Abstract
The use of the food-grade bacterium Lactococcus lactis as a DNA delivery vehicle at the mucosal level is an attractive DNA vaccination strategy. Previous experiments showed that recombinant L. lactis expressing the Listeria monocytogenes inlA gene can deliver a functional gene into mammalian cells. Here, we explored the potential use of noninvasive L. lactis strains as a DNA delivery vehicle. We constructed two Escherichia coli-L. lactis shuttle plasmids, pLIG:BLG1 and pLIG:BLG2, containing a eukaryotic expression cassette with the cDNA of bovine β-lactoglobulin (BLG). The greatest BLG expression after transfection of Cos-7 cells was obtained with pLIG:BLG1, which was then used to transform L. lactis MG1363. The resulting L. lactis strain MG1363(pLIG:BLG1) was not able to express BLG. The potential of L. lactis as a DNA delivery vehicle was analyzed by detection of BLG in Caco-2 human colon carcinoma cells after 3 h of coincubation with (i) purified pLIG:BLG1, (ii) MG1363(pLIG:BLG1), (iii) a mix of MG1363(pLIG) and purified pLIG:BLG1, and (iv) MG1363. Both BLG cDNA and BLG expression were detected only in Caco-2 cells coincubated with MG1363(pLIG:BLG1). There was a decrease in the BLG cDNA level in Caco-2 cells between 24 and 48 h after coincubation. BLG expression by Caco-2 cells started at 24 h and increased between 24 and 72 h. BLG secretion by Caco-2 cells started 48 h after coincubation with MG1363(pLIG:BLG1). We conclude that lactococci can deliver BLG cDNA into mammalian epithelial cells, demonstrating their potential to deliver in vivo a DNA vaccine.
Allergy to cow's milk is an important health problem worldwide, affecting 2 to 3% of infants in the first 2 years of life in various countries of northern Europe (21). Bovine β-lactoglobulin (BLG) is the most abundant whey protein of cow's milk and is considered a dominant allergen; it is an 18-kDa lipocalin glycoprotein with a globular structure, containing two intramolecular disulfide bonds (32).
Lactococcus lactis is a food-grade gram-positive lactic acid bacterium considered to be noninvasive and noncolonizing. L. lactis can deliver antigens and cytokines to the systemic and mucosal immune systems via mucosal routes (42). Therefore, its use as a vaccine delivery system using different antigens and cytokines has been widely studied (5, 6, 7, 17, 28, 33, 34, 35, 44; for a review, see reference 30). We previously showed that administration of L. lactis strains producing either BLG or a BLG epitope induces a mucosal immune response and partially protects against sensitization in mice (9, 10, 3).
In contrast to bacterially mediated delivery of protein antigens, bacterially mediated delivery of DNA vaccines could lead to host expression of posttranslationally modified antigens and therefore to the presentation of conformationally restricted epitopes (15). Attenuated strains of pathogenic bacteria, such as Shigella, Listeria, and Salmonella, have been used as live vectors to deliver DNA into mammalian cells (18, 39). Nevertheless, the risk associated with possible reversion to a virulent phenotype of these pathogenic bacteria is a major concern (13).
The use of food-grade lactic acid bacteria as a DNA delivery vehicle is a promising alternative for a DNA vaccine carrier. We previously investigated whether L. lactis could deliver DNA vaccines by expressing the Listeria monocytogenes inlA gene, which encodes internalin (InlA). The resulting L. lactis inlA+ strains were able to enter intestinal cells in vivo after oral inoculation of guinea pigs (20). Moreover, a functional eukaryotic gfp gene carried by such strains could be delivered into 1% of internalized Caco-2 human colon carcinoma cells (20). The limits of such a strategy were (i) the necessity of working either with transgenic mice expressing human E-cadherin or with guinea pigs and (ii) the need for using recombinant invasive lactococci.
Here, we used native lactococci to deliver BLG cDNA (a eukaryotic expression cassette encoding the BLG antigen) into mammalian cells. Genetic immunization with this cDNA previously induced a preventive and persistent inhibition of specific anti-BLG immunoglobulin E (IgE) responses in mice (2). Caco-2 cells and an L. lactis strain harboring BLG cDNA were cocultured to evaluate the ability of lactococci to deliver DNA into mammalian cells. This coincubation led to the expression and secretion of the BLG protein by Caco-2 cells.
