Abstract
We have investigated the role of CseA in the σE cell envelope stress response of the gram-positive bacterium Streptomyces coelicolor. σE is an extracytoplasmic function RNA polymerase sigma factor required for normal cell envelope integrity in S. coelicolor. σE is encoded within a four-gene operon that also encodes CseA, a protein of unknown function, CseB, a response regulator and CseC, a transmembrane sensor histidine kinase (Cse represents control of sigma E). Previous work has shown that transcription of the sigE gene is completely dependent on the CseBC two-component system and that the CseBC-σE signal transduction system is induced by a wide variety of cell-wall-damaging agents. Here we address the role of CseA, a protein with no homologues outside the streptomycetes. We show that CseA is a novel lipoprotein localized to the extracytoplasmic face of the cell membrane and that loss of CseA results in upregulation of the sigE promoter.
All bacterial cells have stress response pathways that maintain homeostasis in each cellular compartment. One such compartment is the cell envelope, which is the first and major defense against attacks from the environment and from competing microorganisms. Streptomycetes and related actinomycetes are abundant in soil, and they produce two-thirds of known antibiotics (3), most likely as a mechanism to inhibit the growth of their competitors. Since many of the most significant classes of antibiotics target cell envelope biosynthesis, in situ exposure of soil-dwelling actinomycetes to antibiotics targeting this compartment is likely to be a significant source of environmental stress.
To respond to these challenges and maintain homeostasis, bacteria have evolved numerous signal transduction pathways that allow the cell to sense and respond to changes in the integrity of the cell envelope. These pathways are collectively known as the cell envelope stress response. The most detailed studies of such responses have been carried out with the gram-negative bacterium Escherichia coli, in which three dedicated systems have been identified to date: BaeSR, CpxAR, and σE (2, 29, 30, 32). The BaeSR two-component system is induced by xenobiotic agents and confers resistance to such compounds by activating expression of efflux pumps (12, 29). A second two-component system, CpxAR, senses various types of extracytoplasmic stress, including misfolding of periplasmic proteins, and activates expression of target genes encoding periplasmic chaperones, proteases, and trafficking factors that clear such stresses. Signal sensing by the sensor kinase CpxA is inhibited by a periplasmic protein, CpxP, and inhibition is relieved through proteolysis of CpxP (6, 20). The σE pathway responds to protein misfolding in the cell envelope. In the absence of such stress, σE is sequestered at the cell membrane by the cytoplasmic domain of the transmembrane anti-sigma factor RseA. Stress-induced activation of the pathway results in regulated proteolysis of RseA through sequential steps: first, DegS cleaves the periplasmic domain of RseA, leading to subsequent cleavage of the cytoplasmic domain by YaeL, ultimately releasing σE to activate transcription of its target genes (1, 2, 10, 38). The Cpx and σE systems appear to be widely conserved in gram-negative bacteria (30, 31).
Working with the low-G+C gram-positive soil bacterium Bacillus subtilis, Mascher et al. (22) used whole-genome microarrays to study the response to cell envelope stresses induced by the cell-wall-specific antibiotic bacitracin. They identified three two-component systems that could sense and respond to such stresses: YvqEC (now renamed LiaRS) (23), YvcPQ, and BceRS. These three systems contain so-called “intramembrane sensing” sensor kinases, a novel group of sensor proteins that have extracellular domains less than 20 residues long and that are proposed to sense signals associated with damage to the cell membrane (21a). All three systems respond to bacitracin, but only BceRS switches on bacitracin resistance genes (22, 25). Of these three systems, only YvqE also responds to the glycopeptide vancomycin, another cell-wall-specific antibiotic (7, 22). Other cell envelope stress responses in B. subtilis are mediated by extracytoplasmic function (ECF) sigma factors regulated by transmembrane anti-sigma factors presumed to respond to changes in the extracytoplasmic compartment (8a, 13).
We have been studying a cell envelope stress signal transduction system in the high-G+C gram-positive soil bacterium Streptomyces coelicolor (14, 27, 28). This system controls transcription of the gene (sigE) encoding σE, an ECF sigma factor required for normal cell envelope integrity. Despite its name, the σE signal transduction system of S. coelicolor is not analogous to the σE signal transduction system of E. coli. There is no anti-sigma factor involved in the S. coelicolor system; instead, S. coelicolor σE is controlled at the level of transcription of its structural gene. Transcription of sigE is completely dependent on a two-component system consisting of CseB, a response regulator, and CseC, a transmembrane sensor histidine protein kinase (Cse represents control of sigma E). Null mutations in sigE and in cseB appear to cause an identical cell envelope defect phenotype, and a cseB null mutation can be suppressed by expressing sigE from a heterologous, constitutive promoter (27), suggesting that loss of sigE expression is the major determinant of the cseB mutant phenotype. The ligand sensed by the sensor domain of CseC has yet to be identified, but this signal appears to arise as a consequence of cell envelope damage, since expression of sigE is induced by a wide range of structurally unrelated cell-wall-specific antibiotics and also by the cell wall hydrolytic enzyme lysozyme (14). The regulon of target genes under σE control is largely unknown but includes an operon of 12 genes likely to specify biosynthesis of a cell wall glycan (14).
