In this issue of the Journal of Bacteriology, Bieler et al. demonstrate (i) that the toxic activity of microcin E492 is independent of the “route of administration,” viz. whether it is added from the outside (as in nature) or expressed endogenously in the cytoplasm, and (ii) that microcin E492 requires the transporter for mannose to deploy its ion channel-forming activity. This is the strongest and most direct evidence to date that the mannose transporter is involved in microcin activity in gram-negative enterobacteria.
Bacteriocins, colicins, and microcins are proteins produced by gram-positive and gram-negative bacteria to ward off closely related competitors (5). Compared with the other means of bacterial defense, viz. secondary metabolites with antibiotic properties, hydrolytic enzymes, and exotoxins, the microcins have high specificity and potency. “Communication” through bacteriocins may establish a balance of power between bacterial communities and thus maintain the astounding diversity of microbes in nature (2, 14). Bacteriocins are a heterogeneous family of proteins that vary in size from heptapeptides containing modified amino acids (lantibiotics) to proteins of up to 40 kDa. They inhibit the growth of bacteria by forming ion channels in the cytoplasmic membrane, degrading DNA, blocking protein synthesis, or inhibiting peptidoglycan synthesis (24). They are encoded as precursors in operons or gene clusters together with proteases and modifying enzymes for precursor processing, immunity proteins that protect the producing cell against self-killing by the bacteriocin, and dedicated ABC transporters for bacteriocins that are exported rather than released by lysis of the producer cell.
Bieler et al. demonstrate that at least 11 C-terminal residues of microcin E492 (15) which are essential for penetration across the outer membrane probably are not essential for penetration into the inner membrane, and definitely are not essential for activity from inside. On the other hand, all modifications that favor membrane insertion from the inside increase the toxic efficacy of the cytoplasmically expressed microcin E492, viz. a cis-active N-terminal leader/targeting sequence or a trans-active mutant secretion system that can secrete proteins without a signal sequence. Bieler et al. further present convincing evidence that microcin E492 can insert directly from the cytoplasm into the inner membrane without the detour of export and reinsertion from the periplasmic face.
The susceptibility of the target cells to bacteriocins is contingent first on receptors in the outer membrane and second on a number of outer membrane, periplasmic, and inner membrane proteins, such as the Ton and Tol pathways (4, 16, 27). These proteins, which normally mediate the uptake of iron chelates and vitamins, are utilized as carriers for the translocation of the bacteriocin to its molecular target. However, there are some inner membrane proteins that not only are “parasitized” transiently as carriers but also act as “cofactors” of bacteriocin action.
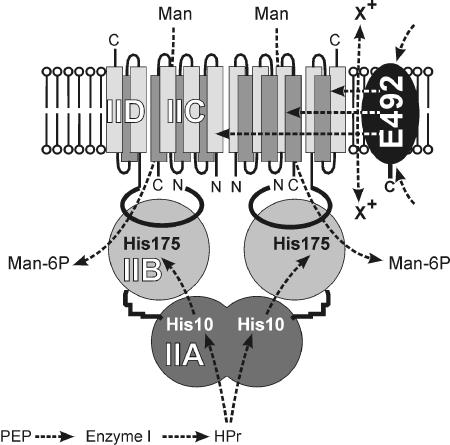
Bieler et al. demonstrate that the mannose transporter of the phosphoenolpyruvate:sugar phosphotransferase system (17, 20, 26) is absolutely essential and directly involved in the toxic effect, viz. membrane depolarization by microcin E492. This transporter consists of three subunits: IIABMan (ManX), IICMan (ManY), and IIDMan (ManZ) (8, 28). IIABMan (35 kDa) is a two-domain hydrophilic subunit with two phosphorylation sites that are necessary for the transport and phosphorylation of mannose. IICMan (29 kDa) and IIDMan (31 kDa) are membrane-embedded subunits that span the membrane six and three (or five) times, respectively (11, 13) (Fig. 1). IIC and IID are necessary and sufficient for microcin action, and, importantly, no mutations in other genes were found to confer resistance against internally produced microcin.
FIG. 1.
Topology model of the mannose transporter complex. His-10 and His-175 of IIABMan (ManX) transfer phosphate from phospho-HPr (PtsH) to the sugar that is translocated by the IIC/IID (ManY/ManZ) complex. The highly specific interaction between microcin E492 and the membrane-spanning IIC and/or IID subunit causes inner membrane depolarization. HPr is phosphorylated with phosphoenolpyruvate (PEP) by enzyme I (PtsI) of the phosphotransferase system. The model is predicted according to reference 18 with the constraints from references 11 and 13.
