personal protective equipment is effective when the location and timing of exposure are predictable (e.g., servicemen training in a known disease hot zone). Metabolic adaptation and internalization within eukaryotic systems (unicellular organisms and plants, etc.) are likely to provide extraordinary environmental advantages to B. pseudomallei. The unusually long environmental persistence in soil and water, as well as the resistance and tolerance to physical factors, pH changes, osmolarity, and chemicals (such as hypochlorite), makes treatment and remediation of environments contaminated with B. pseudomallei extremely challenging. B. pseudomallei will likely remain a pathogen of interest in biodefense since detection is difficult, treatment of melioidosis is long, relapse is frequent, and a vaccine is lacking.
Acknowledgments
This work was supported by the U.S. Department of Defense Chemical and Biological Program, administered by the Defense Threat Reduction Agency.
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Ashdown, L. R., and R. W. Guard. 1984. The prevalence of human melioidosis in Northern Queensland. Am. J. Trop. Med. Hyg. 33:474-478. [DOI] [PubMed] [Google Scholar]
- 2.Brieland, J. K., J. C. Fantone, D. G. Remick, M. LeGendre, M. McClain, and N. C. Engleberg. 1997. The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaire's disease. Infect. Immun. 65:5330-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook, M. D., B. Currie, and P. M. Desmarchelier. 1997. Isolation and identification of Burkholderia pseudomallei from soil using selective culture techniques and the polymerase chain reaction. J. Appl. Microbiol. 82:589-596. [PubMed] [Google Scholar]
- 4.Chambon, L. 1955. Isolement du bacille de Whitmore á partir du milieu extérieur. Ann. Inst. Pasteur 89:229-235. [PubMed] [Google Scholar]
- 5.Champagne, J. 2001. Personal communication.
- 6.Cheng, A. C., and B. J. Currie. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodimella, U., W. L. Hoppes, S. Whalen, A. J. Ognibene, and G. W. Rutecki. 1997. Septicemia and suppuration in a Vietnam veteran. Hosp. Pract. 32:219-221. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie, B. J., D. A. Fisher, D. M. Howard, and J. N. Burrow. 2000. Neurological melioidosis. Acta Trop. 74:145-151. [DOI] [PubMed] [Google Scholar]
- 10.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, S. Selvanayagam, P. L. Snelling, N. M. Anstey, and M. Mayo. 2000. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 74:121-127. [DOI] [PubMed] [Google Scholar]
- 11.Currie, B. J., M. Mayo, N. M. Anstey, P. Donohoe, A. Haase, and D. J. Kemp. 2001. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am. J. Trop. Med. Hyg. 65:177-179. [DOI] [PubMed] [Google Scholar]
- 12.Currie, B. J., and S. P. Jacups. 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 9:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejsirilert, S., E. Kondo, D. Chiewsilp, and K. Kanai. 1991. Growth and survival of Pseudomonas pseudomallei in acidic environments. Jpn. J. Med. Sci. Biol. 44:63-74. [DOI] [PubMed] [Google Scholar]
- 14.Dharakul, T., S. Vejbaesya, W. Chaowagul, P. Laungtrakool, H. A. Stephens, and S. Songsivilai. 1998. HLA-DR and -DQ associations with melioidosis. Hum. Immunol. 59:580-586. [DOI] [PubMed] [Google Scholar]
- 15.Dorman, S. E., V. J. Gill, J. I. Gallin, and S. M. Holland. 1998. Burkholderia pseudomallei infection in a Puerto Rican patient with chronic granulomatous disease: case report and review of occurrences in the Americas. Clin. Infect. Dis. 26:889-894. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons, H. S., S. Lin, R. J. Cotter, and C. R. Raetz. 2000. Oxygen requirement for the biosynthesis of the S-2-hydroxymyristate moiety in Salmonella typhimurium lipid A. Function of LpxO, a new Fe2+/alpha-ketoglutarate-dependent dioxygenase homologue. J. Biol. Chem. 275:32940-32949. [DOI] [PubMed] [Google Scholar]
- 17.Godoy, D., G. Randle, A. J. Simpson, D. M. Aanensen, T. L. Pitt, R. Kinoshita, and B. G. Spratt. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg, J. H. 1969. Public health problems relating to the Vietnam returnee. J. Am. Med. Assoc. 207:697-702. [PubMed] [Google Scholar]
- 19.Hicks, C. L., R. Kinoshita, and P. W. Ladds. 2000. Pathology of melioidosis in marine mammals. Aust. Vet. J. 78:193-195. [DOI] [PubMed] [Google Scholar]
- 20.Holden, M. T., R. W. Titball, S. J. Peacock, A. M. Cerdeno-Tarraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 101:14240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtze, M. W. 1901. The capabilities of the Northern Territory for tropic agriculture. Adelaide Work J. 1901:17-27. [Google Scholar]
