Abstract
DNA methylation by the DNA adenine methyltransferase (Dam) interferes with the coordinated expression of virulence functions in an increasing number of pathogens. While analyzing the effect of Dam on the virulence of the human pathogen Yersinia enterocolitica, we observed type III secretion of Yop effector proteins under nonpermissive conditions. Dam alters the Ca2+ regulation of Yop secretion but does not affect the temperature regulation of Yop/Ysc expression. The phenotype is different from that of classical “Ca2+-blind” mutants of Yersinia, as Dam-overproducing (DamOP) strains still translocate Yops polarly into eukaryotic cells. Although transcription of the lcrGV and yopN-tyeA operons is slightly upregulated, LcrG is absent from lysates of DamOP bacteria, while the amounts of YopN and TyeA are not changed. We present evidence that clpXP expression increases after Dam overproduction and that the ClpP protease then degrades LcrG, thereby releasing a block in type III secretion. This is the first example of posttranslational regulation of type III secretion by the Clp protease and adds a new flavor to the complex regulatory mechanisms underlying the controlled release of effector proteins from bacterial cells.
In Escherichia coli, the Dam enzyme (DNA adenine methyltransferase) catalyzes the methylation of adenine residues at the N-6 position in GATC sequences. The methylation of DNA influences various basic cellular processes ranging from chromosome replication to mismatch repair (72). During replication, delayed methylation of the newly synthesized daughter strand is required for parental strand-directed mismatch repair (48). Therefore, an imbalance in DNA methylation in dam mutant or Dam-overproducing (DamOP) strains results in an increased mutation frequency and sensitivity to base analogues (23, 28, 45). In addition to having these functions, DNA methylation influences the binding of regulatory proteins to promoter regions, thereby imparting an epigenetic mechanism linked to replication on gene expression. The best-studied example is the expression of pyelonephritis-associated pili (pap) of uropathogenic E. coli, where Dam controls the binding of Lrp in the promoter region of the pap operon (29).
Furthermore, Dam has been shown to be essential for virulence in a number of human and animal pathogens, like Salmonella enterica, Yersinia pseudotuberculosis, Yersinia pestis, Haemophilus influenzae, Vibrio cholerae, Pasteurella multocida, and Aeromonas hydrophila (9, 19, 36, 37, 71). For example, Dam-deficient S. enterica serovar Typhimurium show reduced M-cell cytotoxicity and invasion of enterocytes. Furthermore, Dam-deficient as well as DamOP salmonellae deregulate the expression of several virulence-associated genes (22, 27, 43). In Y. pseudotuberculosis, DamOP results in reduced virulence in a mouse model of infection and deregulated expression and secretion of virulence-associated Yop and LcrV proteins (3, 36, 37). Attenuation of these strains was used effectively in the development of vaccine strains of Salmonella as well as Yersinia (17, 18, 26, 36, 57, 66). The phenotypes of strains with defects in DNA methylation are generally diverse, which is not surprising given the fact that GATC sequences are widespread in the chromosome. Interestingly, effects of Dam on the secretion of virulence factors seem to be common among pathogens. DamOP interferes with the regulation of type III secretion (T3S) in S. enterica serovar Typhimurium as well as in Y. pseudotuberculosis (22, 36, 37). Furthermore, dam mutants of S. enterica serovar Typhimurium release large amounts of membrane material containing extracellular proteins into the supernatant (55). In A. hydrophila, the T3S-associated cytotoxicity of a DamOP strain is decreased, while the cytotoxicity associated with the type II secreted Act enterotoxin is increased (19). The regulatory networks behind these phenomena, however, remain elusive. Although DamOP strains as well as dam mutant strains might not reflect a physiologically relevant situation, they provide excellent models for studying the influence of DNA methylation on gene expression. Therefore, employing such strains is primarily a tool for studying effects of differential DNA methylation in promoter regions that are of physiological significance (72). This does not imply a direct regulation by Dam but mirrors the effect of altered binding affinities of methylation-sensitive regulatory proteins.
The virulence plasmid-encoded T3S system (T3SS) is a hallmark of Yersinia virulence. Upon host cell contact, the tightly regulated Yop/Ysc T3SS is expressed and translocates the Yop effector proteins into the host cell cytosol, where they down-regulate the host's immune response, reduce phagocytosis, and induce apoptosis (70). This allows the bacteria to survive extracellularly in lymphoid tissues of the host. Under in vitro conditions, the expression of Yop proteins is induced after culture at 37°C, but secretion starts only after depletion of Ca2+ from the medium (12). Not only Yop translocation and secretion but also their expression is tightly regulated at the transcriptional level. At 37°C, the virulence plasmid-encoded AraC-like regulator VirF initiates the expression of genes for the T3S apparatus as well as for the Yop effector proteins. Furthermore, the histone-like protein YmoA and DNA supercoiling are involved in transcriptional control of and by VirF (39, 58, 59). In addition to having this positive loop, Yop expression is regulated by a negative-feedback mechanism. In the presence of Ca2+, Yops are not secreted and inhibit their further expression (6).
Proteins that control the release of Yops have been identified in “Ca2+-blind” mutants. Defects in e.g., the secreted YopN or cytosolic TyeA and LcrG proteins result in the secretion of effector proteins into the bacterial supernatant irrespective of the presence of Ca2+. Furthermore, the amount of Yops translocated by these strains is decreased compared to that in wild-type bacteria, as Ca2+-blind mutants also apolarly secrete Yops into the surrounding medium and do not only translocate them into the host cell (10, 11, 16, 21, 31, 60, 63, 65). These data indicate that yersiniae are able to respond to host cell contact by a mechanism involving the sensing of Ca2+, which results in the polar translocation of Yop proteins. However, the molecular mechanism behind this regulation is very complex and not completely understood.