These are the first steps toward the use of lactococci as DNA vaccine delivery vectors. The ease of generating such vehicles for DNA delivery makes the use of L. lactis as a carrier for oral DNA vaccination highly attractive for further in vivo research and vaccine development.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
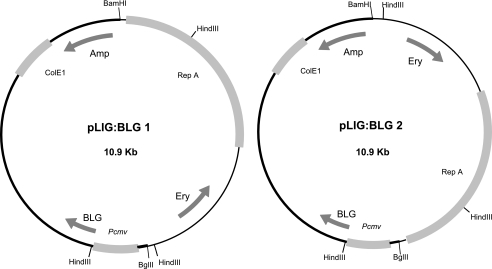
The strains and plasmids used in this study are listed in Table 1. The plasmids pLIG:BLG1 and pLIG:BLG2 are cointegrates obtained by ligating BglII-linearized pcDNA3BLG5 (8) and BamHI-linearized pIL253 (Fig. 1) (40). To avoid self-ligation, pcDNA3BLG5 was dephosphorylated before ligation. The same constructions were performed with pIL253 and pcDNA3, with pLIG1 and pLIG2 used as a negative control. Products of ligation were transformed in CaCl2 competent cells of Escherichia coli strain Top10 (Invitrogen) and plated onto Luria-Bertani agar plates containing 100 μg ampicillin/ml and 150 μg erythromycin/ml. pLIG:BLG1, pLIG:BLG2, pLIG1, and pLIG2 were electrotransformed in L. lactis subsp. cremoris MG1363 (16), as described elsewhere (25). Enzymatic digestion of both cointegrates with HindIII produced a distinct pattern, allowing selection of clones. These strains were grown in M17 medium (43) containing 0.5% glucose (GM17). Transformants were plated on GM17 agar plates containing 5 μg erythromycin/ml after 24 h of incubation at 30°C.
TABLE 1.
Strains and plasmids
| Plasmid(s)a | Strain(s) | Properties | Reference or source |
|---|---|---|---|
| pIL253* | L. lactis MG1363 | High-copy-number lactococcal vector | 40 |
| pLIG1*† and pLIG2*† | E. coli Top10, L. lactis MG1363 | Cointegrate between pIL253 and pcDNA3 (Clontech) | This work |
| pcDNA3BLG5† | E. coli Top10 | pcDNA3 with Pcmv promoter fused to blg cDNA | 8 |
| pLIG:BLG1*† | E. coli Top10, L. lactis MG1363 | Cointegrate between pIL253 and pcDNA3BLG5 | This work |
| pLIG:BLG2*† | E. coli Top10, L. lactis MG1363 | Cointegrate between pIL253 and pcDNA3BLG5 | This work |
| pLEISS-BLG‡ | L. lactis NZ9000 | Lactococcal vector containing a nisin induction system with a LEISS fragment and the blg gene | 31 |
*, erythromycin; †, ampicillin; ‡, chloramphenicol.
FIG. 1.
Structures of pLIG:BLG1 and pLIG:BLG2. Arrows indicate the directions of transcription of BLG cDNA, the ampicillin resistance gene (Amp), and the erythromycin resistance gene (Ery). Boxes indicate Pcmv, the cytomegalovirus eukaryotic promoter; RepA, the origin of replication of L. lactis; and ColE1, the origin of replication of E. coli. The thin part of the vector is derived from plasmid pIL253, and the thick part is derived from pcDNA3BLG5. The BamHI and BglII restriction sites used to obtain the two plasmids and the HindIII restriction site are shown.
DNA manipulations.
DNA manipulations were performed as previously described (37), with the following modifications. For plasmid DNA extraction from L. lactis, TES (25% sucrose, 1 mM EDTA, 50 mM Tris-HCl [pH 8]) containing lysozyme (10 mg/ml) was added for 10 min at 37°C to prepare the protoplasts. Enzymes were used as recommended by suppliers.
BLG expression and extraction in Cos-7 cells.
BLG expression in Cos-7 cells was performed by transfection with LipofectAmine PLUS reagent (Life Technologies, Paisley, United Kingdom). Briefly, 50 to 80% confluent cells cultured in Dulbecco modified Eagle medium, 10% fetal calf serum, 2 mM l-glutamine (BioWhittaker, Cambrex Bio Science, Verviers, Belgium), 100 U penicillin, and 100 μg streptomycin were transfected with pcDNA3BLG5, pLIG:BLG1, and pLIG:BLG2 previously complexed with LipofectAmine. Two days after transfection, the medium was collected and cells were harvested, centrifuged in phosphate-buffered saline (PBS), counted, and sonicated. The cellular extract was centrifuged for 15 min at 10,000 × g at 4°C. The supernatant (S) was collected. The pellet was suspended in 6 M GuHCl-100 mM NaH2PO4-10 mM Tris at pH 8 for 30 min at room temperature and then centrifuged for 10 min at 20,000 × g at 4°C. The supernatant (I) was collected. Medium and I extracts were dialyzed against PBS. Native BLG (nBLG) and denatured BLG (RCM-BLG) were assayed in medium and S and I extracts by a specific two-site enzyme immunometric assay (EIA) described below.