- 22.Hoppe, I., B. Brenneke, M. Rohde, A. Kreft, S. Haussler, A. Reganzerowski, and I. Steinmetz. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard, K., and T. J. Inglis. 2003. Novel selective medium for the isolation of Burkholderia pseudomallei. J. Clin. Microbiol. 41:3312-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard, K., and T. J. Inglis. 2003. The effect of free chlorine on Burkholderia pseudomallei in potable water. Water Res. 37:4425-4432. [DOI] [PubMed] [Google Scholar]
- 25.Howard, K., and T. J. Inglis. 2005. Disinfection of Burkholderia pseudomallei in potable water. Water Res. 39:1085-1092. [DOI] [PubMed] [Google Scholar]
- 26.Howe, C., A. Sampath, and M. Spotnitz. 1971. The Pseudomallei group: a review. J. Infect. Dis. 124:598-606. [DOI] [PubMed] [Google Scholar]
- 27.Inglis, T. J., D. Chiang, G. S. Lee, and L. Chor-Kiang. 1998. Potential misidentification of Burkholderia pseudomallei by API 20NE. Pathology 30:62-64. [DOI] [PubMed] [Google Scholar]
- 28.Inglis, T. J., S. C. Garrow, C. Adams, M. Henderson, M. Mayo, and B. J. Currie. 1999. Acute melioidosis outbreak in Western Australia. Epidemiol. Infect. 123:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglis, T. J. J., S. C. Garrow, M. Henderson, A. Clair, J. Sampson, L. O'Reilly, and B. Cameron. 2000. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg. Infect. Dis. 6:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inglis, T. J., P. Rigby, T. A. Robertson, N. S. Dutton, M. Henderson, and B. J. Chang. 2000. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inglis, T. J., C. L. Golledge, A. Clair, and J. Harvey. 2001. Case report: recovery from persistent septicemic melioidosis. Am. J. Trop. Med. Hyg. 65:76-82. [DOI] [PubMed] [Google Scholar]
- 32.Inglis, T. J., M. Aravena-Roman, S. Ching, K. Croft, V. Wuthiekanun, and B. J. Mee. 2003. Cellular fatty acid profile distinguishes Burkholderia pseudomallei from avirulent Burkholderia thailandensis. J. Clin. Microbiol. 41:4812-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inglis, T. J., N. F. Foster, D. Gal, K. Powell, M. Mayo, R. Norton, and B. J. Currie. 2004. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol. Infect. 132:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inglis, T. J., A. Merritt, G. Chidlow, M. Aravena-Roman, and G. Harnett. 2005. Comparison of diagnostic laboratory methods for identification of Burkholderia pseudomallei. J. Clin. Microbiol. 43:2201-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanai, K., E. Kondo, S. Dejsirilert, and P. Naigowit. 1994. Growth and survival of Pseudomonas pseudomallei in acidic environment with possible relation to the ecology and epidemiology of melioidosis, p. 26-38. In S. D. Puthucheary and Y. A. Malik (ed.), Melioidosis: prevailing problems and future directions. Selected papers from the first international symposium on melioidosis, Kuala Lumpur, Malaysia.
- 36.Kilvington, S., and J. Price. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519-525. [DOI] [PubMed] [Google Scholar]
- 37.Kondo, E., and K. Kanai. 1994. Separation of antigenic glycoprotein fractions from cell-free homogenate of Pseudomonas pseudomallei and characterization as tyrosine phosphatase. Southeast Asian J. Trop. Med. Public Health 25:436-442. [PubMed] [Google Scholar]
- 38.Kunakorn, M., P. Jayanetra, and D. Tanphaichitra. 1991. Man-to-man transmission of melioidosis. Lancet 337:1290-1291. [DOI] [PubMed] [Google Scholar]
- 39.Kunakorn, M., and R. B. Markham. 1995. Clinically practical seminested PCR for Burkholderia pseudomallei quantitated by enzyme immunoassay with and without solution hybridization. J. Clin. Microbiol. 33:2131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, S. W., J. Yi, S. I. Joo, Y. A. Kang, Y. S. Yoon, J. J. Yim, C. G. Yoo, S. K. Han, Y. S. Shim, E. C. Kim, and Y. W. Kim. 2005. A case of melioidosis presenting as migrating pulmonary infiltration: the first case in Korea. J. Korean Med. Sci. 20:139-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leelarasamee, A., and S. Bovornkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413-425. [DOI] [PubMed] [Google Scholar]
- 42.Levy, A., B. J. Chang, L. K. Abbott, J. Kuo, G. Harnett, and T. J. J. Inglis. 2003. Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl. Environ. Microbiol. 69:6250-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manson-Bahr, P. 1946. Synopsis of tropical medicine, p. 72. Cassell and Co., London, United Kingdom.