This is not for the first time that the membrane subunits of the mannose transporter appear to “chaperone” foreign macromolecules in or across the inner membrane: class IIa bacteriocins and bacteriophage lambda DNA also rely on these proteins. Class IIa bacteriocins are produced by gram-positive bacteria (5). They are unrelated to microcin E492 as far as size and sequence are concerned. Resistance of Listeria spp. to IIa bacteriocins was correlated with the following phenotypes: (i) absence of IIABMan in the proteomes of resistant bacteria (however, only the soluble IIAB and not the membrane-inserted IIC and IID can be detected on standard two-dimensional gels) (22), (ii) mutations in the σ54 (rpoN) factor and the σ54-dependent transcription activator ManR of the Listeria mpt operon (homologous to the Escherichia coli man operon) (3, 10, 29), (iii) a (polar?) mutation in the promoter proximal mptA (IIA) cistron (22), and (iv) in-frame deletions in the mptD (IID) gene (which may have compromised the folding and stability of IID and IIC) (3). The interaction with bacteriophage lambda was discovered by Scandella and Arber (25) while they were looking for host-specific restriction properties. They found E. coli mutants that were resistant to low doses of bacteriophage lambda. The adsorption of phage lambda to these mutants was normal, but the phage DNA was not injected. This so called Pel− phenotype (penetration of lambda [6]) is caused by the loss of the IICMan and/or IIDMan subunit. The pel defect is phage specific, conferring resistance to phages λ, 434, Hy-2, and 82 but not to the closely related phage φ80 or to the unrelated phages T4, P1, and M13. Resistance was not absolute but leaky (small plaques and 105-fold reduced efficiency of plating), and it was more pronounced against phages with smaller-than-wild-type genomes (7, 12).
It is not clear which properties make the IIC/IID complex a vulnerable spot for macromolecular invasion. The fact that microcin E492 can penetrate into the membrane from both sides could indicate that the IIC/IID complex is symmetrically oriented in the membrane. Indeed, a functional symmetry of the IIC/IID complex has been proposed by Beneski et al. based on their observation that the complex could phosphorylate mannose on the inside as well as outside of membrane vesicles, while the functionally related but structurally different glucose transporter phosphorylated glucose only on the inside (1). This symmetry, however, has not been confirmed. Although symmetrical, dual-topology membrane proteins exist (19, 23), they are much smaller than the IIC/IID complex.
It is also not known with which of the two subunits (i.e., IIC or IID) microcin E492, IIa bacteriocins, and the tail proteins of bacteriophage lambda interact. IIC and IID cannot be expressed independent of each other, at least not in biochemical amounts, and they also cannot not be separated under nondenaturing conditions (9). There are two apparently conflicting reports, one indicating that a mutation in mptD (IID) suffices to confer resistance (3) and the other indicating that heterologous expression of Listeria monocytogenes mptC (IIC) confers sensitivity to a resistant Lactococcus lactis strain (21). The conflict may be resolved by assuming that Listeria IIC is unstable without IID but that it can be stabilized by heterologous L. lactis IID. A chimeric complex between IIC of E. coli and IID of Klebsiella pneumoniae supports lambda penetration, while the inverse combination is inactive (9). Taken together, these results suggest that the interaction between IIC and IID may be important for stability but that it is most probably IIC that determines the high specificity for microcin E492 and class IIa bacteriocins.