In a Y. pseudotuberculosis DamOP strain, Yop proteins are secreted at nonpermissive temperatures in the absence of Ca2+, demonstrating that DamOP targets the regulation of Ysc/Yop expression but not the Ca2+ regulation of Yop secretion (36, 37). While analyzing the effect of Dam on expression of virulence factors in Y. enterocolitica, we found that, in strict contrast to what occurs in Y. pseudotuberculosis, DamOP alters the Ca2+ regulation of Yop secretion but not the temperature regulation of Yop expression. Therefore, we aimed at identifying the regulatory mechanism behind the Dam-induced T3S phenotype in Y. enterocolitica. Here we present evidence that the protease ClpP mediates the effect of Dam on T3S via the degradation of the regulatory LcrG protein. Our data give new insights into the complex regulatory networks underlying the effect of DNA methylation on virulence gene expression and, in particular, on T3S in pathogenic Yersinia species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Unless otherwise indicated, all strains were grown in Luria-Bertani (LB) broth or on agar plates at 26°C for Y. enterocolitica or 37°C for E. coli. Antibiotics were used as described previously (20).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Y. enterocolitica strains | ||
| JB580v | ΔyenR(r− m+) Nalr, serogroup O:8 | 38 |
| GHY147 | JB580v, pTP166Kan-damΔ | 20 |
| GHY150 | JB580v, pTP166Kan | 20 |
| GHY178 | JB580v yscU:: pEP-yscU | This study |
| GHY179 | JB580v yscU:: pEP-yscU, pTP166Kan-damΔ | This study |
| GHY180 | JB580v yscU:: pEP-yscU, pTP166Kan | This study |
| GHY238 | JB580v lcrG:: lacZYA | This study |
| GHY239 | JB580v orf76::lacZYA | This study |
| GHY240 | JB580v orf83::lacZYA | This study |
| GHY243 | JB580v yscN::lacZYA | This study |
| GHY244 | JB580v lcrG::lacZYA, pTP166Kan-damΔ | This study |
| GHY245 | JB580v lcrG::lacZYA, pTP166Kan | This study |
| GHY246 | JB580v orf76::lacZYA, pTP166Kan-damΔ | This study |
| GHY247 | JB580v orf76::lacZYA, pTP166Kan | This study |
| GHY248 | JB580v orf83::lacZYA, pTP166Kan-damΔ | This study |
| GHY249 | JB580v orf83::lacZYA, pTP166Kan | This study |
| GHY250 | JB580v yscN::lacZYA, pTP166Kan-damΔ | This study |
| GHY251 | JB580v yscN::lacZYA, pTP166Kan | This study |
| GHY256 | JB580v yopN::lacZYA | This study |
| GHY257 | JB580v yopN::lacZYA, pTP166Kan-damΔ | This study |
| GHY258 | JB580v yopN::lacZYA, pTP166Kan | This study |
| GHY266 | JB580v clpP::pEP-clpP | This study |
| GHY267 | JB580v clpP::pEP-clpP, pTP166Kan-damΔ | This study |
| GHY268 | JB580v clpP::pEP-clpP, pTP166Kan | This study |
| GHY274 | JB580v lon::pEP-lon | This study |
| GHY275 | JB580v lon::pEP-lon, pTP166Kan-damΔ | This study |
| GHY276 | JB580v lon::pEP-lon, pTP166Kan | This study |
| GHY347 | JB580v ΔlcrG | This study |
| E. coli strains | ||
| DH5α | φ80dΔ(lacZ)M15 Δ(argF-lac)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | Gibco BRL |
| S17-1λpir | Tpr SmrrecA thi pro hsdR mutant, M+ RP4::2-Tc::Mu::Km Tn7λpir lysogen | 47 |
| Plasmids | ||
| pEP185.2 | Camrmob+ (RP4) R6K ori (suicide vector) | 38 |
| pKN8 | Camrmob+ (RP4) R6K ori (suicide vector) lacZYA | 50 |
| pTP166Kan | Kanamycin-resistant derivative (Amps) of pTP166 | 20 |
| pTP166Kan-damΔ | dam mutant derivative of pTP166Kan | 20 |
| pEP-yscU | Internal fragment of yscU in pEP185.2 | This study |
| pEP-clpP | Internal fragment of clpP in pEP185.2 | This study |
| pEP-lon | Internal fragment of lon in pEP185.2 | This study |
| pKN8-lcrG | lcrG promoter fragment in pKN8 | This study |
| pKN8-orf76 | orf76 promoter fragment in pKN8 | This study |
| pKN8-orf83 | orf83 promoter fragment in pKN8 | This study |
| pKN8-yscN | yscN promoter fragment in pKN8 | This study |
| pKN8-yopN | yopN promoter fragment in pKN8 | This study |
DamOP was achieved by electroporating pTP166Kan or pTP166Kan-damΔ (20) as a negative control into the corresponding Y. enterocolitica strain and inducing the expression of dam from the Ptac promoter by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) 30 min after subculture for each assay.
Analysis of Yop secretion and expression.
Overnight cultures of different Y. enterocolitica strains grown in brain-heart infusion medium (BHI) were used to inoculate 10 ml of fresh BHI to an optical density at 600 nm of 0.1. To analyze Yop secretion and expression, either BHI was supplemented with 20 mM MgCl2 and 20 mM sodium oxalate to remove free Ca2+ ions or the bacteria were subcultivated in the presence of Ca2+. All cultures were incubated with aeration at 26°C for 2 h, followed by incubation at 26°C or at 37°C for a further 4 h and collection of the bacterial cells by centrifugation at 11,500 × g for 10 min.
For the analysis of Yop secretion, proteins in the supernatant of 2 ml of culture were precipitated by trichloroacetic acid (TCA; final concentration of 10%, vol/vol) and washed with acetone. Dried protein pellets were resuspended in 30 to 40 μl of sample buffer and normalized according to the cell count. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 15% polyacrylamide gels and visualized by Coomassie brilliant blue staining.
For the analysis of Yop expression, whole-cell lysates corresponding to 3.7 × 108 bacteria were separated by SDS-15% PAGE and transferred to nitrocellulose for immunoblot analysis. Rabbit polyclonal antisera were used at the following dilutions: for anti-YopE, 1:10,000; for anti-YopT, 1:3,000; for anti-YopH, 1:1,024,000; and for anti-YopM, 1:51,200. Binding was detected after incubation with alkaline phosphatase-conjugated secondary antibody (goat anti-rabbit).
To determine the amount of LcrG, LcrV, YopN, or TyeA after DamOP, bacteria were subcultured for 30 min at 26°C before IPTG was added to induce Dam expression. Growth was continued for 1.5 h, and then cultures were shifted to 37°C for an additional 2 h. Whole-cell lysates and supernatants corresponding to 3 × 108 bacteria were separated by tricine-SDS-14% PAGE (61) and transferred to nitrocellulose for immunoblot analysis. Rabbit polyclonal antisera specific for LcrG (dilution, 1:150), LcrV (1:7,500), YopN (1:200), and TyeA (1:200) and peroxidase-conjugated secondary antibodies (goat anti-rabbit) were used to detect binding. Chemiluminescence was detected using ECL Western blotting detection reagents (Amersham).
Immunoprecipitation of translocated YopE protein.
Infection of cells and immunoprecipitation were performed as previously described (34) with the exception that 8 × 106 CHO (Chinese hamster ovary) cells were infected with 4.8 × 108 bacteria from overnight cultures grown at 26°C in BHI (multiplicity of infection of 60:1). In some instances, 0.5 μM cytochalasin D was included to avoid bacterial uptake. After 60 min of infection, CHO cells were washed twice with phosphate-buffered saline to remove nonadherent bacteria and cultured for a further 5 h. To stop the infection, cell culture flasks were transferred to 4°C for 10 min and CHO cells were washed with ice-cold phosphate-buffered saline. The cells were collected with a cell scraper, followed by centrifugation at 500 × g. To analyze the polarity of Yop secretion/translocation, supernatant proteins were precipitated by 10% TCA and quantified by Western blot analysis. The pelleted cells were solubilized (50 mM Tris-HCl, pH 8.0; 50 mM NaCl; 0.1% Triton X-100), and cell debris and bacteria were removed by centrifugation. Unspecifically binding proteins were removed from the supernatant by preincubation with protein G-Sepharose. The resulting supernatant was incubated overnight with anti-YopE antiserum and subsequently subjected to protein G-Sepharose. Antibody-YopE-protein G-Sepharose complexes were resuspended in sample buffer for immunoblot analysis and detected using peroxidase-conjugated goat anti-rabbit antibody and the SuperSignal chemiluminescent-substrate kit (Pierce).
Construction of mutant strains.