Detection of BLG under native and denatured conformation by two-site EIA.
Two-site EIAs for nBLG and RCM-BLG were performed as previously described (29). Briefly, assays were performed in 96-well microtiter plates coated with a monoclonal antibody (capture antibody) specific for either nBLG or RCM-BLG. Fifty microliters of standard (nBLG or RCM-BLG) or 50 μl of the samples was added; then, 50 μl of tracer was added, consisting of a second monoclonal antibody labeled with acetylcholinesterase (AChE), a conjugate recognizing either nBLG or RCM-BLG. The capture and tracer antibodies were directed against different complementary epitopes. After an 18-h reaction at 4°C, the plates were washed and solid-phase-bound AChE activity was measured by Ellman's method (14). Detection limits of 30 and 200 pg/ml were obtained for nBLG and RCM-BLG, respectively.
Total RNA extraction and purification from bacteria and Caco-2 cells and detection of specific mRNA by RT-PCR.
Total mRNA was extracted and purified with the RNeasy Fibrous Tissue Mini kit (QIAGEN) as described by the supplier. Reverse transcriptase (RT) PCR was performed on 1 μg of total RNA with the OneStep RT-PCR kit from QIAGEN. In order to detect β-actin mRNA or BLG mRNA, we used oligonucleotides specific for β-actin, ATGGATGACGATATCGCTGCGCTGGTCGTC (β-actin Fwd) and CTAGAAGCACTTGCGGTGCACGATGGAG (β-actin Rev), and oligonucleotides specific for BLG, CTCATCGTCACCCAGACCATGAAGGGCC (BLG5′) and GATGTGGCACTGCTCCTCCAGCTGGGTTGGG (BLG3′).
Coculture assays of L. lactis strains and Caco-2 human epithelial cells.
Caco-2 epithelial cells were cocultivated with (i) purified pLIG:BLG1, (ii) L. lactis MG1363(pLIG:BLG1), (iii) a mix of L. lactis MG1363(pLIG1) and purified pLIG:BLG1, and (iv) L. lactis MG1363(pLIG1) in order to determine the potential of lactococci to deliver DNA in epithelial host cells. The coculture assays were performed with the human colon carcinoma cell line Caco-2 (ATCC HTB37), as described by Dramsi et al. (12). These cells were cultured in RPMI supplemented with 2 mM l-glutamine (BioWhittaker, Cambrex Bio Science, Verviers, Belgium) and 20% fetal calf serum (complete RPMI). Under these experimental conditions, Caco-2 cells from passages 9 to 12 were used, maintained without antibiotics. The number of cells tested was 5 × 105 per dish. Four 50-mm petri dishes (Corning Glass Works) were used to analyze each coculture assay. L. lactis strains were grown to an optical density at 600 nm of 0.9 to 1.0, washed, and diluted in 1× PBS so that the multiplicity of infection was about 103 bacteria per cell, giving about 5 × 108 per dish. pLIG:BLG1 is a derivative of pIL253 usually present at a rate of 80 copies per cell (40); consequently, the quantity of pLIG:BLG1 contained in 5 × 108 bacteria was estimated to be 90 ng. Either the bacterial suspension or the purified pLIG:BLG1 was added to mammalian Caco-2 cells. After 3 h of coculturing, the cells were incubated for 24, 48, or 72 h in complete RPMI medium with gentamicin (20 mg/liter) to kill noninternalized lactococci (12). Bacterial counting was performed after 24, 48, and 72 h of incubation in the presence of gentamicin to estimate bacterial survival. A stable survival level of ∼102 lactococci was observed (data not shown).
Detection of DNA transfer from L. lactis to Caco-2 cells by PCR.
At 24, 48, and 72 h, genomic DNA from gentamicin-treated Caco-2 cells was extracted with the DNeasy tissue kit (QIAGEN), as described by the supplier. Plasmid DNA was detected by PCR with the oligonucleotides pcDNA3 start (ATCCCCTATGGTCGACTCTCAGTACAATCT), priming at the N terminus of pcDNA3; pcDNA3 end (GACGTCAGGTGGCACTTTTCGGGGAAATGT), priming at the C terminus of pcDNA3; BLGn2 (CTAATCGTCACCCAGACCATGAAGGGC), priming at the N terminus of BLG cDNA; and BLGc2 (GATGTGGCACTGCTCCTCCAGCTGGGTTGGG), priming at the C terminus of BLG cDNA.
BLG extraction and detection in Caco-2 cells.
At 24, 48, and 72 h after gentamicin treatment, the medium was collected and Caco-2 cells were harvested. Proteins were extracted as described above for Cos-7 cells, and BLG was assayed in various extracts with both EIAs described above.
BLG extraction and detection from cultures of bacteria.