σE, CseB, and CseC are encoded in an operon (sigE cseA cseB cseC) in which >90% transcription from the sigE promoter terminates just downstream of sigE (28). The presence of a fourth gene in the operon, cseA, suggested that it might also be involved in the σE cell envelope stress response. However, CseA has no known function and has no homologues outside the streptomycetes. Here we show that CseA is a lipoprotein localized to the extracytoplasmic face of the cell membrane and that loss of the CseA results in upregulation of the sigE promoter.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Bacterial strains and plasmids are described in Table 1. The level of kanamycin resistance conferred on M600 and J2172 by pIJ6880 was assessed on MMCGT, which is agar minimal medium (MM) (21) supplemented with 0.6% (wt/vol) Difco Casamino Acids, 0.75% (vol/vol) Tiger Milk (21), and 0.5% (wt/vol) glucose. Except where described below, other media and culture conditions were as given previously (14, 18, 21). For the globomycin experiments, wild-type S. coelicolor was grown for 16 h in NMMP liquid medium containing 2 mM MgCl2 in the presence or absence of the antibiotic (150 μg ml−1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype/comments | Source/reference |
|---|---|---|
| Strains | ||
| S. coelicolor A3(2) | ||
| M600 | SCP1− SCP2− | 21 |
| J2130 | ΔsigE SCP1− SCP2− | 27 |
| J2142 | ΔcseB::hyg SCP1− SCP2− | 28 |
| J2172 | ΔcseA SCP1− SCP2− | This study |
| E. coli | ||
| ET12567(pUZ8002) | ET12567 containing helper plasmid pUZ8002 | 27 |
| Plasmids | ||
| pGEX-KG | E. coli overexpression vector | 11 |
| pKC1132 | Conjugative vector for gene disruption in Streptomyces (Aprr) | 5 |
| pSET152 | φC31 attP-int-derived integration vector for the conjugal transfer of DNA from E. coli to Streptomyces spp. (Aprr) | 5 |
| pIJ486 | Multicopy Streptomyces promoter-probe plasmid containing neo as reporter gene (Thior) | 39 |
| pIJ6880 | 0.75-kb sigE promoter region (BglII) in pIJ486 (BamHI) | 14 |
| pIJ6809 | pKC1132 carrying an in-frame cseA deletion allele | This study |
| pIJ6810 | pSET152 carrying the cseA gene driven by its native promoter (sigEp) | This study |
| pIJ6946 | pGEX-KG derivative overexpressing CseA lacking the first 44 amino acids | This study |
| pIJ6962 | pSET152 carrying the cseA gene driven by its native promoter (sigEp) | This study |
| pIJ6963 | As pIJ6962, but carrying the TTG1-to-CTC mutation | This study |
| pIJ6964 | As pIJ6962, but with a TGA stop codon introduced between TTG1 and GTG2 | This study |
| pIJ6065 | As pIJ6962, but carrying the GTG2-to-GTC mutation | This study |
| pIJ6966 | As pIJ6962, but carrying the GTG3-to-GTC mutation | This study |
| pIJ6967 | As pIJ6962, but carrying the C37A substitution | This study |
Protoplast transformation and conjugal plasmid transfer from E. coli to Streptomyces spp.
To bypass the methyl-specific restriction system of S. coelicolor, cosmids and plasmids were passed through the dam dcm hsdS E. coli strain ET12567 prior to protoplast transformation or conjugation. E. coli ET12567 carrying the nontransmissible, oriT-mobilizing “driver” plasmid pUZ8002 was used for conjugation. Streptomyces protoplast transformation and conjugation from E. coli to Streptomyces were carried out as described by Kieser et al. (21).
Construction of a ΔcseA null mutant.
An in-frame ΔcseA mutant allele was constructed by introducing an Asp718 site 12 bp upstream of the stop codon of cseA and deleting the DNA lying between this introduced site and a naturally occurring Asp718 site 118 bp downstream of the TTG start codon, thereby deleting 537 bp of the gene. Briefly, the extreme 3′ end of cseA and 1.2 kb of DNA lying downstream were amplified by PCR using two oligonucleotides (ASP-1, 5′-GGTTGGTACCGAGCCGGACAGCTGAACCG-3′; and a universal primer, UP-1, 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′) simultaneously introducing the Asp718 site just upstream of the cseA stop codon (both underlined). The PCR product was cleaved with EcoRI and Asp718 and cloned into pIJ5950 (28) cut with the same enzymes. The resulting plasmid, pIJ6808, carried the desired in-frame cseA deletion flanked by 1.5 kb of DNA upstream and 1.2 kb of DNA downstream. The constructed ΔcseA mutant allele was removed from pIJ6808 as a 2.7-kb BglII fragment and cloned into the conjugative delivery vector pKC1132 (5) cut with BamHI, to create pIJ6809. pIJ6809 was introduced into S. coelicolor M600 by mating from E. coli, and exconjugants in which the plasmid had presumptively integrated into the sigE operon by single crossing over were selected with apramycin. The chromosomal structures of three of these isolates were confirmed by Southern blot analysis. These three strains were subjected to two rounds of nonselective growth, and isolates in which the delivery plasmid had been lost were identified by virtue of their sensitivity to apramycin. These isolates were a mixture of cseA+ strains and the desired ΔcseA mutant, depending on whether integration and excision of the plasmid had taken place through the same or alternative flanking intervals of DNA. Southern blot analysis was used to identify a ΔcseA mutant and to confirm its chromosomal structure; the strain was designated J2172.
Construction of pIJ6810, a plasmid to test in trans complementation of the ΔcseA mutant.