Y. enterocolitica mutant strains were constructed as follows. Internal fragments were amplified by PCR using the primer pairs SF-yscU1/SF-yscU2, SF-clpPRT1/SF-clpP5, and SF-lonRT1/SF-lon5 (Table 2) and ligated into the suicide vector pEP185.2. The resulting plasmids, pEP-yscU, pEP-lon, and pEP-clpP, were transferred to Y. enterocolitica JB580v from E. coli S17-1λpir by conjugation and integrated into the virulence plasmid or the chromosome by homologous recombination with selection for chloramphenicol, directly resulting in yscU, lon, and clpP mutant strains.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′→3′)a | Restriction site |
|---|---|---|
| SF-yscU1 | CCATCGATGGCGATCGCTTCTCATGTTGTGCA | ClaI |
| SF-yscU2 | GCTCTAGAGCAATATGGGTCGGATTAGCTACC | XbaI |
| SF-orf76-1 | GCTCTAGATGAGCCAGCTTCCAGAACACG | XbaI |
| SF-orf76-2 | GAAGATCTAAAACGATTCGCCCCTGAAAG | BglII |
| SF-orf83-1 | GCTCTAGATTGCTCGCGAATAACACTCAC | XbaI |
| SF-orf83-2 | GAAGATCTATTGGTCGCCAGCTGATAAAA | BglII |
| SF-yscN1 | GCTCTAGACTGGAACTTTGCTAAGGTATT | XbaI |
| SF-yscN2 | GAAGATCTTTGAGTGACACGTCCTCTAAT | BglII |
| SF-lcrGRT1 | CAAGCAGAACTGGCAATAGC | |
| SF-lcrGRT2 | CCTCGCATCATCGTTGGTTT | |
| SF-lcrG3 | GCTCTAGACACTTGGACGAGTTTGCTTAT | XbaI |
| SF-lcrG4 | GAAGATCTTAATTTTGCGCGGTGATCGCT | BglII |
| SF-yopNRT1 | GACGTTGAGGAGCAGGTTAA | |
| SF-yopNRT2 | ATAACCCATCACTGCATCGC | |
| SF-yopN3 | GCTCTAGACAGTATTAGAAGATATCCCGT | XbaI |
| SF-yopN4 | GAAGATCTATCAGCTATAGACTGCAGAGT | BglII |
| SF-lonRT1 | GCCATTCAGAAAGAACTGGGC | |
| SF-lonRT2 | GGCACCTGCAACATCCAATC | |
| SF-lon5 | GAAGATCTTACCCGGCATAGAGCCAATA | BglII |
| SF-clpPRT1 | TTTTCCTGACAGGCCAGGGTTG | |
| SF-clpPRT2 | ATGCACCCATTGAACATGCC | |
| SF-clpP5 | GAAGATCTTGCCATCAGTTCATTCATGC | BglII |
| SF-yhbURT1 | CAGCCGCAAACGGAAATTG | |
| SF-yhbURT2 | TTCATCATTGGTCGCCGAGG | |
| SF-rnaYE1 | AATACCGCATAACGTCTTCG | |
| SF-rnaYE2 | CTTCTTCTGCGAGTAACGTC | |
| SF-lcrG5 | CCGCTCGAGTTAATATCCAACCACTTGGAC | XhoI |
| SF-lcrG6 | GAAGATCTAATCTATCTCCTATTCTATTA | BglII |
| SF-lcrG7 | GAAGATCTTATGATTAGAGCCTACGAACA | BglII |
| SF-lcrG8 | TCCCCGCGGGTAAGCTCAGCTAATTCTTCA | SacII |
The restriction site is underlined.
For the construction of a nonpolar lcrG deletion mutant strain, 500 bp upstream and 500 bp downstream of the lcrG coding sequence were amplified with the primer pairs SF-lcrG5/SF-lcrG6 and SF-lcrG7/SF-lcrG8, respectively. After digestion with BglII and ligation of the two fragments, the resulting 1-kb fragment was amplified with the primers SF-lcrG5 and SF-lcrG8 and ligated into pEP185.2, resulting in plasmid pEP-ΔlcrG5/8. After conjugation of the plasmid into Y. enterocolitica JB580v from E. coli S17-1λpir and homologous recombination into the virulence plasmid, cycloserine enrichment was used to identify chloramphenicol-sensitive exintegrants. Subsequently, as for all mutants, the mutant genotype was confirmed by Southern blotting and PCR analysis (data not shown).
Quantification of gene expression.
The expression of different genes involved in Yop secretion was quantified by reporter gene technology and/or quantitative reverse transcription (qRT)-PCR. For the construction of Y. enterocolitica promoter-lacZ fusions, ∼600-bp fragments containing the 5′ end of the respective gene, including the promoter region, were amplified by PCR using the primer pairs SF-lcrG3/SF-lcrG4, SF-yopN3/SF-yopN4, SF-yscN1/SF-yscN2, SF-orf76-1/SF-orf76-2, and SF-orf83-1/SF-orf83-2 (see Table 2), with genomic DNA of Y. enterocolitica JB580v as the template. The PCR products were ligated into pKN8 (20), resulting in pKN8-lcrG, pKN8-yopN, pKN8-yscN, pKN8-orf76, and pKN8-orf83. After conjugation of the plasmids to Y. enterocolitica JB580v from E. coli S17-1λpir and integration into the virulence plasmid, merodiploid lcrG-lacZYA/lcrG+, yopN-lacZYA/yopN+, yscN-lacZYA/yscN+, orf76-lacZYA/orf76+, and orf83-lacZYA/orf83+ strains were generated. Proper integration of the plasmids into the Y. enterocolitica virulence plasmid was confirmed by Southern blot analysis (data not shown).
β-Galactosidase assays were performed as previously described (46). Briefly, overnight cultures grown at 26°C were subcultivated in BHI in the presence of Ca2+ and 1 mM IPTG to induce DamOP from pTP166Kan at 26°C for 2 h and at 37°C for 2 h. The bacterial cells were collected by centrifugation and washed in 0.85% (wt/vol) NaCl before enzyme activity assays. Enzyme activities are expressed as Miller units (46) and were averaged from at least three independent experiments, each performed in triplicate.
For the quantification of gene expression by qRT-PCR, cultures were grown in the presence of Ca2+ and 1 mM IPTG to induce DamOP from pTP166Kan at 26°C for 2 h and shifted to 37°C for 2 h. Total RNA was isolated with the RNeasy mini kit (QIAGEN). cDNA was generated by randomly primed reverse transcription (Revert Aid kit; Fermentas) after removal of contaminating DNA (Turbo DNA-free kit; Ambion). Relative gene expression was determined by qRT-PCR with the Light-Cycler system (Roche) and the QuantiTect SYBR green PCR kit (QIAGEN) according to the manufacturers' manuals. Experiments were performed at least in triplicate for each gene with the primer pairs SF-yopNRT1/SF-yopNRT2, SF-lcrGRT1/SF-lcrGRT2, SF-lonRT1/SF-lonRT2, SF-clpPRT1/SF-clpPRT2, SF-yhbURT1/SF-yhbURT2, and SF-rnaYE1/SF-rnaYE2 (Table 2). The expression of the 16S rRNA subunit was used as the housekeeping gene control. Specificities of the amplifications were confirmed by melting curve analysis.
RESULTS
DamOP alters the Ca2+ regulation of Yop secretion.