The molecular determinants of IIC and/or IID for interaction with microcin E492 could be identified with mutations that confer microcin resistance but continue to allow the transportation and phosphorylation of mannose. Such mutants could be selected with mannose as the only carbon source and with microcin E492 as the counterselectant.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Beneski, D. A., T. P. Misko, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Preparation and characterization of membrane vesicles from mutant and wild type Salmonella typhimurium. J. Biol. Chem. 257:14565-14575. [PubMed] [Google Scholar]
- 2.Czaran, T. L., R. F. Hoekstra, and L. Pagie. 2002. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. USA 99:786-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A sigma(54)-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 4.Destoumieux-Garzon, D., X. Thomas, M. Santamaria, C. Goulard, M. Barthelemy, B. Boscher, Y. Bessin, G. Molle, A. M. Pons, L. Letellier, J. Peduzzi, and S. Rebuffat. 2003. Microcin E492 antibacterial activity: evidence for a TonB-dependent inner membrane permeabilization on Escherichia coli. Mol. Microbiol. 49:1031-1041. [DOI] [PubMed] [Google Scholar]
- 5.Drider, D., G. Fimland, Y. Hechard, L. M. McMullen, and H. Prevost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott, J., and W. Arber. 1978. Escherichia coli mutants which block phage lambda DNA injection coincide with ptsM which determines a component of a sugar transport system. Mol. Gen. Genet. 161:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Emmons, S. W., V. MacCosham, and R. L. Baldwin. 1975. Tandem genetic duplications in phage lambda. III. The frequency of duplication mutants in two derivatives of phage lambda is independent of known recombination systems. J. Mol. Biol. 91:133-146. [DOI] [PubMed] [Google Scholar]
- 8.Erni, B., B. Zanolari, and H. P. Kocher. 1987. The mannose permease of Escherichia coli consists of three different proteins: amino-acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J. Biol. Chem. 262:5238-5247. [PubMed] [Google Scholar]
- 9.Esquinas-Rychen, M., and B. Erni. 2001. Facilitation of bacteriophage lambda DNA injection by inner membrane proteins of the bacterial phosphoenol-pyruvate:carbohydrate phosphotransferase system (PTS). J. Mol. Microbiol. Biotechnol. 3:361-370. [PubMed] [Google Scholar]
- 10.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Hechard, J. W. Hastings, and S. Knochel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 11.Huber, F., and B. Erni. 1996. Membrane topology of the mannose transporter of Escherichia coli K12. Eur. J. Biochem. 239:810-817. [DOI] [PubMed] [Google Scholar]
- 12.Katsura, I. 1983. Tail assembly and injection, p. 331-346. In R. W. Hendrix (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 13.Kaufmann, M. 1997. Subklonierung und Charakterisierung der zytoplasmatischen Domäne der IID-Untereinheit des Mannosetransporters von Escherichia coli. Diploma thesis, University of Bern, Bern, Switzerland.
- 14.Kerr, B., M. A. Riley, M. W. Feldman, and B. J. Bohannan. 2002. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature 418:171-174. [DOI] [PubMed] [Google Scholar]
- 15.Lagos, R., J. E. Villanueva, and O. Monasterio. 1999. Identification and properties of the genes encoding microcin E492 and its immunity protein. J. Bacteriol. 181:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazdunski, C. J., E. Bouveret, A. Rigal, L. Journet, R. Lloubes, and H. Benedetti. 1998. Colicin import into Escherichia coli cells. J. Bacteriol. 180:4993-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meadow, N. D., D. K. Fox, and S. Roseman. 1990. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu. Rev. Biochem. 59:497-542. [DOI] [PubMed] [Google Scholar]
- 18.Melen, K., A. Krogh, and G. Von Heijne. 2003. Reliability measures for membrane protein topology prediction algorithms. J. Mol. Biol. 327:735-744. [DOI] [PubMed] [Google Scholar]
- 19.Pornillos, O., Y. J. Chen, A. P. Chen, and G. Chang. 2005. X-ray structure of the EmrE multidrug transporter in complex with a substrate. Science 310:1950-1953. [DOI] [PubMed] [Google Scholar]
- 20.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, Curtiss R., J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 21.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Hechard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 22.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapp, M., E. Granseth, S. Seppala, and G. Von Heijne. 2006. Identification and evolution of dual-topology membrane proteins. Nat. Struct. Mol. Biol. 13:112-116. [DOI] [PubMed] [Google Scholar]
- 24.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 25.Scandella, D., and W. Arber. 1974. An Escherichia coli mutant which inhibits the injection of phage lambda DNA. Virology 58:504-513. [DOI] [PubMed] [Google Scholar]
- 26.Siebold, C., K. Flükiger, R. Beutler, and B. Erni. 2001. Carbohydrate transporters of the bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS). FEBS Lett. 504:104-111. [DOI] [PubMed] [Google Scholar]
- 27.Strahsburger, E., M. Baeza, O. Monasterio, and R. Lagos. 2005. Cooperative uptake of microcin E492 by receptors FepA, Fiu, and Cir and inhibition by the siderophore enterochelin and its dimeric and trimeric hydrolysis products. Antimicrob. Agents Chemother. 49:3083-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams, N., D. K. Fox, C. Shea, and S. Roseman. 1986. Pel, the protein that permits lambda DNA penetration of Escherichia coli, is encoded by a gene in ptsM and is required for mannose utilization by the phosphotransferase system. Proc. Natl. Acad. Sci. USA 83:8934-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue, J., I. Hunter, T. Steinmetz, A. Peters, B. Ray, and K. W. Miller. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]