- 44.Marolda, C., B. Hauroder, M. J. R. Michel, and M. Valvano. 1999. Intracellular survival and saprophytic growth of isolates from the Burkholderia cepacia complex in free-living amoebae. Microbiology 145:1509-1517. [DOI] [PubMed] [Google Scholar]
- 45.McCormick, J. B., D. J. Sexton, J. G. McMurray, E. Carey, P. Hayes, and R. A. Feldman. 1975. Human-to-human transmission of Pseudomonas pseudomallei. Ann. Intern. Med. 83:512-513. [DOI] [PubMed] [Google Scholar]
- 46.McCormick, J. B., R. E. Weaver, P. S. Hayes, J. M. Boyce, and R. A. Feldman. 1977. Wound infection by an indigenous Pseudomonas pseudomallei-like organism isolated from the soil: case report and epidemiologic study. J. Infect. Dis. 135:103-107. [DOI] [PubMed] [Google Scholar]
- 47.Merianos, A., M. Patel, J. M. Lane, C. N. Noonan, D. Sharrock, P. A. Mock, and B. Currie. 1993. The 1990-1991 outbreak of melioidosis in the Northern Territory of Australia: epidemiology and environmental studies. Southeast Asian J. Trop. Med. Public Health 24:425-435. [PubMed] [Google Scholar]
- 48.Merritt, A., T. J. J. Inglis, G. Chidlow, and G. Harnett. PCR-based identification of Burkholderia pseudomallei Rev. Inst. Med. Trop. São Paulo, in press. [DOI] [PubMed]
- 49.Miralles, I. S., M. do C. Maciel, M. R. Angelo, M. M. Gondini, L. H. Frota, C. M. dos Reis, and E. Hofer. 2004. Burkholderia pseudomallei: a case report of a human infection in Ceará, Brazil. Rev. Inst. Med. Trop. São Paulo 46:51-54. [DOI] [PubMed] [Google Scholar]
- 50.Moulin, L., A. Munive, B. Dreyfus, and C. Boivin-Masson. 2001. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948-950. [DOI] [PubMed] [Google Scholar]
- 51.Nachiangmai, N., P. Patamasucon, B. Tipayamonthein, A. Kongpon, and S. Nakaviroj. 1985. Pseudomonas pseudomallei in southern Thailand. Southeast Asian J. Trop. Med. Public Health 16:83-87. [PubMed] [Google Scholar]
- 52.Na-ngam, N., S. Angkititakul, P. Noimay, and V. Thamlikitkul. 2004. The effect of quicklime (calcium oxide) as an inhibitor of Burkholderia pseudomallei. Trans. R. Soc. Trop. Med. Hyg. 98:337-341. [DOI] [PubMed] [Google Scholar]
- 53.Ngauy, V., Y. Lemeshev, L. Sadkowski, and G. Crawford. 2005. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J. Clin. Microbiol. 43:970-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nierman, W. C., D. DeShazer, H. S. Kim, H. Tettelin, K. E. Nelson, T. Feldblyum, R. L. Ulrich, C. M. Ronning, L. M. Brinkac, S. C. Daugherty, T. D. Davidsen, R. T. Deboy, G. Dimitrov, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, H. Khouri, J. F. Kolonay, R. Madupu, Y. Mohammoud, W. C. Nelson, D. Radune, C. M. Romero, S. Sarria, J. Selengut, C. Shamblin, S. A. Sullivan, O. White, Y. Yu, N. Zafar, L. Zhou, and C. M. Fraser. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 101:14246-14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nussbaum, J. J., D. S. Hull, and M. J. Carter. 1980. Pseudomonas pseudomallei in an anophthalmic orbit. Arch. Ophthalmol. 98:1224-1225. [DOI] [PubMed] [Google Scholar]
- 56.Parry, C. M., V. Wuthiekanun, N. T. Hoa, T. S. Diep, L. T. Thao, P. V. Loc, B. A. Wills, J. Wain, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Melioidosis in Southern Vietnam: clinical surveillance and environmental sampling. Clin. Infect. Dis. 29:1323-1326. [DOI] [PubMed] [Google Scholar]
- 57.Partida-Martinez, L. P., and C. Hertweck. 2005. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437:884-888. [DOI] [PubMed] [Google Scholar]