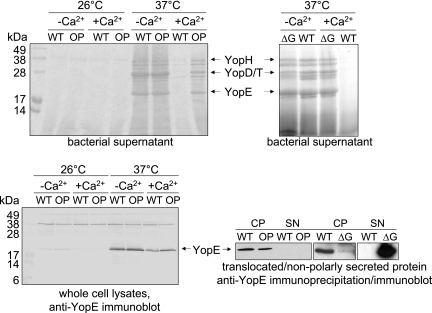
In Y. pseudotuberculosis, DamOP deregulates the control of Yop expression and secretion, as DamOP strains secrete Yops even at low (28°C) growth temperatures (36, 37). In order to characterize the effect of DamOP on Yop secretion in Y. enterocolitica, we analyzed supernatants of wild-type and DamOP strains grown under conditions either inducing or not inducing Yop secretion. As shown in Fig. 1, a DamOP strain secretes Yops at 37°C irrespective of the presence of Ca2+ ions in the growth medium, although the Yop levels in the presence of Ca2+ are slightly reduced compared to their secretion in the absence of Ca2+. As DamOP alters the temperature regulation of Yop expression in Y. pseudotuberculosis (37), we analyzed whole-cell lysates of wild-type and DamOP Y. enterocolitica organisms grown under different conditions by immunoblotting. As exemplified for YopE, the temperature regulation of expression of YopE, YopT, YopM, and YopH was not influenced in a DamOP strain (Fig. 1 and data not shown). Our data indicate that, in contrast to what occurs in Y. pseudotuberculosis, overproduction of the Dam enzyme in Y. enterocolitica interferes with the Ca2+ regulation of Yop secretion, whereas the temperature regulation of Yop expression remains unaffected. This phenotype resembles the Ca2+-blind phenotype of strains lacking YopN, TyeA, SycN, YscB, or LcrG (Fig. 1), which are involved in the control of Yop release and have been described as “gatekeepers” of Yop secretion (10, 15, 21, 31, 51, 63).
FIG. 1.
DamOP affects T3S in Y. enterocolitica. While DamOP affects the Ca2+ regulation of Yop secretion, it does not influence the temperature regulation of Yop expression or the polarity of Yop translocation into the host cell cytoplasm, which is in contrast to a Ca2+-blind ΔlcrG strain. To analyze T3S, supernatants of strains grown under different secretion-inducing and noninducing conditions were precipitated by TCA, separated by SDS-PAGE, and stained with Coomassie brilliant blue. Yop expression was analyzed by Western blotting. Translocated YopE protein was collected from the cytoplasm (CP) of infected CHO cells by immunoprecipitation. Apolarly secreted YopE was TCA precipitated from the culture supernatant. Proteins were subjected to SDS-PAGE and transferred to nitrocellulose. YopE was detected using anti-YopE polyclonal antiserum. Lanes are labeled as follows: OP, GHY150 (DamOP); WT, GHY147 (wild-type control); ΔG, GHY347 (Y. enterocolitica ΔlcrG); CP, immunoprecipitated YopE from the cytoplasm of Y. enterocolitica-infected CHO cells; SN, TCA-precipitated supernatant of Y. enterocolitica-infected CHO cells, corresponding to 3 ml of supernatant (only 250 μl in the case of the ΔlcrG strain).
DamOP does not influence the polar translocation of YopE.
Typical Ca2+-blind mutants show a nonpolar secretion into the medium and diminished translocation of Yops into host cells (10, 11, 16, 31, 60, 65). As DamOP strains of Y. enterocolitica show a response to low Ca2+ concentrations resembling that of Ca2+-blind mutants, we examined the effect of DamOP on the polarized secretion and translocation of YopE. To this end, CHO epithelial cells were infected with wild-type or DamOP Y. enterocolitica. Subsequently, the amount of translocated YopE was determined by immunoprecipitation. Furthermore, the supernatants of infected cells were analyzed for the presence of apolarly secreted YopE. As shown in Fig. 1, an influence of DamOP on polar translocation could not be observed. Similar amounts of YopE were translocated into the cytosol of cells infected with wild-type and a DamOP strain, while no YopE could be detected in the supernatants. This is in contrast to what occurs with an lcrG mutant strain, which apolarly secretes massive amounts of Yops into the supernatant of infected cells, resulting in decreased Yop translocation (Fig. 1). This shows that the observed phenotype of the DamOP strain is certainly different from that of classical Ca2+-blind strains (11, 14, 16, 65) and represents a new T3S-associated phenotype of Yersinia.
In a recent study, Lee et al. (40) suggested that Y. enterocolitica needs a serum signal to activate the T3S pathway. As we analyzed the translocation of Yops in the absence of serum, we could not exclude the possibility that this might interfere with proper Yop translocation. To analyze the effect of serum on Yop translocation after DamOP, cells were infected with DamOP and wild-type strains in the presence of 0.5% fetal calf serum. Differences in the amounts of translocated YopE could not be detected, although the amount of serum is well above the concentration of 0.2% previously described to activate T3S in cell culture medium (40; data not shown). Therefore, we conclude that a serum signal is not needed for the polar translocation of Yop proteins from a DamOP Y. enterocolitica strain.
We could show previously that DamOP leads to an increased ability of Y. enterocolitica to invade epithelial cells (20). Therefore, translocated as well as bacterium-associated Yop proteins could potentially be detected in the cytosol of host cells after immunoprecipitation with Yop-specific antisera, and this might be a reason for the differences in translocation between DamOP and Ca2+-blind strains. To exclude this possibility, we infected cells with DamOP and wild-type strains in the presence or absence of 0.5 μM cytochalasin D to prevent bacterial uptake. We could not detect any difference in the amounts of translocated YopE in the presence or absence of cytochalasin D, indicating that an increased invasion is not responsible for the distinct translocation phenotype of the DamOP strain compared to that of Ca2+-blind strains (data not shown).
Deregulation of Yop secretion after DamOP is dependent on the Ysc secretion system.
In Y. enterocolitica, three T3SSs implicated in virulence have been described: the pYV virulence plasmid-encoded Ysc secretion system (13), the chromosomally encoded Ysa secretion system (25), and the flagellar secretion system (76). Dependent on growth conditions, identical effector proteins can be secreted via all three systems (74, 75). Therefore, we wondered if the secretion of Yop proteins in the presence of Ca2+ is dependent on a functional Ysc system or if Yops might be secreted via an alternative system after DamOP. To this end, we constructed a yscU mutant strain with a nonfunctional Ysc secretion system (2). After DamOP, Yop secretion was analyzed as described above. Secreted Yop proteins could not be detected in the supernatants, indicating that DamOP interferes specifically with Yop secretion via the Ysc T3SS.
While Yop secretion after DamOP depends on the Ysc T3SS, translocation could still occur via an alternative system. However, when cells were infected with a yscU mutant strain overproducing the Dam enzyme, we could not detect any YopE either in the host cell's cytosol or in the supernatant. Our data indicate that the effect of DamOP on the regulation of Yop secretion and translocation strictly depends on a functional Ysc secretion system.
Effect of DamOP on the transcription of pYV-borne genes.