A 1-kb PvuII-Asp718 fragment extending from the PvuII site in the mutY gene upstream of sigE to the Asp718 site in the 5′ end of cseA, but carrying the sigE in-frame deletion (27), was ligated to a 0.75-kb Asp718-XhoI fragment extending from the Asp718 site in the 5′ end of cseA to the XhoI site in the 5′ end of cseB, creating a 1.75-kb contiguous stretch of DNA in which the sigE promoter directed transcription of cseA downstream from the ΔsigE allele. This 1.75-kb fragment was cloned into the integrative vector pSET152 to create pIJ6810, which was introduced into the ΔcseA mutant (J2172) by conjugation from E. coli.
RNA preparation and S1 nuclease protection assays.
RNA was prepared as described previously (14, 15). Germinated spores of S. coelicolor strains were inoculated into NMMP liquid medium (21) containing 2 mM MgCl2 and grown to an optical density at 450 nm (OD450) of 0.3 to 0.6 at 30°C. Immediately after the first 10 ml of sample was taken, inducer (10 μg/ml vancomycin) was added to the remaining 40 ml of culture broth and 10-ml samples were taken at subsequent 30-min intervals up to 90 min. The sigE transcript was quantified using a 0.4-kb 5′-end-labeled probe generated by PCR from pIJ5950 as described previously (28). For all assays, 30 μg RNA and 25 pmol labeled probe were dissolved in 20 μl of Na trichloroacetic acid buffer and hybridized at 45°C overnight after denaturation at 65°C for 15 min.
Construction of pIJ6946, overexpression of glutathione S-transferase-CseA, purification of truncated CseA protein, and raising of antisera.
A 576-bp PCR fragment, containing the cseA gene lacking the first 44 codons, was generated using the forward primer cseAfor (5′-CGGGGGTACCGGCGCCATGG-3′) to incorporate an NcoI site at the 5′ end and the reverse primer cseArev (5′-TCGGGGCGGCCGGCGGTTCA-3′), cloned into pIJ2925 for sequencing on both strands, and then subcloned into NcoI/BamHI-cut pGEX-KG (11). The resulting plasmid, pIJ6946, was then used to transform E. coli strain BL21. Cells were grown at 37°C in LB to an OD600 of 0.6, induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and 100 μg ml−1 carbenicillin, and grown for a further 2 h at 37°C. Cells were harvested, washed once in thrombin cleavage buffer (TCB: 100 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM CaCl2) and sonicated in TCB plus EDTA-free protease inhibitor cocktail (Roche). Cell debris was removed by centrifugation at 10,000 rpm for 15 min at 4°C, and the cell extract was applied to a 2-ml glutathione Sepharose column (Amersham Biosciences) equilibrated in TCB. The column was washed with 50 volumes of ice-cold TCB, and then 10 U of human plasma thrombin (Sigma-Aldrich) in 2 ml TCB was added to the column, the matrix was mixed, and the column was left at room temperature for 2 h. The TCB was collected and the column was further eluted with 5 ml TCB. Most of the purified CseA protein was collected in the first 2 ml of buffer. σE was overexpressed and purified as described previously (27). Purified CseA and σE (1 mg each) were sent to CovalAb UK to raise antiserum in rabbit.
Construction of cseA point mutants.
Using pIJ6810 as a template mutations were introduced by PCR. Two flanking primers, cseAflank1 (5′-ACCGTGTTCTCTGTGACCGC-3′) and cseAflank2 (5′-TCGGGGCGGCCGGCGGTTCA-3′), were used to amplify a 1,000-bp fragment containing a wild-type copy of the cseA gene plus 400 bp of sigE promoter sequence. The mutagenesis primers fM1Lfor (5′-AGCGGGAGCGCTCCGCGGCCT-3′) and fM1Lrev (5′-AGGCCGCGGAGCGCTCCCGCT-3′), R15STOPfor (5′-GGGGACCCGGTGAACCACGCA-3′) and R15STOPrev (5′-TGCGTGGTTCACCGGGTCCCC-3′), fM24Vfor (5′-GTACGGCGGTCGCCGTGTTCG-3′) and fM24Vrev (5′-CGAACACGGCGACCGCCGTAC-3′), fM26Vfor (5′-CGGTGGCCGTCTTCGTCGCCC-3′) and fM26Vrev (5′-GGGCGACGAAGACGGCCACCG-3′) and C37Afor (5′-CCTGGCCGGCGCCGGGACCGGG-3′) and C37Arev (5′-CCCGGTCCCGGCGCCGGCCAGG-3′) were used in conjunction with the flank primers to generate two overlapping PCR products for each mutant. The two PCRs were gel purified, combined, extended, and then amplified with the flank primers. The wild-type and mutant PCR products were cloned into pUC19, sequenced, subcloned into integrative vector pSET152 (5), and introduced into the ΔcseA mutant (J2172) by conjugation from E. coli.
Subcellular fractionation and immunoblotting of CseA and σE.
For cell fractionation, crude S. coelicolor extracts were prepared from strain M600 grown at 30°C for 16 h in NMMP. Briefly, mycelium was harvested by centrifugation at 3,000 rpm for 1 min, washed in P buffer, and sonicated for three bursts of 5 s, on ice, in P buffer plus EDTA-free protease inhibitor cocktail (Roche). A sample of the crude extract was retained for analysis, and the remainder was centrifuged at 80,000 rpm for 1 h at 4°C. The supernatant (soluble fraction) was removed to a fresh tube, the pellet was washed in P buffer plus 50 mM NaCl, and the sample was centrifuged again at 80,000 rpm for 1 h. The supernatant (wash fraction) was removed to a fresh tube, and the pellet (membrane fraction) was resuspended in P buffer plus 1% sarcosyl. All fractions were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gels and blotted onto Hybond C membrane (Amersham Pharmacia Biotech) at 150 mA for 30 min on a Bio-Rad semidry blotter. The membrane was incubated in blocking solution (10% dried milk powder in Tris-buffered saline plus 0.01% Tween 20) overnight, and immunoblotting was carried out essentially as described previously (17) using 1/100 dilutions of CseA antiserum or σE antiserum and 1/5,000 dilutions of horseradish peroxidase-linked goat anti-rabbit immunogobulin G antibody (Amersham Pharmacia Biotech). Blots were developed using the ECL enhanced chemiluminescence system from Amersham Pharmacia Biotech and were typically exposed to X-ray film for between 30 s and 3 min.