- 58.Perez, J. M., A. Petiot, C. Adjide, F. Gerry, R. Goursaud, and B. Juminer. 1997. First case report of melioidosis in Guadeloupe, a French West Indies archipelago. Clin. Infect. Dis. 25:164-165. [DOI] [PubMed] [Google Scholar]
- 59.Pitt, T. L. 1995. Pseudomonas, Burkholderia and related genera, p. 1109-1138. In W. J. Haussler and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, vol. 2. Arnold, London, United Kingdom. [Google Scholar]
- 60.Pourtaghva, M., A. Machoun, and A. Dodin. 1975. Demonstration of Pseudomonas pseudomallei (Whitmore's bacillus) in the mud of Iranian ricefields (author's transl.). Bull. Soc. Pathol. Exot. Filiales 68:367-370. (In French.) [PubMed] [Google Scholar]
- 61.Preston, P. J., N. Lightfoot, and P. Clarke. 1976. A retrospective serological survey of Royal Marines previously exposed to Pseudomonas pseudomallei in South East Asia. Trans. R. Soc. Trop. Med. Hyg. 70:335-337. [DOI] [PubMed] [Google Scholar]
- 62.Rogul, M., and S. R. Carr. 1972. Variable ammonia production among smooth and rough strains of Pseudomonas pseudomallei: resemblance to bacteriocine production. J. Bacteriol. 112:372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolim, D. B. 2005. Melioidosis. Northeastern Brazil. Emerg. Infect. Dis. 11:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose, L. J., E. W. Rice, B. Jensen, R. Murga, A. Peterson, R. M. Donlan, and M. J. Arduino. 2005. Chlorine inactivation of bacterial bioterrorism agents. Appl. Environ. Microbiol. 71:566-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell, P., S. M. Eley, J. Ellis, M. Green, D. L. Bell, D. J. Kenny, and R. W. Titball. 2000. Comparison of efficacy of ciprofloxacin and doxycycline against experimental melioidosis and glanders. J. Antimicrob. Chemother. 45:813-818. [DOI] [PubMed] [Google Scholar]
- 66.Sagripanti, J. L., and A. Bonifacino. 1996. Comparative sporicidal effects of liquid chemical agents. Appl. Environ. Microbiol. 63:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sagripanti, J. L., and A. Bonifacino. 2000. Resistance of Pseudomonas aeruginosa to liquid disinfectants on contaminated surfaces before formation of biofilms. J. AOAC Int. 83:1415-1422. [PubMed] [Google Scholar]
- 68.Sanford, J. P., and W. L. Moore. 1971. Recrudescent melioidosis: a Southeast Asian legacy. Am. Rev. Respir. Dis. 104:452-453. [DOI] [PubMed] [Google Scholar]
- 69.Santiphop, T. 2000. The relevant population dynamics to land degradation in the northeast region. Warasan Prachakon Lae Sangkhom 8:67-89. [PubMed] [Google Scholar]
- 70.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and T. L. Pitt. 1993. Latex agglutination test for identification of Pseudomonas pseudomallei. J. Clin. Pathol. 46:374-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1995. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:488-490. [DOI] [PubMed] [Google Scholar]
- 72.Smith, M. D., B. J. Angus, V. Wuthiekanun, and N. J. White. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65:4319-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song, Y., C. Xie, Y. M. Ong, Y. H. Gan, and K. L. Chua. 2005. The BpsIR quorum-sensing system of Burkholderia pseudomallei. J. Bacteriol. 187:785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spotnitz, M., J. Rudnitsky, and J. J. Rambaud. 1967. Melioidosis pneumonitis: analysis of nine cases of a benign form of melioidosis. J. Am. Med. Assoc. 202:950-954. [DOI] [PubMed] [Google Scholar]