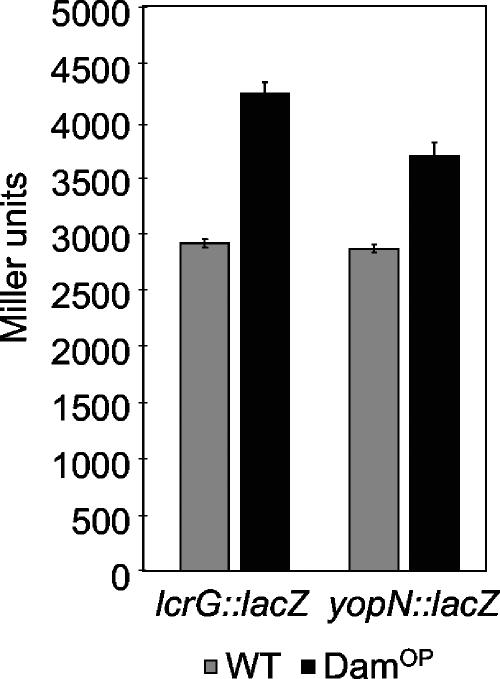
We assumed that DamOP might result in the deregulation of Yop secretion by changing the methylation pattern of GATC sequences present in promoter regions of T3S regulatory genes. As most regulators of the Ysc T3S system are encoded by the virulence plasmid, we screened the nucleotide sequence of the pYV plasmid of Y. enterocolitica 8081v (64) for GATC sequences. pYVe8081 contains a total of 222 GATC sequences, of which 25 sites are located in 5′ regions of genes or operons. Of these sequences, eight are upstream of genes/operons encoding regulatory proteins or proteins with unknown functions. These include orf76, orf83, the yopN-tyeA-sycN-yscXY-lcrDR (virA operon), the yscNOPQRSTU (virB operon), and the lcrGVH-yopBD operon. Notably, the region between the divergently transcribed virA and virB loci contains five GATC sequences. To monitor the expression of these genes/operons after DamOP, lacZ transcriptional fusions to orf76, orf83, yopN, yscN, and lcrG were constructed. After DamOP, the expression of lcrG and yopN was slightly upregulated at 37°C in the presence of Ca2+ (P = 0.0003 for lcrG and P = 0.0001 for yopN), whereas transcription of orf76, orf83, and yscN was not affected (Fig. 2 and data not shown), indicating that DamOP does not result in a general increase in the transcription of pYV-borne genes. The modest upregulation of lcrG and yopN expression was confirmed by qRT-PCR in relation to 16S rRNA expression. The transcription of lcrG is upregulated 1.32- ± 0.25-fold (P = 0.017) and yopN 1.51- ± 0.28-fold (P = 0.0059) in this assay. A comparable modest effect on transcription has also been described for most Dam-regulated genes in E. coli (41, 53). We analyzed whether DamOP results in the methylation of GATC sequences in the 5′ region of the yopN or lcrG operon after digestion with methylation-specific restriction enzymes by a method adapted from van Steensel and Henikoff (69). We could not detect differences in the restriction patterns, indicating that all GATC sequences present within the 5′ regions of the operons are at least hemimethylated and that there is no switch from nonmethylated to methylated GATCs in the DNA regions analyzed after DamOP (data not shown). However, a switch between hemimethylated and fully methylated DNA cannot be detected with the method used and remains a possibility. We conclude that the modest upregulation of lcrG and yopN expression after DamOP cannot simply be explained by a change from nonmethylated to methylated promoter regions and that hemimethylated DNA and/or more likely other indirect mechanisms might be involved in the regulation.
FIG. 2.
Transcription of the yopN-tyeA-sycN-yscXY-lcrDR and lcrGVH-yopBD operons is upregulated after DamOP. Expression was determined by transcriptional analysis using promoter-lacZ fusions and β-galactosidase activity assays after growth of a Y. enterocolitica DamOP strain (GHY150) and a control strain (WT; GHY147) in the presence of Ca2+ at 37°C. Data are means and standard deviations from at least three independent experiments.
DamOP results in reduced amounts of LcrG.
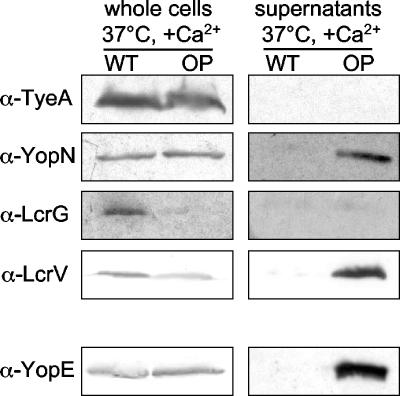
To determine if the Dam-induced modest upregulation of transcription of the lcrG and yopN operons leads to elevated protein levels, we analyzed whole-cell lysates and supernatants of cells grown at 37°C in the presence of Ca2+ by immunoblotting with antibodies directed against the regulatory proteins YopN, TyeA, LcrV, and LcrG. As shown in Fig. 3, similar amounts of TyeA are present within whole-cell lysates of wild-type and DamOP Y. enterocolitica. In accordance with recent publications, secretion of TyeA could not be detected (31, 65). As YopN is a secreted protein (10), and as DamOP induces secretion of Yops at 37°C even in the presence of Ca2+, we observed YopN in the supernatant of DamOP Y. enterocolitica. Similar amounts of YopN can be found in whole-cell lysates of DamOP and wild-type strains, while the total amount of YopN (whole cells and supernatant) is increased in the DamOP strain. These data suggest that the upregulation of the virA operon containing yopN and tyeA is rather a secondary event following Yop secretion induced by DamOP. LcrG is considered a cytoplasmic protein (16, 51), although it also has been reported that small amounts of LcrG are secreted in Y. pestis (63). Similar amounts of LcrG are present in whole-cell lysates of bacteria grown in the absence (Yop secretion) and in the presence (no Yop secretion) of Ca2+ (16). Interestingly, as shown in Fig. 3, we could not detect any LcrG protein in whole-cell lysates or bacterial supernatants after DamOP, although the expression of the lcrG operon is upregulated. This effect is not due to the instability of RNA, as qRT-PCR analyses revealed increased amounts of lcrG mRNA after DamOP. Furthermore, the effect seems to be specific for LcrG; controls with LcrV-specific antiserum revealed that the amount of cytoplasmic LcrV might be somewhat decreased. However, this is most likely due to the observation that LcrV is secreted after DamOP, resulting in slightly reduced intracellular levels of LcrV but an increased amount of total LcrV (cytoplasmic and secreted), as was also seen for YopN (Fig. 3). As an lcrG mutant strain displays a Ca2+-blind phenotype, Yop secretion after DamOP in the presence of Ca2+ might at least partially be explained by the strongly reduced amount of LcrG inside the bacterial cell.
FIG. 3.
The amount of LcrG protein in whole-cell lysates is reduced after DamOP. A DamOP Y. enterocolitica strain (GHY150) (lanes OP) and a control strain (GHY147) (lanes WT) were grown at 37°C in the presence of Ca2+. Whole-cell lysates and TCA-precipitated supernatant proteins were transferred to nitrocellulose and subjected to Western blot analysis using the indicated antisera. α, anti.
LcrG is stable in a clpP mutant strain overproducing Dam.
LcrG cannot be detected in whole-cell lysates of DamOP Y. enterocolitica. As this is not due to reduced mRNA stability and as the total amount of LcrV seems to increase under the same conditions, this implies that LcrG might specifically be degraded when Dam is overproduced. ClpXP and Lon proteases were recently reported to be involved in the regulation of the expression of the T3SSs of enterohemorrhagic E. coli and Y. pestis (32, 33). Furthermore, ClpP modulates the expression of ail in Y. enterocolitica (54). In Y. pestis, ClpXP and Lon degrade YmoA, a small histone-like protein that acts as a negative regulator of yop/ysc expression at temperatures below 37°C. Because of this function in the regulation of T3S, ClpP and Lon may also play a role in the proteolysis of LcrG. When screening the 5′ regions of the clpP and lon genes of Y. enterocolitica for the presence of GATC sequences, we identified five GATC sequences in the 5′ region of clpP and two GATC sequences upstream of lon. The expression of these genes was analyzed by qRT-PCR, and both were found to be upregulated after DamOP (clpP is upregulated 1.3- ± 0.13-fold [P = 0.0004]; lon is upregulated 1.47- ± 0.28-fold [P = 0.0036]). However, as for the yopN and lcrG upstream regions, a differential methylation (switching between nonmethylated and methylated sequences) of GATC sequences in the 5′ untranslated region of the lon and clpXP genes/operons after DamOP could not be detected (data not shown). In contrast, transcription of the yhbU homologue, encoding a putative protease that is regulated by Dam in E. coli (53), is downregulated (0.58- ± 0.13-fold [P = 0.0001]), indicating that increased transcription is not a general effect after DamOP in Y. enterocolitica. This suggests that degradation of LcrG after DamOP might be the result of an increased expression of Lon and ClpP proteases. To analyze this hypothesis in more detail, we constructed lon and clpP mutant strains of Y. enterocolitica and transferred plasmids pTP166Kan and pTP166Kan-damΔ into these strains to overproduce Dam. As the lon mutant shows severe growth defects, especially after DamOP and/or induction of Yop secretion, it was impossible to reliably analyze T3S in this strain. However, the growth of the clpP mutant was not affected. As shown in Fig. 4, the clpP strain overproducing Dam is no longer deregulated for Yop secretion, and Yop proteins are not secreted during growth at 37°C in the presence of Ca2+. If this is due to the absence of ClpP's proteolytic activity on LcrG in the clpP strain, then LcrG should be detected in the cytosol after DamOP. Indeed, Western blot analysis clearly shows the presence of LcrG after DamOP in the clpP strain, while it is missing in the wild-type strain (Fig. 4). These data indicate that LcrG is a target of the ClpP protease after DamOP, resulting in the deregulation of Yop secretion.