“Protoplast shaving” experiments.
Protoplasts were prepared as described previously (21) and incubated in P buffer or in P buffer with trypsin (0.2 μg mg−1 total protein) for 1 h at 30°C. An equal concentration of trypsin inhibitor was then added to both samples, and the protoplasts were harvested at 1,000 rpm for 5 min, resuspended in 200 μl P buffer, and sonicated on ice for two bursts of 5 s at half power, separated by 30 s on ice. The total protein concentration was measured using the Bio-Rad protein assay system, and 10 μg of total protein per sample was loaded onto a 10% SDS-polyacrylamide gel and blotted as described above.
RESULTS AND DISCUSSION
Transcription of sigE is upregulated in a cseA null mutant.
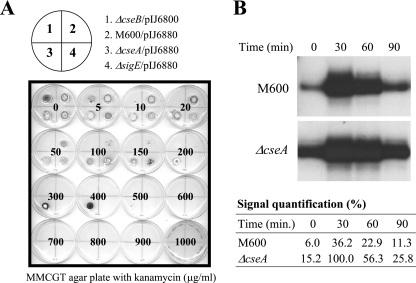
BLAST sequence analyses (E value cutoff, 0.01) indicated that CseA has no homologues outside the streptomycetes. To investigate the function of CseA, we constructed an in-frame cseA deletion mutant in the M600 background, as described in Materials and Methods. We then introduced the reporter plasmid pIJ6880 (14), carrying a sigE promoter-kanamycin resistance gene fusion (sigEp-neo), into the cseA null mutant (J2172) and M600. In the absence of applied inducers, pIJ6880 conferred resistance to 80 μg ml−1 kanamycin in M600 (Fig. 1A and data not shown), resulting from the basal activity of the sigE promoter. In contrast, pIJ6880 conferred resistance to 400 μg ml−1 kanamycin in the ΔcseA mutant, (Fig. 1A), showing that loss of CseA results in upregulation of the sigE promoter.
FIG. 1.
(A) Kanamycin resistance arising from the basal activity of the sigE promoter in the ΔcseA null mutant (J2142) carrying the sigEp-neo reporter plasmid pIJ6880. Approximately 105 spores (in 10 μl of 20% glycerol) were spotted onto MMCGT plates containing different amounts of kanamycin. Plates were incubated at 30°C for 4 days. The results shown for the wild type (M600) and for the cseB and sigE null mutants have been reported previously (14). (B) S1 nuclease protection analysis of the sigE transcript in S. coelicolor M600 and the ΔcseA null mutant (J2172) following induction with vancomycin. RNA was isolated at 30-min intervals up to 90 min from S. coelicolor M600 and J2172 grown in NMMP liquid medium containing 2 mM MgCl2 after induction with 10 μg/ml vancomycin. Relative signal strengths were quantified on a phosphorimager; each signal is expressed as a percentage of the strongest signal.
To ensure that the in-frame cseA mutation was not polar on the downstream genes in the operon (cseB and cseC), a plasmid was constructed to determine if the ΔcseA phenotype could be complemented in trans. This plasmid (pIJ6810; Materials and Methods) carried a 1.75-kb insert containing the first half of the sigE operon—the sigE promoter, sigE, and cseA—except that the sigE gene carried an in-frame deletion. Thus, in pIJ6810, which integrates site specifically into the chromosome at the phage φC31 attB site, the sigE promoter directs transcription of cseA downstream from the ΔsigE allele. pIJ6810 fully complemented the ΔcseA phenotype, reducing the basal level of kanamycin resistance conferred by the sigEp-neo fusion plasmid (pIJ6880) back to 80 μg ml−1—the same level observed in the congenic parent, M600. In contrast, pSET152, the parent vector on which pIJ6810 was based, had no influence on the ΔcseA phenotype. Therefore, the phenotype of J2172 (ΔcseA) was caused by loss of cseA function, and not by any polar effect on cseB and cseC. (In fact, if the ΔcseA mutation had been polar on cseB, it would have had the opposite phenotype from the one observed, because the sigE promoter is inactive in a cseB mutant [Fig. 1A] [14, 28].)
To confirm and extend the results obtained using the sigEp-neo bioassay, the level of the sigE transcript was monitored in its native chromosomal context in liquid cultures of the ΔcseA mutant, before and after exposure to vancomycin (10 μg ml−1), using S1 nuclease protection analysis (Fig. 1B). Consistent with the results obtained using the sigEp-neo fusion, the basal level of the sigE transcript prior to vancomycin induction was approximately threefold higher in the ΔcseA mutant than in M600 (cseA+). Despite this increased basal activity, the level of the sigE transcript in the ΔcseA mutant still increased approximately sevenfold after a 30-min exposure to vancomycin, showing that the CseBC signal transduction system remained inducible in this genetic background and led to higher maximally induced levels of the sigE transcript in the ΔcseA mutant than in the wild type. As in M600, sigE transcript levels fell back almost to uninduced levels by 90 min in the ΔcseA mutant (Fig. 1B).