Total proteins were extracted from cultures of bacteria, as previously described (9). BLG was then detected in extracts with both EIAs described above.
RESULTS
BLG expression in Cos-7 cells is higher with pLIG:BLG1.
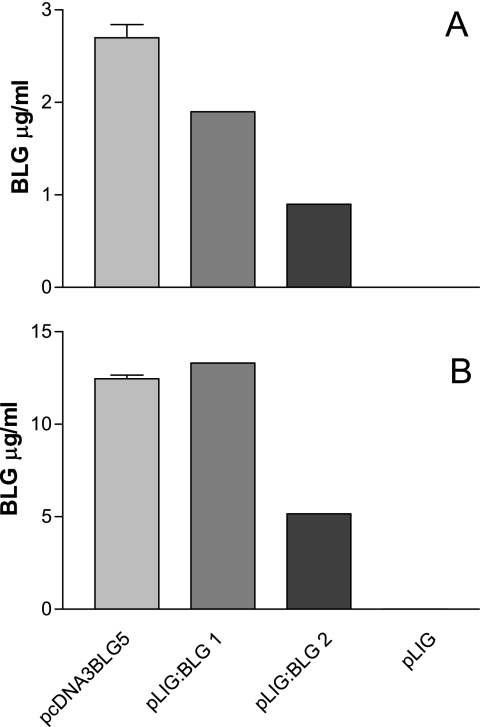
Plasmids pLIG:BLG1 and pLIG:BLG2, carrying a eukaryotic expression cassette of the blg gene, are depicted in Fig. 1. Cos-7 cells were transfected with the purified plasmids pcDNA3BLG5 (positive control), pLIG1 (negative control), pLIG:BLG1, and pLIG:BLG2. Forty-eight hours after transfection, nBLG and RCM-BLG were assayed in culture supernatants and cell extracts. RCM-BLG was never detected in the culture supernatant. The amount of BLG detected in cell extracts was significantly lower with pLIG:BLG2 than with pcDNA3BLG5 or pLIG:BLG1 (Fig. 2A). In contrast, the amount of BLG detected in culture supernatants was not significantly different between pLIG:BLG1 and pLIG:BLG2 (Fig. 2B). About 10% of the BLG found in the cell extract was denatured. We used pLIG:BLG1 for further experiments.
FIG. 2.
BLG expression in Cos-7 cells after transfection. Cos-7 cells were transfected with plasmids pcDNA3BLG5, pLIG:BLG1, and pLIG:BLG2. Two days later, the transfection medium was collected and cells were harvested. BLG was assayed in the culture medium and in cell extracts. Results are expressed in micrograms per milliliter of culture medium (A) and of cell extracts (B). The results correspond to the averages of two independent assays. Error bars correspond to standard deviations. Statistical significance was set at a P value of <0.05.
Lactococcus lactis MG1363(pLIG:BLG1) is not able to express BLG.
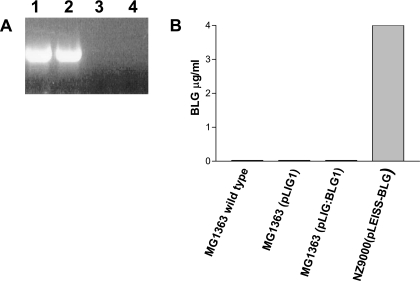
Total mRNA was extracted from cultures of MG1363(pLIG1) and MG1363(pLIG:BLG1). Figure 3A shows RT-PCR experiments performed with total mRNA from MG1363(pLIG1) and MG1363(pLIG:BLG1), using β-actin-specific oligonucleotides as an RNA positive control (lanes 1 and 2, respectively). No BLG mRNAs were detected in MG1363(pLIG1) or in MG1363(pLIG:BLG1) (Fig. 3A, lanes 3 and 4, respectively). An EIA was performed to confirm the RT-PCR data (Fig. 3B). Total protein was extracted from cultures of wild-type MG1363, MG1363(pLIG1), and MG1363(pLIG:BLG1). BLG was assayed from supernatant and total protein extracts. No BLG was detected in any strain. No trace of BLG mRNA or BLG protein was detected in wild-type L. lactis MG1363, MG1363(pLIG1), or MG1363(pLIG:BLG1). We checked that BLG was produced only by our positive control, a nisin-induced culture of L. lactis NZ900(pLEISS-BLG) (31).
FIG. 3.
BLG expression in MG1363(pLIG1) and MG1363(pLIG:BLG1). (A) Detection of β-actin and BLG-specific mRNA by RT-PCR in total RNA from saturated growth of MG1363(pLIG1) and MG1363(pLIG:BLG1). Lanes 1 and 2, β-actin mRNA detection by RT-PCR from MG1363(pLIG1) and MG1363(pLIG:BLG1), respectively; lanes 3 and 4, BLG mRNA detection by RT-PCR from MG1363(pLIG1) and MG1363(pLIG:BLG1), respectively. (B) BLG assay of L. lactis strains grown to saturation.