FIG. 4.
ClpP is necessary for the Dam-induced deregulation of Yop secretion. Supernatant proteins of a DamOP or a vector control strain of GHY266 (clpP::pEP-clpP) or of JB580v (wild type) grown at 37°C in the presence of Ca2+ were precipitated by TCA, separated by SDS-PAGE, and stained with Coomassie brilliant blue. The DamOP wild-type strain secretes Yops irrespective of the presence of Ca2+, while the clpP mutant strain does not (A). Whole-cell lysates of corresponding cultures were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-LcrG antiserum (α-LcrG). While LcrG is not detectable in lysates of DamOP wild-type cells, it is present in the DamOP clpP mutant strain (B).
DISCUSSION
The DNA adenine methyltransferase is a well-characterized enzyme with a role in various basic cellular processes ranging from DNA replication to mismatch repair. Besides having these functions, there is recent evidence that Dam interferes with the proper expression of virulence genes in an increasing number of human and animal pathogens (42, 43, 72). Previously we could show that dam is essential for growth in Y. enterocolitica and that DamOP results in increased invasion into eukaryotic cells (20). Here, we extended our initial analyses of Dam's influence on virulence functions in Y. enterocolitica and identified a role of DNA methylation in the regulation of T3S in Y. enterocolitica. The observed Yop secretion phenotype is different from that of a DamOP strain of Y. pseudotuberculosis, since it is the Ca2+ regulation of Yop secretion, not the temperature regulation of Yop expression, that is affected by DamOP in Y. enterocolitica. This implies that both pathogens employing the highly conserved Ysc/Yop T3SS and having similar lifestyles during infection may react differently to DNA methylation signals. This effect is strongest immediately after introduction of the plasmid carrying the dam gene under Ptac control, and we observed that it decreased after prolonged passage or even storage. This is not surprising, as overproduction of the Dam enzyme results in increased mutation frequencies (20). We therefore routinely transformed the Ptac-dam plasmid used for DamOP immediately before we performed the assays to avoid the introduction of suppressor mutations.
Dam influences the transcription of genes by methylating GATC sequences in promoter regions, thereby interfering with protein-DNA interactions (72). We assumed that Dam interferes with transcriptional regulation, resulting in the deregulation of Yop secretion, and analyzed the expression of pYV-borne genes or operons that contain GATC sites within their regulatory regions. Indeed, the expression of the lcrGVH-yopBD operon as well as the virA operon containing yopN and tyeA is slightly upregulated, while transcription of other genes/operons on pYV containing GATC sequences in their respective 5′ untranslated regions was not affected. The modest increase in transcription after DamOP is in accordance with the results of previous studies of E. coli, where the differences in transcription of most genes affected by Dam are mostly minor (41, 53). However, our current data do not support the idea that DNA methylation directly influences the transcriptional regulation of the lcrGVH-yopBD or the virA operon. The upregulation of transcription might be a secondary effect after the secretion of Yops has started, as the expression of Yop proteins is controlled by a negative-feedback inhibition mechanism where intracellular Yop proteins inhibit their synthesis until they are secreted (8, 13, 56). As DamOP induces Yop secretion even in the presence of Ca2+, the feedback inhibition no longer occurs, and as a secondary event, the regulons encoding secreted proteins like YopN might be upregulated. As DNA methylation also regulates replication, we analyzed whether differences in the levels of transcription of pYV genes might be due to a changed copy number of the plasmid. Quantitative real-time PCR analysis indicates, however, that the pYV copy number is not changed after DamOP (data not shown).
We conclude from our data that DamOP affects T3S by initially interfering with transcriptional regulation; however, the direct target of Dam remains elusive. The observed increase in the transcription of yopN-tyeA-sycN-yscXY-lcrDR and lcrGVH-yopBD and also of lon and clpXP is most likely indirect or includes hemimethylated DNA, as a differential methylation of GATC sequences in the respective 5′ untranslated regions could not be detected after DamOP. The conjugation of pSLT in S. enterica is inhibited by the small, untranslated RNA FinP. The transcription of finP is indirectly regulated by DNA methylation, as a GATC sequence in the −10 region of the finP promoter is dispensable for Dam-mediated regulation. Interestingly, H-NS is involved in this mechanism (7). In E. coli, Dam-mediated methylation of DNA interferes with protection by global regulatory proteins like cyclic Amp receptor protein (CRP), Fnr, Lrp, and integration host factor (44, 52, 53, 72). Therefore, we suggest that global regulatory proteins might also be involved in mediating the transcriptional response in Y. enterocolitica after DamOP.
Surprisingly, in whole-cell extracts of DamOP bacteria grown in the presence of Ca2+, LcrG is no longer detectable, while the amounts of TyeA and YopN were not affected and the total amount of LcrV (cytoplasmic and secreted) seemed to increase. Besides transcriptional regulation by DNA binding proteins which directly interact with promoters, specific degradation of regulators by proteases like Clp and Lon has been shown to be a key process in bacterial regulation (24, 35). This also has implications in the regulation of virulence properties. For example, Lon and ClpXP proteases are involved in the regulation of expression of T3S in Salmonella, enterohemorrhagic E. coli, and Y. pestis and also in the expression of the ail gene in Y. enterocolitica (32, 33, 54, 67, 68). Interestingly, transcription of the lon and clpXP genes is upregulated after DamOP in Y. enterocolitica, and we speculated that Lon and/or ClpP activities might be responsible for the proteolysis of LcrG. As expected, the clpP strain did not secrete Yop proteins at 37°C in the presence of Ca2+ after DamOP and secreted Yop proteins to levels comparable to those in a wild-type strain without DamOP. In addition, LcrG was still detectable in whole-cell lysates after DamOP. This shows that ClpP is involved in LcrG degradation in a DamOP Y. enterocolitica strain and adds a previously unanticipated level of regulation to the T3S of Yop proteins. The possibility of a posttranscriptional effect of Dam also cannot be excluded, and examples for such a possibility have been described for E. coli (4). Although clpXP transcription increases in a DamOP strain, this is probably not the only mechanism contributing to LcrG degradation by ClpP. Substrate choice and proteolytic degradation by ClpP is regulated at various levels, including at the levels of peptide motifs, adapter proteins like RssB and SspB, and SsrA tagging of proteins (1). Proteins destined for degradation by proteolysis are usually nonfunctional, mutated, or misfolded in response to stress. Other substrates for degradation by proteases are regulatory proteins that are often needed for a specific time during the growth cycle. For example, Clp inactivates by degradation the stationary phase sigma factor RpoS (49, 62). Whether LcrG is degraded by the Clp protease as a result of misfolding, e.g., because it does not properly interact with a stabilizing protein like LcrV, or whether it is a substrate of ClpP as part of another regulatory mechanism has to be analyzed in future experiments. However, the role for Clp in the posttranslational regulation of T3S described here is clearly different from its role in the transcriptional regulation of T3S in Salmonella, enterohemorrhagic E. coli, and Y. pestis (32, 33, 67, 68) and has so far not been described for another T3SS.