The CseA start codon is misassigned.
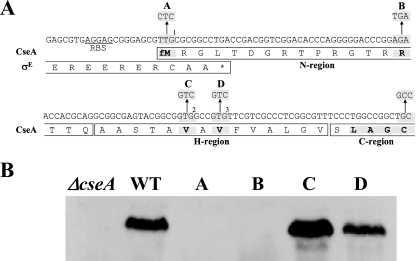
Examination of the primary sequence of CseA revealed an LAGC motif 10 residues downstream of the assigned N-terminal fMet. This sequence matches the lipobox motif characteristic of lipoproteins, typically Leu−3-Ala/Ser−2-Gly/Ala−1-Cys+1, in which the cysteine residue at position +1 is invariant (34). Production of a lipoprotein requires not just a lipobox but also a specific signal sequence. This signal sequence contains an N region, rich in the basic amino acid lysine and/or arginine, followed by an H region, rich in hydrophobic amino acids, followed by a cleavage (C) region, which incorporates the lipobox (34). The signal sequence directs protein translocation, typically via the Sec general secretion pathway, and following export, the enzyme prolipoprotein diacylglyceryl transferase attaches a diacylglyceride unit by thioether linkage to the conserved cysteine residue in the lipobox (33). The lipid-modified prolipoprotein is then cleaved of its signal sequence by lipoprotein signal peptidase II (37), and the mature lipoprotein becomes anchored in the cytoplasmic membrane by the lipid moiety, with the protein on the outside of the membrane.
The GTG start codon (GTG2 in Fig. 2A) assigned by Paget et al. (28) and by the S. coelicolor genome sequence annotation (http://www.sanger.ac.uk/Projects/S_coelicolor/) (3) is only 13 codons upstream of the lipobox cysteine codon (Fig. 2A). However, as noted by Paget et al. (28), the cseA start codon was hard to identify by bioinformatic means and was only tentatively assigned to GTG2. There is a second in-frame GTG (GTG3 in Fig. 2A) two codons further downstream, but both GTGs lack a credible ribosome binding site. Further analysis of the DNA sequence upstream of GTG2 revealed a potential TTG start codon, 7 bp upstream of the sigE stop codon (TTG1 in Fig. 2A). TTG start codons are rare in the high-G+C streptomycetes, with only 3% of genes starting at TTG in S. coelicolor (65% start at ATG and 32% start at GTG). However, if translation of CseA started at TTG1, the N terminus of the protein would represent a good match for a lipoprotein signal sequence. Furthermore, TTG1 is preceded with appropriate spacing (8 bp) by a strongly predicted Streptomyces ribosome binding site (AGGAG). In order to determine the true cseA start codon, TTG1, GTG2, and GTG3 were individually mutated to synonymous codons and a stop codon was introduced between TTG1 and GTG2 (Fig. 2A). Each of the resulting alleles was cloned into the integrative vector pSET152 (5) to generate a series of plasmids identical to pIJ6962 (carrying wild-type cseA), except for the intended mutation. The wild-type cseA gene and the four mutant alleles were then introduced in trans in single copy into the ΔcseA mutant (J2172), and extracts of each strain were immunoblotted with polyclonal anti-CseA antiserum (raised as described in Materials and Methods). Changing TTG1 to CTC abolished detection of CseA, as did the introduction of the stop codon between TTG1 and GTG2 (Fig. 2B). In contrast, changing GTG2 or GTG3 to GTC had no effect on translation of the protein (Fig. 2B). Thus, translation of cseA initiates at TTG1, internal to the sigE gene, and CseA therefore carries a long but credible N-terminal lipoprotein signal sequence (Fig. 2A).
FIG. 2.
Mutational analysis of cseA translation and sequence of the CseA lipoprotein signal peptide. (A) The three potential cseA start codons, TTG1, GTG2, and GTG3, and the substitutions made in them are highlighted, as is the TGA stop codon introduced between TTG1 and GTG2. Translation from TTG1, internal to the sigE gene, would result in a protein with an N-terminal lipoprotein signal sequence containing N, H, and C regions and an LAGC lipobox motif (highlighted). (B) Immunoblot analysis of cseA mutants. The lane lettering refers to the codon substitutions highlighted in panel A. Changing TTG1 to CTC abolishes detection of CseA, as does the introduction of the stop codon between TTG1 and GTG2. In contrast, changing GTG2 or GTG3 to GTC has no effect on translation of the protein. Strains were grown for 16 h in NMMP liquid medium containing 2 mM MgCl2. WT, wild type.
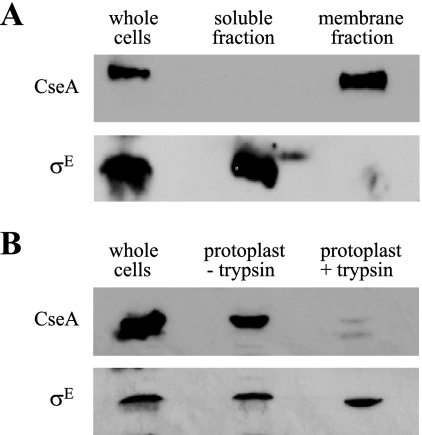
CseA is localized to the membrane.