Lactococcus lactis is able to transfer genetic material to Caco-2 cells.
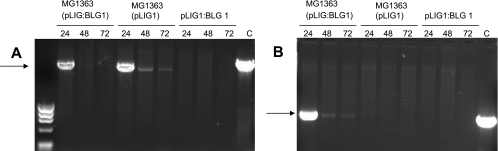
In order to evaluate the feasibility of plasmid delivery from L. lactis to eukaryotic cells, MG1363(pLIG1), MG1363(pLIG:BLG1), or purified plasmid pLIG:BLG1 was cocultivated for 3 h with Caco-2 cells, which are routinely used as a model of epithelial cells. DNA was extracted, purified, and quantified from these cells at 24, 48, and 72 h after incubation with gentamicin. Specific amplification by PCR was performed on 1 μg of total DNA with specific oligonucleotides priming at the N terminus and C terminus of pcDNA3 (Fig. 4A) and the N terminus and C terminus of BLG cDNA (Fig. 4B). No amplification was observed with any primer pair when purified pLIG:BLG1 was added to Caco-2 cells. In cocultures of Caco-2 cells with MG1363(pLIG1), the plasmid was detected at 24, 48, and 72 h with pcDNA3-specific oligonucleotides (Fig. 4A). Nevertheless, a dramatic decrease in pLIG1 concentration was observed after 24 h (Fig. 4A). In cocultures of Caco-2 cells with MG1363(pLIG:BLG1), the plasmid was detected at 24 h (Fig. 4A) and at 24, 48, and 72 h (Fig. 4B), with either the pcDNA3 or BLG primer pairs, respectively. A similarly dramatic decrease in the pLIG:BLG1 concentration after 24 h was observed (Fig. 4B). L. lactis was able to transfer genetic material to Caco-2 cells, and this DNA remained detectable even 72 h after coincubation.
FIG. 4.
Plasmid transfer detection in Caco-2 cells determined by amplification by PCR from total DNA purified 24, 48, and 72 h after gentamicin treatment of Caco-2 cells cocultured with MG1363(pLIG:BLG1), MG1363(pLIG1), and purified plasmid pLIG:BLG1. (A) Specific amplification by PCR using oligonucleotides primed at the N terminus and the C terminus of pcDNA3. (B) Specific amplification by PCR using oligonucleotides primed at the N terminus and the C terminus of BLG cDNA. Control (C) amplifications were made with purified plasmid pcDNA3BLG5.
The Caco-2 epithelial cell line is able to express and secrete BLG after coculture with MG1363(pLIG:BLG1).
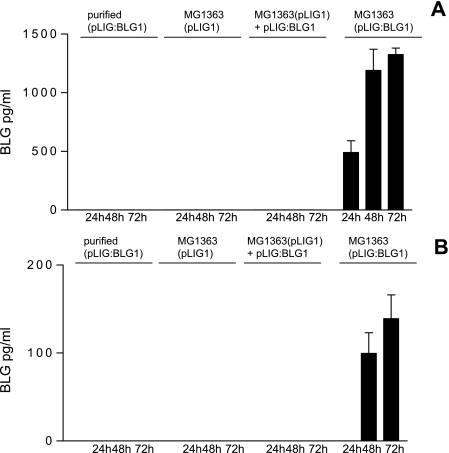
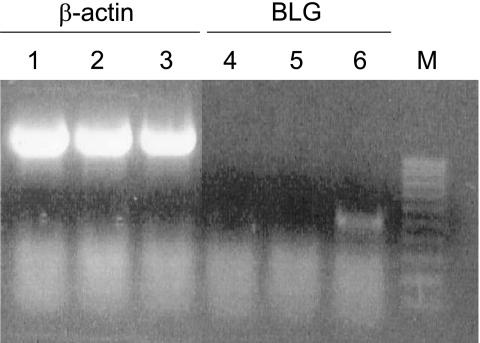
In order to determine whether L. lactis is able to deliver a functional plasmid, we used the coculture assay with Caco-2 cells and either (i) purified pcDNA3:BLG1, (ii) MG1363(pLIG1), (iii) purified pLIG:BLG1 mixed with MG1363(pLIG1), or (iv) MG1363(pLIG:BLG1). The cellular extracts and media from 24-, 48-, and 72-h gentamicin-treated Caco-2 cells were analyzed by a highly specific BLG EIA. We did not detect BLG expression when Caco-2 cells were cocultured with purified pLIG:BLG1, MG1363(pLIG1), or purified pLIG:BLG1 mixed with MG1363(pLIG1) (Fig. 5). BLG expression and secretion were detected only when Caco-2 cells were cocultured with MG1363(pLIG:BLG1), showing that the plasmid should be inside the bacteria to be successfully delivered. In this case, we observed an increase in BLG expression in the cellular extract from 500 pg/ml to about 1,500 pg/ml between 24 and 48 h, reaching a maximum at 72 h (Fig. 5). We also observed an increase in BLG secretion from 100 pg/ml to 150 pg/ml between 48 and 72 h in the cellular supernatant. Seventy hours after coculture, 80% of the BLG was found in the medium. Denatured BLG conformation was never detected. Taken together, these results demonstrate that (i) L. lactis was able to deliver fully functional plasmid into Caco-2 cell lines and (ii) these host cells were able to incorporate this DNA, expressing and secreting the BLG protein. Moreover, the presence of BLG mRNA in Caco-2 cells was confirmed by RT-PCR performed with BLG-specific oligonucleotides (Fig. 6).