The decrease in LcrG levels in a DamOP strain results in an intermediate Ca2+-blind phenotype of Yop secretion clearly different from that of an lcrG mutant strain, indicating that (i) the decrease in LcrG is presumably not the only effect of DamOP leading to this intermediate Ca2+-blind phenotype of Yop secretion and translocation and that (ii) DamOP leads to a complex phenotype differing from that of an lcrG mutant. That DamOP affects additional functions associated with T3S besides LcrG would not be surprising if we keep in mind the pleiotropic effects of Dam previously described (41, 42, 53). Therefore, the effect of DamOP in Y. enterocolitica has to be analyzed in future experiments at the genome and proteome levels to help identify the putative additional factors involved in the T3S phenotype. In addition, it is important to keep in mind that minute amounts of LcrG could still be present in DamOP cells that cannot be detected in Western blots. This could indicate that only small amounts of LcrG are needed for targeted Yop translocation but that a larger amount of LcrG is required to block Yop secretion.
Although the lifestyles of Y. enterocolitica and Y. pseudotuberculosis are very similar, their T3S phenotypes after DamOP are distinctly different. It is evident that different regulatory mechanisms are targeted by DNA methylation in both species, but the molecular background is currently unknown. Further analyses of Y. pseudotuberculosis and also Y. pestis are necessary to determine if LcrG and Clp are involved in the differential regulation of T3S after DamOP and to reveal how epigenetic signals evolved in the regulation of T3S in closely related species. One has furthermore to keep in mind that although Y. enterocolitica and Y. pseudotuberculosis have similar lifestyles, they are evolutionarily not as close as Y. pseudotuberculosis and Y. pestis (73).
The question concerning the biological significance of the observed phenotype of a DamOP strain remains elusive. DNA methylation regulates phase variation in different systems (72), so it might be tempting to speculate that this is also true for Yop secretion in Y. enterocolitica. This would mean that the control of Yop secretion can be “relaxed” under certain conditions during an infection, influenced by increased DNA methylation (mimicked by DamOP), allowing the secretion of Yops, while the bacteria are still able to effectively translocate Yops polarly after contact with host cells to avoid damaging host responses. This hypothesis implicates putative extracellular functions for Yop proteins; support may come from the fact that antibodies against some Yops can be detected after experimental infection of mice and that, e.g., YopM might have extracellular functions during pathogenesis (5, 30). Further experimental data on the putative extracellular functions of Yops are needed to prove or disprove this hypothesis.
Acknowledgments
We thank G. R. Cornelis for antisera directed against TyeA, YopN, and LcrG; J. Heesemann and A. Sing for antisera against YopE, YopT, and LcrV; E. Bohn and I. Autenrieth for antisera against YopM and YopH; and M. G. Marinus for the gift of plasmid pTP166.
This work was supported by Innovative Medical Research grants (IMF HE120201 and HE110401) of the Medical School of the University of Münster and in part by grants of the Deutsche Forschungsgemeinschaft (DFG SFB293/B5 and SCHM770/10).
REFERENCES
- 1.Ades, S. E. 2004. Proteolysis: adaptor, adaptor, catch me a catch. Curr. Biol. 14:R924-R926. [DOI] [PubMed] [Google Scholar]
- 2.Allaoui, A., S. Woestyn, C. Sluiters, and G. R. Cornelis. 1994. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J. Bacteriol. 176:4534-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badie, G., D. M. Heithoff, and M. J. Mahan. 2004. LcrV synthesis is altered by DNA adenine methylase overproduction in Yersinia pseudotuberculosis and is required to confer immunity in vaccinated hosts. Infect. Immun. 72:6707-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, D. C., and C. G. Cupples. 2001. Very-short-patch repair in Escherichia coli requires the dam adenine methylase. J. Bacteriol. 183:3631-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benner, G. E., G. P. Andrews, W. R. Byrne, S. D. Strachan, A. K. Sample, D. G. Heath, and A. M. Friedlander. 1999. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infect. Immun. 67:1922-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451-1460. [DOI] [PubMed] [Google Scholar]
- 7.Camacho, E. M., A. Serna, C. Madrid, S. Marques, R. Fernandez, F. de la Cruz, A. Juarez, and J. Casadesus. 2005. Regulation of finP transcription by DNA adenine methylation in the virulence plasmid of Salmonella enterica. J. Bacteriol. 187:5691-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cambronne, E. D., J. A. Sorg, and O. Schneewind. 2004. Binding of SycH chaperone to YscM1 and YscM2 activates effector yop expression in Yersinia enterocolitica. J. Bacteriol. 186:829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., D. B. Paulsen, D. W. Scruggs, M. M. Banes, B. Y. Reeks, and M. L. Lawrence. 2003. Alteration of DNA adenine methylase (Dam) activity in Pasteurella multocida causes increased spontaneous mutation frequency and attenuation in mice. Microbiology 149:2283-2290. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, L. W., and O. Schneewind. 2000. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J. Bacteriol. 182:3183-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell. Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day, J. B., F. Ferracci, and G. V. Plano. 2003. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47:807-823. [DOI] [PubMed] [Google Scholar]
- 15.Day, J. B., and G. V. Plano. 1998. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol. Microbiol. 30:777-788. [DOI] [PubMed] [Google Scholar]
- 16.DeBord, K. L., V. T. Lee, and O. Schneewind. 2001. Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183:4588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2003. Salmonella DNA adenine methylase mutants elicit early and late onset protective immune responses in calves. Vaccine 21:3249-3258. [DOI] [PubMed] [Google Scholar]
- 18.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 69:7950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erova, T. E., L. Pillai, A. A. Fadl, J. Sha, S. Wang, C. L. Galindo, and A. K. Chopra. 2006. DNA adenine methyltransferase influences the virulence of Aeromonas hydrophila. Infect. Immun. 74:410-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fälker, S., M. A. Schmidt, and G. Heusipp. 2005. DNA methylation in Yersinia enterocolitica: role of the DNA adenine methyltransferase in mismatch repair and regulation of virulence factors. Microbiology 151:2291-2299. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg, A., A. M. Viitanen, M. Skurnik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977-986. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:11578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glickman, B., P. van den Elsen, and M. Radman. 1978. Induced mutagenesis in dam− mutants of Escherichia coli: a role for 6-methyladenine residues in mutation avoidance. Mol. Gen. Genet. 163:307-312. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 25.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 26.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 28.Herman, G. E., and P. Modrich. 1981. Escherichia coli K-12 clones that overproduce dam methylase are hypermutable. J. Bacteriol. 145:644-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernday, A. D., B. A. Braaten, and D. A. Low. 2003. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol. Cell 12:947-957. [DOI] [PubMed] [Google Scholar]