To determine if CseA is membrane associated, we fractionated S. coelicolor crude cell extracts by ultracentrifugation and immunoblotted each fraction with anti-CseA antibodies. CseA was found exclusively in the membrane fraction (Fig. 3A). As a control, antiserum against σE was used to probe the same extracts. As expected, σE was detected in the whole-cell and cytoplasmic extracts but was absent from the membrane fraction (Fig. 3A). To determine the orientation of CseA, protoplasts (cells lacking cell walls) were prepared and incubated at 37°C for 1 h in the presence or absence of trypsin. Following incubation, extracts were prepared and analyzed by immunoblotting. The amount of CseA protein detected in the protoplasts treated with trypsin was dramatically decreased compared with that in untreated protoplasts, strongly suggesting that CseA is exposed on the outside of the cytoplasmic membrane (Fig. 3B). The σE control was unaffected by trypsin treatment of protoplasts (Fig. 3B).
FIG. 3.
(A) Subcellular localization of CseA. M600 was grown for 16 h in NMMP liquid medium containing 2 mM MgCl2. (B) “Protoplast shaving” experiments to determine if CseA is exposed on the external face of the membrane. M600 was grown in YEME liquid medium containing 0.5% (wt/vol) glycine for 40 h. Protoplasts were prepared and incubated with or without trypsin before immunoblotting.
CseA is a lipoprotein.
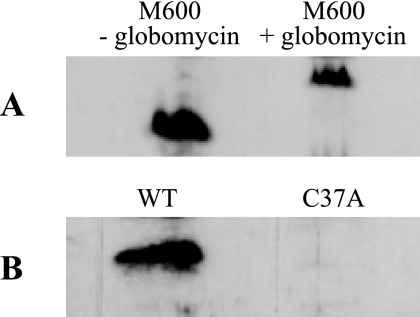
To determine if CseA is a lipoprotein, we used the antibiotic globomycin, a potent inhibitor of lipoprotein signal peptidase II (19). Normally, prolipoproteins carrying their signal peptide are not detected in whole-cell extracts. However, inhibition of lipoprotein signal peptidase II blocks removal of the lipoprotein signal peptide, causing a shift in mobility of the lipoprotein on SDS-polyacrylamide gels (35, 36). Whole-cell extracts were prepared from wild-type S. coelicolor grown in the presence or absence of globomycin, and CseA was detected by immunoblotting. Exposure of the mycelium to globomycin (150 μg ml−1) resulted in a shift in the mobility of CseA consistent with retention of the signal sequence (Fig. 4A), showing that CseA is a lipoprotein.
FIG. 4.
Immunoblot analysis of CseA processing. (A) Wild-type S. coelicolor was grown for 16 h in NMMP liquid medium containing 2 mM MgCl2 in the presence or absence of globomycin (150 μg ml−1), an inhibitor of lipoprotein signal peptidase II; whole-cell extracts were prepared, and CseA was detected by immunoblotting. (B) The cseA deletion mutant (J2172) carrying pIJ6962 (wild-type [WT] cseA) or pIJ6967 (C37A mutant) was grown for 16 h in NMMP liquid medium containing 2 mM MgCl2, and CseA was detected by immunoblotting.
To confirm and extend our analysis, we mutated the conserved lipobox cysteine to alanine. This cysteine would represent the site of attachment of the lipid, and so its conversion to alanine should block the action of prolipoprotein diacylglyceryl transferase and cause the translocated protein either to become trapped in the cell membrane (via its uncleaved signal peptide) or be exported into the medium as the result of the exposure of cryptic signal peptidase 1 sites in the signal peptide. CseA C37A was undetectable in whole-cell extracts (Fig. 4B) and in the culture supernatant (data not shown), suggesting that the failure to correctly process the preprolipoprotein results in its proteolytic degradation, possibly preventing deleterious effects of preprolipoprotein accumulation on membrane function.
Two-component systems genetically associated with lipoproteins are common in S. coelicolor.
The realization that CseA is a lipoprotein prompted bioinformatic analysis of the genome sequence of S. coelicolor to see if there are other examples of two-component systems genetically associated with lipoproteins in this organism. This analysis revealed five other sensor kinase genes which form operons with genes encoding putative lipoproteins (Fig. 5). Four of these are also linked to response regulator genes, presumably forming two-component systems (Fig. 5). However, while the five sensor kinase proteins are all ∼40% identical to CseC, the five lipoproteins do not share significant similarity with CseA. The sigE operon is also the only operon in S. coelicolor to encode both a sigma factor and a two-component system. One of the other five operons, the afsQ operon, is divergently transcribed from a sigma factor gene (sigQ, Fig. 5) whose product is closely related to σE, but expression of sigQ is not regulated by the AfsQ system, at least under the conditions tested (M. I. Hutchings, unpublished). The six sensor kinases represented in Fig. 6 have predicted extracytoplasmic sensor domains of between 99 and 167 amino acids.
FIG. 5.
Two-component systems genetically associated with lipoproteins in S. coelicolor. Unnamed genes are identified by their SCO numbers (http://www.sanger.ac.uk/Projects/S_coelicolor/). There is no response regulator gene associated with the SCO5304-5 operon.
FIG. 6.