FIG. 5.
BLG expression in Caco-2 cells. Caco-2 cells were cocultivated with purified plasmid pLIG:BLG1, MG1363(pLIG1), purified plasmid pLIG:BLG1 mixed with MG1363(pLIG1), and MG1363(pLIG:BLG1). Twenty-four, 48, and 72 h after treatment with gentamicin, the cells were harvested and culture supernatants were collected. (A) BLG assayed in the cellular extract. (B) BLG assayed in the culture supernatant. The results correspond to the averages of three independent assays. Error bars correspond to standard deviations.
FIG. 6.
Detection of BLG mRNA in Caco-2 cells. Caco-2 cells were cocultivated with purified plasmid pLIG:BLG1 (lanes 1 and 4), MG1363(pLIG1) (lanes 2 and 5), and MG1363(pLIG:BLG1) (lanes 3 and 6). Forty-eight hours after treatment with gentamicin, the cells were harvested and total RNA was collected and used for RT-PCR experiments with β-actin- and BLG-specific oligonucleotides. M, nucleic acid marker.
DISCUSSION
We evaluated the potential of native noninvasive lactococci as DNA delivery vectors by coculture of Caco-2 epithelial cells and L. lactis strains carrying an expression cassette encoding BLG cDNA under the transcriptional control of the human cytomegalovirus eukaryotic promoter (Pcmv). We showed that L. lactis was able to deliver the blg gene with subsequent expression of the BLG protein by the eukaryotic host cell. It was previously shown that native lactococcus strains are able to adhere to the human cell line Caco-2 (23). Moreover, a similar adhesion background was also observed after coincubation of recombinant invasive L. lactis strains and epithelial cells (4, 20, 27, 36, 41). Our hypothesis is that after coculture of L. lactis and Caco-2 epithelial cells, some lactococcal strains are internalized and probably lysed within the phagolysosome, releasing BLG cDNA into the cytosol via leakage from host cell phagosomes. This type of leakage between these two compartments has already been proposed for the transfer of certain protein antigens from the phagosome to the cytosol in primary macrophages and in dendritic cells (1, 19, 22, 24).
After 24 h, the BLG-specific DNA concentration significantly decreased inside Caco-2 cells, suggesting that it progressively disappeared by degradation by cytoplasmic nucleases and/or by entering the nucleus and being stable inside it (26). The turnover of DNA delivered by microinjection into the cytosol has been shown to be rapid, with an apparent half-life of 50 to 90 min in HeLa and COS-1 cells (26). Bacterial DNA delivery to eukaryotic cells was previously achieved at a low rate with noninvasive strains of E. coli (38). The low rate of BLG expression by Caco-2 cells could be due to the low efficiency of nuclear DNA importation into the nucleus (11). No production of BLG was observed when Caco-2 cells were coincubated with purified plasmid mixed or not mixed with the bacterium, suggesting that the plasmid must be inside the bacterium to achieve transfer and consequent BLG production.
Some advantages of using lactococci as a DNA delivery vehicle can be considered. (i) Oral administration of such a DNA vaccine vector could induce specific protection against food allergies by inducing a local IgA immune response (9). (ii) In contrast to immunization with naked plasmid DNA, no further plasmid amplification and purification steps are needed, considerably reducing cost and labor. Finally, (iii) an improved safety profile can be attained due to the fact that lactococci are food-grade bacteria that deliver genes encoding only the desired antigens, in contrast to similar delivery systems with pathogens. This strategy could be improved by increasing the quantities of delivered DNA, which could be performed by analyzing the incidence of plasmid size and copy number. This could optimize DNA nuclear importation and consequently BLG expression by the host cell. The results presented here are highly encouraging; the next step is to evaluate the immunological response after oral administration in mice of this Lactococcus strain as a bacterial carrier to determine whether it can be used as a DNA vaccine against BLG in vivo.