- 75.Stanton, A. T., and W. Fletcher. 1925. Melioidosis and its relation to glanders. J. Hyg. 23:347-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suputtamongkol, Y., A. J. Hall, D. A. Dance, W. Chaowagul, A. Rajchanuvong, M. D. Smith, and N. J. White. 1994. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int. J. Epidemiol. 23:1082-1090. [DOI] [PubMed] [Google Scholar]
- 77.Suputtamongkol, Y., W. Chaowagul, P. Chetchotisakd, N. Lertpatanasuwun, S. Intaranongpai, T. Ruchutrakool, D. Budhsarawong, P. Mootsikapun, V. Wuthiekanun, N. Teerawatasook, and A. Lulitanond. 1998. Risk factors for melioidosis and bacteremic melioidosis. Clin. Infect. Dis. 29:408-413. [DOI] [PubMed] [Google Scholar]
- 78.Thomas, A., and J. C. Forbes-Faulkner. 1981. Persistence of Pseudomonas pseudomallei in soil. Aust. Vet. J. 57:535-536. [DOI] [PubMed] [Google Scholar]
- 79.Tong, S., S. Yang, Z. Lu, and W. He. 1996. Laboratory investigation of ecological factors influencing the environmental presence of Burkholderia pseudomallei. Microbiol. Immunol. 40:451-453. [DOI] [PubMed] [Google Scholar]
- 80.Vasu, C., J. Vadivelu, and S. D. Puthucheary. 2003. The humoral immune response in melioidosis patients during therapy. Infection 3:24-30. [DOI] [PubMed] [Google Scholar]
- 81.Vorachit, M., K. Lam, P. Jayanetra, and J. W. Costerton. 1993. Resistance of Pseudomonas pseudomallei growing as a biofilm on silastic discs to ceftazidime and co-trimoxazole. Antimicrob. Agents Chemother. 37:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vuddhakul, V., P. Tharavichitkul, N. Na-Ngam, S. Jitsurong, B. Kunthawa, P. Noimay, P. Noimay, A. Binla, and V. Thamlikitkul. 1999. Epidemiology of Burkholderia pseudomallei in Thailand. Am. J. Trop. Med. Hyg. 60:458-461. [DOI] [PubMed] [Google Scholar]
- 83.Wadowsky, R. M., T. M. Wilson, N. J. Kapp, A. J. West, J. M. Kuchta, S. J. States, J. N. Dowling, and R. B. Yee. 1991. Multiplication of Legionella spp. in tap water containing Hartmannella vermiformis. Appl. Environ. Microbiol. 57:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warawa, J., and D. E. Woods. 2002. Melioidosis vaccines. Expert Rev. Vaccines 1:477-482. [DOI] [PubMed] [Google Scholar]
- 85.Whitmore, A., and C. S. Krisnaswami. 1912. An account of the discovery of a hitherto undescribed infective disease occurring among the population of Rangoon. Ind. Med. Gaz. 47:262-267. [PMC free article] [PubMed] [Google Scholar]
- 86.Woo, P. C., P. K. Leung, S. S. Wong, P. L. Ho, and K. Y. Yuen. 2001. groEL encodes a highly antigenic protein in Burkholderia pseudomallei. Clin. Diagn. Lab. Immunol. 8:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woo, P. C., S. K. Lau, G. K. Woo, A. M. Fung, A. H. Ngan, W. T. Hui, and K. Y. Yuen. 2003. Seronegative bacteremic melioidosis caused by Burkholderia pseudomallei with ambiguous biochemical profile: clinical importance of accurate identification by 16S rRNA gene and groEL gene sequencing. J. Clin. Microbiol. 41:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wuthiekanun, V., M. D. Smith, D. A. Dance, and N. J. White. 1995. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:41-43. [DOI] [PubMed] [Google Scholar]
- 89.Wuthiekanun, V., M. D. Smith, and N. J. White,. 1995. Survival of Burkholderia pseudomallei in the absence of nutrients. Trans. R. Soc. Trop. Med. Hyg. 89:491. [DOI] [PubMed] [Google Scholar]
- 90.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immun. 36:1251-1275. [DOI] [PubMed] [Google Scholar]
- 91.Yabuuchi, E., Y. Kosako, M. Arakawa, H. Hotta, and I. Yano. 1992. Identification of Oklahoma isolate as a strain of Pseudomonas pseudomallei. Microbiol. Immunol. 36:1239-1249. [DOI] [PubMed] [Google Scholar]