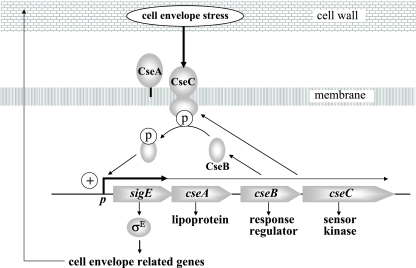
Model for the σE cell envelope stress response. Expression of the gene encoding σE (sigE) is regulated at the level of transcription by the CseB/CseC two-component signal transduction system. In response to signals originating in the cell envelope when it is under stress, the sensor kinase, CseC, becomes autophosphorylated and transfers this phosphate to the response regulator, CseB. Phospho-CseB activates the promoter of the sigE operon, and σE is recruited by core RNA polymerase to transcribe genes with cell envelope-related functions. Note that >90% transcription from the sigE promoter terminates just downstream of sigE and that the sigE promoter itself is not a σE target (28). CseA is a lipoprotein localized to the extracytoplasmic face of the cell membrane, and loss of CseA results in upregulation of the sigE promoter. CseA might interact directly with the sensor domain of CseC, or, alternatively, loss of CseA might lead to destabilization of the cell envelope, which in turn leads to induction of the CseBC signal transduction system.
The function of these systems is unknown, but one operon, SCO3011-SCO3013, appears to be an orthologue of the mtrAB-lpqB operon of Mycobacterium tuberculosis. This operon is conserved in all published actinomycete genomes with the exception of the obligate intracellular human pathogen Tropheryma whipplei, which has a greatly reduced genome (4, 16). In Corynebacterium glutamicum, the mtrA-mtrB-lpqB operon has been associated with several phenotypes linked to cell envelope organization, including altered sensitivities to cell-wall-specific antibiotics (24). In M. tuberculosis, the mtrA gene could be disrupted only when a copy was introduced in trans, suggesting that this response regulator, which shares 75% identity with SCO3013, is essential (40). Recently, Rajagopalan and colleagues demonstrated that MtrA binds to and activates the dnaA promoter in vivo, suggesting that MtrA has a role in the regulation of DNA replication (9). Unexpectedly, the sensor kinase, MtrB, which is 50% identical to S. coelicolor SCO3012, is nonessential in M. tuberculosis (40). It therefore seems likely that MtrA can be phosphorylated in the absence of MtrB, either by another sensor kinase or by a small-molecule phosphodonor such as acetyl phosphate. The function of the lipoprotein, LpqB, encoded by the third gene in the operon, has not been studied.
How does CseA exert its effect?
From the data given above, it is clear that loss of CseA from the external face of the cytoplasmic membrane leads to upregulation of the sigE promoter (Fig. 6). One possible interpretation of these data is that loss of CseA leads to destabilization of the cell envelope, which in turn leads to induction of the CseBC signal transduction system. By analogy, loss of the YfgL lipoprotein from E. coli results in a broad range of cell envelope defects and upregulation of σE activity (26). Alternatively, given that CseA and CseC are encoded in the same operon, CseA might interact directly with the sensor domain of CseC to modulate its activity. Again, by analogy, the KapB lipoprotein is required for sporulation in B. subtilis, and Dartois et al. (8) have postulated that KapB acts as an accessory protein to the KinB sensor kinase involved in triggering this process. Thus far, we have been unable to show direct interaction between CseA and the sensor domain of CseC using in vivo E. coli two-hybrid analysis, in vitro pull-down experiments, or membrane cross-linking followed by immunoblotting. However, working with isolated domains of membrane proteins is problematic, and to date, a direct biochemical interaction between a sensor kinase and a proposed accessory lipoprotein has not been demonstrated in any system.
Acknowledgments
We thank David Hopwood, Ray Dixon, Mike Merrick, Govind Chandra, and Paul Straight for helpful discussion and/or comments on the manuscript and Masatoshi Inukai (Sankyo Co. Ltd., Japan) for the kind gift of globomycin.
This work was funded by Biotechnology and Biological Sciences Research Council (BBSRC) grant 208/P14575 (to M.J.B.) and by a grant-in-aid to the John Innes Centre from the BBSRC.
REFERENCES
- 1.Alba, B. M., J. A. Leeds, C. Onufryk, C. Z. Lu, and C. A. Gross. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE-dependent extracytoplasmic stress response. Genes Dev. 16:2156-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, S. D., K. F. Chater, A. M. Cerdeño-Tárraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, W. C. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 4.Bentley, S. D., M. Maiwald, L. D. Murphy, M. J. Pallen, C. A. Yeats, L. G. Dover, H. T. Norbertczak, G. S. Besra, M. A. Quail, D. E. Harris, A. von Herbay, A. Goble, S. Rutter, R. Squares, S. Squares, B. G. Barrell, J. Parkhill, and D. A. Relman. 2003. Sequencing and analysis of the genome of the Whipple's disease bacterium Tropheryma whipplei. Lancet 361:637-644. [DOI] [PubMed] [Google Scholar]
- 5.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 6.Buelow, D. R., and T. L. Raivio. 2005. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J. Bacteriol. 187:6622-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, M., T. Wang, R. Ye, and J. D. Helmann. 2002. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 45:1267-1276. [DOI] [PubMed] [Google Scholar]
- 8.Dartois, V., T. Djavakhishvili, and J. A. Hoch. 1997. KapB is a lipoprotein required for KinB signal transduction and activation of the phosphorelay to sporulation in Bacillus subtilis. Mol. Microbiol. 26:1097-1108. [DOI] [PubMed] [Google Scholar]