Acknowledgments
Valeria Dellaretti Guimarães was a recipient of grants from the Region Ile-de-France, the INRA Animal Health Department (France), French-Brazilian CAPES-Cofecub, and CNPq (Brazil). Silvia Innocentin is a recipient of a European Ph.D. Marie Curie grant from the LABHEALTH program.
Footnotes
Published ahead of print on 8 September 2006.
REFERENCES
- 1.Ackerman, A. L., C. Kyritsis, R. Tampe, and P. Cresswell. 2003. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. USA 100:12889-12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adel-Patient, K., C. Creminon, D. Boquet, J. M. Wal, and J. M. Chatel. 2001. Genetic immunisation with bovine beta-lactoglobulin cDNA induces a preventive and persistent inhibition of specific anti-BLG IgE response in mice. Int. Arch. Allergy Immunol. 126:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Adel-Patient, K., S. Ah-Leung, C. Creminon, S. Nouaille, J. M. Chatel, P. Langella, and J. M. Wal. 2005. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin. Exp. Allergy 35:539-546. [DOI] [PubMed] [Google Scholar]
- 4.Avall-Jaaskelainen, S., A. Lindholm, and A. Palva. 2003. Surface display of the receptor-binding region of the Lactobacillus brevis S-layer protein in Lactococcus lactis provides nonadhesive lactococci with the ability to adhere to intestinal epithelial cells. Appl. Environ. Microbiol. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez-Humaran, L. G., P. Langella, A. Miyoshi, A. Gruss, R. T. Guerra, R. Montes de Oca Lunaqq, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermúdez-Humarán, L. G., P. Langella, N. Cortez-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Sauceda-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal administration of recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermúdez-Humarán, L. G., N. G. Cortes-Perez, Y. Le Loir, J. M. Alcocer-Gonzalez, R. S. Tamez-Guerra, R. M. de Oca-Luna, and P. Langella. 2004. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J. Med. Microbiol. 53:427-433. [DOI] [PubMed] [Google Scholar]
- 8.Chatel, J. M., K. Adel-Patient, C. Creminon, and J. M. Wal. 1999. Expression of a lipocalin in prokaryote and eukaryote cells: quantification and structural characterization of recombinant bovine beta-lactoglobulin. Protein Expr. Purif. 16:70-75. [DOI] [PubMed] [Google Scholar]
- 9.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatel, J. M., S. Nouaille, K. Adel-Patient, Y. Le Loir, H. Boe, A. Gruss, J. M. Wal, and P. Langella. 2003. Characterization of a Lactococcus lactis strain that secretes a major epitope of bovine beta-lactoglobulin and evaluation of its immunogenicity in mice. Appl. Environ. Microbiol. 69:6620-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean, D. A., Strong, D. D., and W. E. Zimmer. 2005. Nuclear entry of nonviral vectors. Gene Ther. 12:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 13.Dunham, S. P. 2002. The application of nucleic acid vaccines in veterinary medicine. Res. Vet. Sci. 73:9-16. [DOI] [PubMed] [Google Scholar]
- 14.Ellman, G. L., K. D. Courtney, V. Andres, and R. M. Featherstone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88-95. [DOI] [PubMed] [Google Scholar]
- 15.Fouts, T. R., A. L. DeVico, D. Y. Onyabe, M. T. Shata, K. C. Bagley, G. K. Lewis, and D. M. Hone. 2003. Progress toward the development of a bacterial vaccine vector that induces high-titer long-lived broadly neutralizing antibodies against HIV-1. FEMS Immunol. Med. Microbiol. 37:129-134. [DOI] [PubMed] [Google Scholar]
- 16.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grangette, C., H. Muller-Alouf, P. Hols, D. Goudercourt, J. Delcour, M. Turneer, and A. Mercenier. 2004. Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect. Immun. 5:2731-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillot-Courvalin, C., S. Goussard, and P. Courvalin. 1999. Bacteria as gene delivery vectors for mammalian cells. Curr. Opin. Biotechnol. 10:477-481. [DOI] [PubMed] [Google Scholar]
- 19.Guermonprez, P., L. Saveanu, M. Kleijmeer, J. Davoust, P. Van Endert, and S. Amigorena. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425:397-402. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes, V. D., J. E. Gabriel, F. Lefevre, D. Cabanes, A. Gruss, P. Cossart, V. Azevedo, and P. Langella. 2005. Internalin-expressing Lactococcus lactis is able to invade small intestine of guinea pigs and deliver DNA into mammalian epithelial cells. Microb. Infect. 7:836-844. [DOI] [PubMed] [Google Scholar]