- 30.Heusipp, G., K. Spekker, S. Brast, S. Fälker, and M. A. Schmidt. 2006. YopM of Yersinia enterocolitica specifically interacts with alpha1-antitrypsin without affecting the anti-protease activity. Microbiology 152:1327-1335. [DOI] [PubMed] [Google Scholar]
- 31.Iriarte, M., M. P. Sory, A. Boland, A. P. Boyd, S. D. Mills, I. Lambermont, and G. R. Cornelis. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyoda, S., and H. Watanabe. 2005. ClpXP protease controls expression of the type III protein secretion system through regulation of RpoS and GrlR levels in enterohemorrhagic Escherichia coli. J. Bacteriol. 187:4086-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson, M. W., E. Silva-Herzog, and G. V. Plano. 2004. The ATP-dependent ClpXP and Lon proteases regulate expression of the Yersinia pestis type III secretion system via regulated proteolysis of YmoA, a small histone-like protein. Mol. Microbiol. 54:1364-1378. [DOI] [PubMed] [Google Scholar]
- 34.Jacobi, C. A., A. Roggenkamp, A. Rakin, R. Zumbihl, L. Leitritz, and J. Heesemann. 1998. In vitro and in vivo expression studies of yopE from Yersinia enterocolitica using the gfp reporter gene. Mol. Microbiol. 30:865-882. [DOI] [PubMed] [Google Scholar]
- 35.Jenal, U., and R. Hengge-Aronis. 2003. Regulation by proteolysis in bacterial cells. Curr. Opin. Microbiol. 6:163-172. [DOI] [PubMed] [Google Scholar]
- 36.Julio, S. M., D. M. Heithoff, D. Provenzano, K. E. Klose, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect. Immun. 69:7610-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julio, S. M., D. M. Heithoff, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2002. DNA adenine methylase overproduction in Yersinia pseudotuberculosis alters YopE expression and secretion and host immune responses to infection. Infect. Immun. 70:1006-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 39.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 40.Lee, V. T., S. K. Mazmanian, and O. Schneewind. 2001. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol. 183:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Løbner-Olesen, A., M. G. Marinus, and F. G. Hansen. 2003. Role of SeqA and Dam in Escherichia coli gene expression: a global/microarray analysis. Proc. Natl. Acad. Sci. USA 100:4672-4677. [Epub ahead of print 7 April 2003.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Løbner-Olesen, A., O. Skovgaard, and M. G. Marinus. 2005. Dam methylation: coordinating cellular processes. Curr. Opin. Microbiol. 8:154-160. [DOI] [PubMed] [Google Scholar]
- 43.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marinus, M. G. 1996. Methylation of DNA, p. 782-791. In R. I. C. F. C. Neidhardt, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 45.Marinus, M. G., and N. R. Morris. 1974. Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J. Mol. Biol. 85:309-322. [DOI] [PubMed] [Google Scholar]
- 46.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Modrich, P. 1987. DNA mismatch correction. Annu. Rev. Biochem. 56:435-466. [DOI] [PubMed] [Google Scholar]
- 49.Muffler, A., D. Fischer, S. Altuvia, G. Storz, and R. Hengge-Aronis. 1996. The response regulator RssB controls stability of the sS subunit of RNA polymerase in Escherichia coli. EMBO J. 15:1333-1339. [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson, K. M., G. M. Young, and V. L. Miller. 2001. Identification of a locus involved in systemic dissemination of Yersinia enterocolitica. Infect. Immun. 69:6201-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilles, M. L., A. W. Williams, E. Skrzypek, and S. C. Straley. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nou, X., B. Braaten, L. Kaltenbach, and D. A. Low. 1995. Differential binding of Lrp to two sets of pap DNA binding sites mediated by Pap I regulates Pap phase variation in Escherichia coli. EMBO J. 14:5785-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oshima, T., C. Wada, Y. Kawagoe, T. Ara, M. Maeda, Y. Masuda, S. Hiraga, and H. Mori. 2002. Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol. Microbiol. 45:673-695. [DOI] [PubMed] [Google Scholar]
- 54.Pederson, K. J., S. Carlson, and D. E. Pierson. 1997. The ClpP protein, a subunit of the Clp protease, modulates ail gene expression in Yersinia enterocolitica. Mol. Microbiol. 26:99-107. [DOI] [PubMed] [Google Scholar]
- 55.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148:1171-1182. [DOI] [PubMed] [Google Scholar]
- 56.Ramamurthi, K. S., and O. Schneewind. 2002. Type III protein secretion in Yersinia species. Annu. Rev. Cell Dev. Biol. 18:107-133. [DOI] [PubMed] [Google Scholar]
- 57.Robinson, V. L., P. C. Oyston, and R. W. Titball. 2005. A dam mutant of Yersinia pestis is attenuated and induces protection against plague. FEMS Microbiol. Lett. 252:251-256. [DOI] [PubMed] [Google Scholar]
- 58.Rohde, J. R., J. M. Fox, and S. A. Minnich. 1994. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol. Microbiol. 12:187-199. [DOI] [PubMed] [Google Scholar]
- 59.Rohde, J. R., X. S. Luan, H. Rohde, J. M. Fox, and S. A. Minnich. 1999. The Yersinia enterocolitica pYV virulence plasmid contains multiple intrinsic DNA bends which melt at 37 degrees C. J. Bacteriol. 181:4198-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarker, M. R., M. P. Sory, A. P. Boyd, M. Iriarte, and G. R. Cornelis. 1998. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect. Immun. 66:2976-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 62.Schweder, T., K. H. Lee, O. Lomovskaya, and A. Matin. 1996. Regulation of Escherichia coli starvation sigma factor (sS) by ClpXP protease. J. Bacteriol. 178:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skryzpek, E., and S. C. Straley. 1993. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175:3520-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snellings, N. J., M. Popek, and L. E. Lindler. 2001. Complete DNA sequence of Yersinia enterocolitica serotype 0:8 low-calcium-response plasmid reveals a new virulence plasmid-associated replicon. Infect. Immun. 69:4627-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundberg, L., and A. Forsberg. 2003. TyeA of Yersinia pseudotuberculosis is involved in regulation of Yop expression and is required for polarized translocation of Yop effectors. Cell. Microbiol. 5:187-202. [DOI] [PubMed] [Google Scholar]
- 66.Taylor, V. L., R. W. Titball, and P. C. Oyston. 2005. Oral immunization with a dam mutant of Yersinia pseudotuberculosis protects against plague. Microbiology 151:1919-1926. [DOI] [PubMed] [Google Scholar]
- 67.Tomoyasu, T., T. Ohkishi, Y. Ukyo, A. Tokumitsu, A. Takaya, M. Suzuki, K. Sekiya, H. Matsui, K. Kutsukake, and T. Yamamoto. 2002. The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomoyasu, T., A. Takaya, E. Isogai, and T. Yamamoto. 2003. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol. Microbiol. 48:443-452. [DOI] [PubMed] [Google Scholar]
- 69.van Steensel, B., and S. Henikoff. 2000. Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nat. Biotechnol. 18:424-428. [DOI] [PubMed] [Google Scholar]
- 70.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 71.Watson, M. E., Jr., J. Jarisch, and A. L. Smith. 2004. Inactivation of deoxyadenosine methyltransferase (dam) attenuates Haemophilus influenzae virulence. Mol. Microbiol. 53:651-664. [DOI] [PubMed] [Google Scholar]
- 72.Wion, D., and J. Casadesus. 2006. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nat. Rev. Microbiol. 4:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wren, B. W. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 1:55-64. [DOI] [PubMed] [Google Scholar]
- 74.Young, B. M., and G. M. Young. 2002. Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica. J. Bacteriol. 184:5563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 184:1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]