- 8a.Ellermeier, C. D., and R. Losick. 2006. Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis. Genes. Dev. 20:1911-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fol, M., A. Chauhan, N. K. Nair, E. Maloney, M. Moomey, C. Jagannath, M. V. V. Madiraju, and M. Rajagopalan. 2006. Modulation of Mycobacterium tuberculosis proliferation by MtrA, an essential two-component response regulator. Mol. Microbiol. 60:643-657. [DOI] [PubMed] [Google Scholar]
- 10.Grigorova, I. L., R. Chaba, H. J. Zhong, B. M. Alba, V. Rhodius, C. Herman, and C. A. Gross. 2004. Fine-tuning of the Escherichia coli σE envelope stress response relies on multiple mechanisms to inhibit signal-independent proteolysis of the transmembrane anti-sigma factor, RseA. Genes Dev. 18:2686-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:113-1126. [DOI] [PubMed] [Google Scholar]
- 13.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 14.Hong, H.-J., M. S. B. Paget, and M. J. Buttner. 2002. A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Mol. Microbiol. 44:1199-1211. [DOI] [PubMed] [Google Scholar]
- 15.Hong, H.-J., M. I. Hutchings, J. M. Neu, G. D. Wright, M. S. B. Paget, and M. J. Buttner. 2004. Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol. Microbiol. 52:1107-1121. [DOI] [PubMed] [Google Scholar]
- 16.Hoskisson, P. A., and M. I. Hutchings. MtrAB-LpqB, a conserved three-component system in the actinobacteria? Trends Microbiol., in press. [DOI] [PubMed]
- 17.Hutchings, M. I., N. Shearer, S. Wastell, R. J. M. Van Spanning, and S. Spiro. 2000. Heterologous NNR-mediated nitric oxide signalling in Escherichia coli. J. Bacteriol. 182:6434-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchings, M. I., H-J. Hong, and M. J. Buttner. 2006. The vancomycin resistance VanRS signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 59:923-935. [DOI] [PubMed] [Google Scholar]
- 19.Inukai, M., M. Takeuchi, K. Shimizu, and M. Arai. 1978. Mechanism of action of globomycin. J. Antibiot. (Tokyo) 31:1203-1205. [DOI] [PubMed] [Google Scholar]
- 20.Isaac, D. D., J. S. Pinkner, S. J. Hultgren, and T. J. Silhavy. 2005. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. USA 102:17775-17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 21a.Mascher, T. Intramembrane-sensing histidine kinases: a new family of bacterial cell envelope stress sensors. FEMS Microbiol. Lett., in press. [DOI] [PubMed]
- 22.Mascher, T., N. G. Margulis, T. Wang, W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50:1591-1604. [DOI] [PubMed] [Google Scholar]
- 23.Mascher, T., S. L. Zimmer, T.-A. Smith, and J. D. Helmann. 2004. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob. Agents Chemother. 48:2888-8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möker, N., M. Brocker, S. Schaffer, R. Krämer, S. Morbach, and M. Bott. 2004. Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol. Microbiol. 54:420-438. [DOI] [PubMed] [Google Scholar]
- 25.Ohki, R., T. Giyanto, K. Tateno, W. Masuyama, S. Moriya, K. Kobayashi, and N. Ogasawara. 2003. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49:1135-1144. [DOI] [PubMed] [Google Scholar]
- 26.Onufryk, C., M.-L. Crouch, F. C. Fang, and C. A. Gross. 2005. Characterization of six lipoproteins in the σE regulon. J. Bacteriol. 187:4552-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paget, M. S. B., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paget, M. S. B., E. Leibovitz, and M. J. Buttner. 1999. A putative two-component signal transduction system regulates σE, a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Mol. Microbiol. 33:97-107. [DOI] [PubMed] [Google Scholar]
- 29.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599-1611. [DOI] [PubMed] [Google Scholar]
- 30.Raivio, T. L. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119-1128. [DOI] [PubMed] [Google Scholar]
- 31.Rhodius, V. A., W. C. Suh, G. Nonaka, J. West, and C. A. Gross. 2006. Conserved and variable functions of the stress response in related genomes. PLoS Biol. 4:43-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz, N., and T. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 33.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 34.Sutcliffe, I. C., and D. J. Harrington. 2002. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology 148:2065-2077. [DOI] [PubMed] [Google Scholar]
- 35.Sutcliffe, I. C., L. Tao, J. J. Ferretti, and R. R. B. Russell. 1993. MsmE, a lipoprotein involved in sugar transport in Streptococcus mutans. J. Bacteriol. 175:1853-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjalsma, H., V. P. Kontinen, Z. Pragai, H. Wu, R. Meima, G. Venema, S. Bron, M. Sarvas, and J. M. van Dijl. 1999. The role of lipoprotein processing by signal peptidase II in the Gram-positive eubacterium Bacillus subtilis. Signal peptidase II is required for the efficient secretion of alpha-amylase, a non-lipoprotein. J. Biol. Chem. 274:1698-1707. [DOI] [PubMed] [Google Scholar]
- 37.Tokunaga, M., J. M. Loranger, and H. H. Wu. 1984. Prolipoprotein modification and processing enzymes in Escherichia coli. J. Biol. Chem. 259:3825-3830. [PubMed] [Google Scholar]
- 38.Walsh, N. P., B. M. Alba, B. Bose, C. A. Gross, and R. T. Sauer. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61-71. [DOI] [PubMed] [Google Scholar]
- 39.Ward, J. M., G. R. Janssen, T. Kieser, M. J. Bibb, M. J. Buttner, and M. J. Bibb. 1986. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol. Gen. Genet. 203:468-478. [DOI] [PubMed] [Google Scholar]
- 40.Zahrt, T. C., and V. Deretic. 2000. An essential two-component signal transduction system in Mycobacterium tuberculosis. J. Bacteriol. 182:3832-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]