- 21.Host, A., and S. Halken. 1998. Epidemiology and prevention of cow's milk allergy. Allergy 53:111-113. [DOI] [PubMed] [Google Scholar]
- 22.Houde, M., S. Bertholet, E. Gagnon, S. Brunet, G. Goyette, A. Laplante, M. F. Princiotta, P. Thibault, D. Sacks, and M. Desjardins. 2003. Phagosomes are competent organelles for antigen cross-presentation. Nature 425:402-406. [DOI] [PubMed] [Google Scholar]
- 23.Kimoto, H., J. Kurisaki, N. M. Tsuji, S. Ohmomo, and T. Okamoto. 1999. Lactococci as probiotic strains: adhesion to human enterocyte-like Caco-2 cells and tolerance to low pH and bile. Lett. Appl. Microbiol. 29:313-316. [DOI] [PubMed] [Google Scholar]
- 24.Kovacsovics-Bankowski, M., and K. L. Rock. 1995. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267:243-246. [DOI] [PubMed] [Google Scholar]
- 25.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechardeur, D., K. J. Sohn, M. Haardt, P. B. Joshi, M. Monck, R. W. Graham, B. Beatty, J. Squire, H. O'Brodovich, and G. L. Lukacs. 1999. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 6:482-497. [DOI] [PubMed] [Google Scholar]
- 27.Lindholm, A., A. Smeds, and A. Palva. 2004. Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl. Environ. Microbiol. 70:2061-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannan, P., K. F. Jones, and B. L. Geller. 2004. Mucosal vaccine made from live, recombinant Lactococcus lactis protects mice against pharyngeal infection with Streptococcus pyogenes. Infect. Immun. 72:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negroni, L., H. Bernard, G. Clement, J. M. Chatel, P. Brune, Y. Frobert, J. M. Wal, and J. Grassi. 1998. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J. Immunol. Methods 220:25-37. [DOI] [PubMed] [Google Scholar]
- 30.Nouaille, S., L. A. Ribeiro, A. Miyoshi, D. Pontes, Y. Le Loir, S. C. Oliveira, P. Langella, and V. Azevedo. 2003. Heterologous protein production and delivery systems for Lactococcus lactis. Genet. Mol. Res. 31:102-111. [PubMed] [Google Scholar]
- 31.Nouaille, S., L. G. Bermudez-Humaran, K. Adel-Patient, J. Commissaire, A. Gruss, J. M. Wal, V. Azevedo, P. Langella, and J. M. Chatel. 2005. Improvement of bovine ss-lactoglobulin production and secretion by Lactococcus lactis. Braz. J. Med. Biol. Res. 38:353-359. [DOI] [PubMed] [Google Scholar]
- 32.Papiz, M. Z., L. Sawyer, E. E. Eliopoulos, A. C. North, J. B. Findlay, R. Sivaprasadarao, T. A. Jones, M. E. Newcomer, and P. J. Kraulis. 1986. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 324:383-385. [DOI] [PubMed] [Google Scholar]
- 33.Pontes, D. S., F. A. Dorella, L. A. Ribeiro, A. Miyoshi, Y. Le Loir, A. Gruss, S. C. Oliveira, P. Langella, and V. Azevedo. 2003. Induction of partial protection in mice after oral administration of Lactococcus lactis producing Brucella abortus L7/L12 antigen. J. Drug Target. 11:489-493. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step toward food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson, K., L. M. Chamberlain, M. C. Lopez, C. M. Rush, H. Marcotte, R. W. Le Page, and J. M. Wells. 2004. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 72:2753-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roche, F. M., M. Meehan, and T. J. Foster. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759-2767. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Schaffner, W. 1980. Direct transfer of cloned genes from bacteria to mammalian cells. Proc. Natl. Acad. Sci. USA 77:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoen, C., J. Stritzker, W. Goebel, and S. Pilgrim. 2004. Bacteria as DNA vaccine carriers for genetic immunization. Int. J. Med. Microbiol. 294:319-335. [DOI] [PubMed] [Google Scholar]
- 40.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-567. [DOI] [PubMed] [Google Scholar]
- 41.Sinha, B., P. François, Y. A. Que, M. Hussain, C. Heilman, P. Moreillon, D. Lew, K. H. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steidler, L. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 43.Terzaghi, B., and W. E. Sandine. 1975. Improved medium for lactic acid streptococci and their bacteriophages. Appl. Environ. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theisen, M., S. Soe, K. Brunstedt, F. Follmann, L. Bredmose, H. Israelsen, S. M. Madsen, and P. Druilhe. 2004. A Plasmodium falciparum GLURP-MSP3 chimeric protein: expression in Lactococcus lactis, immunogenicity and induction of biologically active antibodies. Vaccine 22:1188-1198. [DOI] [PubMed] [Google Scholar]